ABSTRACT
Rheumatic heart disease (RHD) remains an important global health challenge. Administration of benzathine penicillin (BPG) every 3 to 4 weeks is recommended as a secondary prophylaxis to prevent recurrent episodes of acute rheumatic fever and subsequent RHD. Following intramuscular injection, BPG is hydrolyzed to penicillin G (benzylpenicillin). However, little is known of the pharmacokinetics (PK) of BPG in pediatric populations at high risk of RHD or of the pharmacokinetic-pharmacodynamic relationship between penicillin exposure and clinically relevant outcomes. Dried blood spot (DBS) assays can facilitate PK studies in situations where frequent venous blood sampling is logistically difficult. A liquid chromatography-mass spectroscopy assay for penicillin G in plasma and DBS was developed and validated. Application of the DBS assay for PK studies was confirmed using samples from adult patients receiving penicillin as part of an infection management plan. The limit of quantification for penicillin G in DBS was 0.005 mg/liter. Penicillin G is stable in DBS for approximately 12 h at room temperature (22°C), 6 days at 4°C, and >1 month at −20°C. Plasma and DBS penicillin G concentrations for patients receiving BPG and penicillin G given via bolus doses correlated well and had comparable time-concentration profiles. There was poor correlation for patients receiving penicillin via continuous infusions, perhaps as a result of the presence of residual penicillin in the peripherally inserted central catheter, from which the plasma samples were collected. The present DBS penicillin G assay can be used as a surrogate for plasma concentrations to provide valid PK data for studies of BPG and other penicillin preparations developed to prevent rheumatic fever and RHD.
KEYWORDS: dried blood spot, penicillin, rheumatic fever
INTRODUCTION
Acute rheumatic fever (ARF) is an autoimmune condition caused by untreated group A streptococcal (GAS) infection of the upper respiratory tract and possibly of skin that can lead to rheumatic heart disease (RHD) (1). While ARF and RHD are rare in most developed countries, their prevalence and associated morbidity and mortality are high in the developing world (2). The global prevalence of RHD has been recently estimated at 34 million with a death rate of 345,000 per year (3). In Australia and New Zealand, the burden of disease from GAS and RHD among indigenous Australian, Maori, and Pasifika children is among the highest in the world (4).
One way to reduce the risk of RHD is by preventing ARF through intramuscular (i.m.) injections of 1.2 million units of benzathine penicillin G (BPG) given every 3 to 4 weeks. After i.m. injection, BPG is slowly absorbed into the circulation and hydrolyzed to penicillin G. This form of secondary prophylaxis is recommended to be given for at least 10 years (5, 6). Adherence to regular BPG injections is generally poor due to the pain and regularity of injections and to the difficulties associated with access to health care in the case of patients living in deprived and remote communities (7).
It is widely accepted that protection from GAS infection requires a plasma penicillin G concentration above the laboratory-derived MIC or a breakpoint for GAS of 0.02 mg/liter for most of the time between i.m. injections. There are, however, no convincing data demonstrating an inverse relationship between exposure to penicillin G and both GAS infection and subsequent ARF episodes. Furthermore, there is a lack of data relating to BPG pharmacokinetics (PK) in populations at highest risk of ARF. Most of the evidence for ARF dosing is from studies conducted in the 1950s that were undertaken in healthy white male military recruits. Data from these studies were used as a basis for BPG regimens in all patient groups, including Indigenous, Maori, and Pacifika children with ARF (8–12).
The development of population PK and pharmacokinetic/pharmacodynamic (PK/PD) models of penicillin G after BPG injection is a crucial step toward understanding how penicillin G concentration profiles are related to GAS colonization, infection, and ARF. Such models could be used in the formulation of dose regimens for BPG and newer longer-acting penicillins. A PK model might also inform individualized adaptive dosing regimens for at-risk vulnerable populations currently receiving secondary prophylaxis for ARF.
The measurement of drug concentrations in dried blood spots (DBS) can overcome the challenges of multiple, large-volume venesections in children and a lack of laboratory infrastructure and the logistic difficulties associated with blood sample processing, storage, and transportation in areas in which ARF is common. We have previously demonstrated the accuracy and feasibility of this method for assay of other antibiotics (13, 14). Very low (20-μl) volumes of whole blood can be collected from finger or heel prick samples and impregnated into filter paper, which is then stored in foil bags without the need for processing of plasma and immediate frozen storage. A small-diameter disc, or chad, can be punched out from the filter paper and the drug eluted into a liquid matrix prior to analysis using liquid chromatography-mass spectroscopy (LC-MS).
In the present study, we have developed and validated a DBS assay for penicillin G. To ensure its applicability to field studies of patients, in particular, children receiving BPG for secondary ARF prophylaxis, we required a limit of quantification of <0.02 mg/liter and evidence of thermal stability during collection, transport, and storage of DBS samples.
RESULTS
Assay performance.
The limit of quantitation (LOQ) and limit of detection (LOD) were 0.004 and 0.002 mg/liter for plasma assays, respectively, and 0.005 and 0.003 mg/liter for DBS assays, respectively. Data corresponding to matrix effects, process efficiency, absolute recovery, intraday variation, interday variation, and accuracy for plasma and DBS assays are shown in Table 1. Changes in hematocrit levels from 0.24 to 0.59 did not have a significant impact on the matrix effect, process efficiency, or recovery of drugs from DBS (data not shown).
TABLE 1.
Validation data for penicillin G in plasma and dried blood spots
| Parameter | Value(s) | |||
|---|---|---|---|---|
| Penicillin G concn (mg/liter) | 0.1 | 1 | 10 | 100 |
| Plasma | ||||
| % matrix effect (± SD); n = 5) | 85.5 (8.4) | 106.24 (5.2) | 110.84 (8.9) | 94.5 (3.5) |
| % process efficiency (± SD); n = 5 | 86.4 (5.2) | 116.2 (8.3) | 107.8 (3.7) | 89.8 (6.4) |
| % absolute recovery (± SD); n = 5 | 101.5 (6.8) | 109.6 (9.5) | 97.7 (8.1) | 95.8 (5.3) |
| Interday variation (RSD%)a; n = 5 | 6.33 | 5.34 | 3.96 | 4.79 |
| Intraday variation (RSD%); n = 15 | 6.64 | 5.97 | 3.74 | 5.63 |
| Accuracy (%); n = 15 | 93.5 | 107.25 | 102.4 | 111.54 |
| Red cell partition ratio (± SD)b | 0.98 (±0.02) | 0.95 (±0.08) | 1.04 (±0.11) | 0.96 (±0.11) |
| Dried blood spots | ||||
| % matrix effect (± SD); n = 5) | 95.5 (4.3) | 84.8 (2.9) | 88.2 (11.1) | 82.1 (3.3) |
| % process efficiency (± SD); n = 5 | 101.5 (5.5) | 92.5 (4.5) | 82.6 (2.4) | 87.1 (5.4) |
| % absolute recovery (± SD); n = 5 | 107.1 (8.8) | 109.1 (4.9) | 94.5 (10.4) | 106.3 (7.2) |
| Interday variation (RSD%); n = 5 | 9.73 | 8.37 | 8.63 | 5.33 |
| Intraday variation (RSD%); n = 15 | 7.33 | 7.20 | 9.05 | 4.13 |
| Accuracy (%); n = 15 | 116 | 87.5 | 92.34 | 101.8 |
RSD%, percent relative standard deviation.
Calculated using the following formula:
Penicillin concentration profiles.
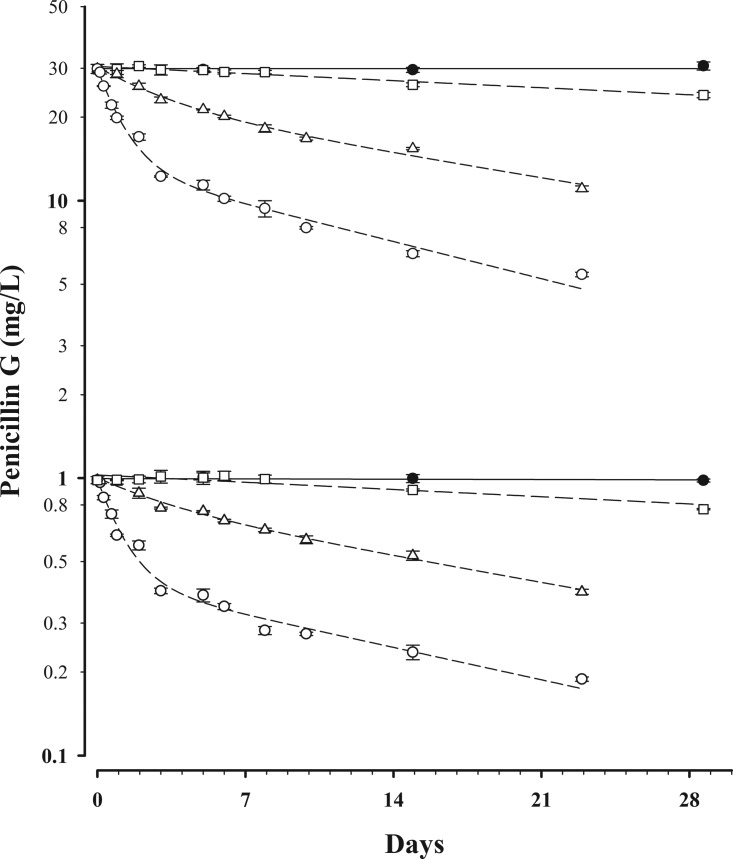
Seventeen patients (12 males and 5 females) were recruited, 4 in group 1, 4 in group 2, and 9 in group 3. The median (interquartile range [IQR]) age for the study population was 53 years (38 to 72 years). The clinical characteristics, indications, and penicillin G formulations and doses are shown in Table 2. There were a total of 57 paired DBS and plasma samples from which penicillin G concentrations were obtained. Eleven paired samples from the four group 1 patients were below the LOQ for both plasma and DBS assays. Concentration-time data for penicillin G stability in DBS are shown in Fig. 1. At 35°C, the time required to reach 95% of the initial concentration (t95) was 2.5 h for both concentrations. At room temperature (22°C), the t95 was 13 h at 30 mg/liter and 16 h at 1 mg/liter. The t95 was 6 days at 4°C, and there was no detectable degradation for at least 4 weeks at −20°C.
TABLE 2.
Clinical characteristics and dosage for participants enrolleda
| Group | Age (yrs) | Sex (M/F) | Wt (kg) | Creatinine level (μmol/liter) | HCT level | Clinical indication | Penicillin dose and formulation |
|---|---|---|---|---|---|---|---|
| 1 | 19 | M | 62 | 89 | 0.47 | Primary syphilis | Benzathine penicillin G 1.8 g |
| 1 | 40 | M | 70 | 67 | 0.42 | Primary syphilis | Benzathine penicillin G 1.8 g |
| 1 | 40 | M | 89 | 71 | 0.51 | Secondary syphilis | Benzathine penicillin G 1.8 g |
| 1 | 46 | M | 198 | 70 | 0.46 | Recurrent lower leg cellulitis | Benzathine penicillin G 1.8 g |
| 2 | 35 | M | 81 | 68 | 0.37 | Infective endocarditis | Benzylpenicillin 1.8 g 4 hourly |
| 2 | 58 | M | 81 | 116 | 0.33 | Infective endocarditis | Benzylpenicillin 1.8 g 4 hourly |
| 2 | 87 | F | 95 | 74 | 0.23 | Prosthetic joint infection | Benzylpenicillin 1.8 g 4 hourly |
| 2 | 36 | M | 92 | 66 | 0.25 | Endovascular infection | Benzylpenicillin 1.8 g 4 hourly |
| 3 | 64 | M | 54 | 50 | 0.36 | Osteoarticular infection | Benzylpenicillin infusion 10.8 g over 24 h |
| 3 | 88 | F | 69 | 79 | 0.31 | Intra-abdominal actinomycosis | Benzylpenicillin infusion 7.2 g over 24 h |
| 3 | 83 | F | 63 | 52 | 0.35 | Osteoarticular infection of the spine | Benzylpenicillin infusion 10.8 g over 24 h |
| 3 | 71 | M | 90 | 75 | 0.41 | Bacteremia | Benzylpenicillin infusion 10.8 g over 24 h |
| 3 | 28 | F | 53 | 57 | 0.33 | Bacteremia | Benzylpenicillin infusion 10.8 g over 24 h |
| 3 | 73 | F | 110 | 359 | 0.33 | Bacteremia | Benzylpenicillin infusion 5.4 g over 24 h |
| 3 | 51 | M | 89 | 102 | 0.36 | Infective endocarditis | Benzylpenicillin infusion 14.4 g over 24 h |
| 3 | 53 | M | 130 | 94 | 0.42 | Osteoarticular infection | Benzylpenicillin infusion 10.8 g over 24 h |
| 3 | 70 | M | 115 | 0.43 | Infective endocarditis | Benzylpenicillin infusion 10.8 g over 24 h |
F, female; M, male; HCT, hematocrit.
FIG 1.
Concentration-time data for penicillin G stability in DBS at initial concentrations of 1 and 30 mg/liter, stored at 35°C (○), 22°C (△), 4°C (□), and −20°C (●). Data are means ± standard deviations (SD). A monoexponential (first-order) equation was fitted to the data at 4°C and −20°C, and a biexponential equation was fitted to the data at 35°C and 21°C.
The relationship between plasma concentrations and mixed capillary blood taken directly from finger prick samples and placed onto filter paper is shown, together with a Bland-Altman plot, in Fig. 2. In group 1 and group 2 patients, plasma concentrations correlated well with DBS concentrations taken directly from the finger (Spearman's rank correlation coefficient [rs] [95% confidence interval {CI95}] = 0.98 [0.95 to 0.99] and rs [CI95] = 0.98 [0.95 to 0.99], respectively; P < 0.0001 for both). The slopes (CI95) of the linear regression line for unadjusted DBS concentrations compared with plasma concentrations collected directly from the finger were 1.13 (1.08 to 1.17) and 0.76 (0.72 to 0.80) for groups 1 and 2, respectively. The Bland-Altman plot also showed that there was evidence of a bias for lower penicillin concentrations in DBS samples in group 2 patients at concentrations of >10 mg/liter. The concentration-time profiles of penicillin G in plasma (Fig. 3A) and DBS (Fig. 3B) for group 1 patients were similar.
FIG 2.
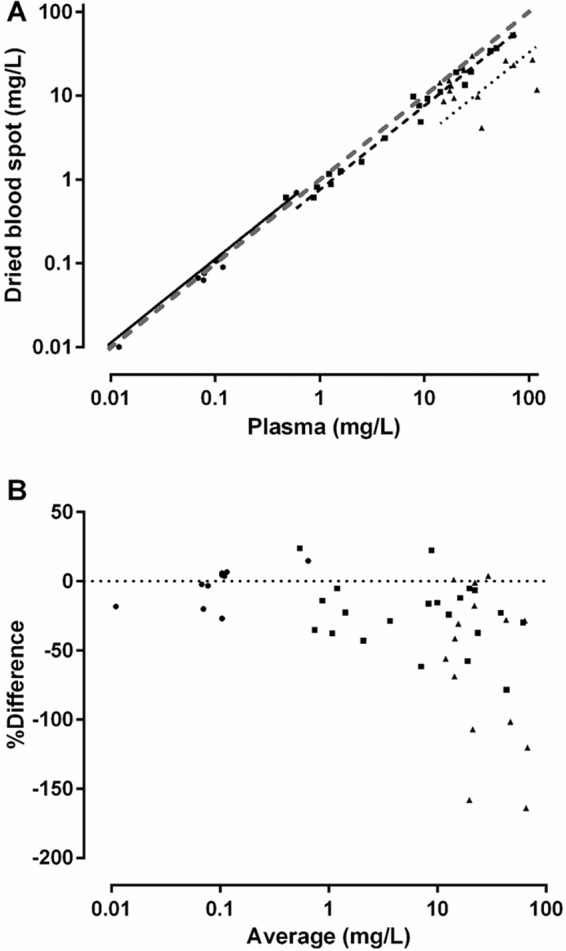
Comparison between penicillin G concentrations measured in dried blood spots and plasma (panel A) and Bland-Altman plot (panel B). Data are from patients in group 1 (●; solid linear regression line), group 2 (■; dashed linear regression line), and group 3 (▲; dotted linear regression line). A line of identity is also shown in panel A (gray dashed line).
FIG 3.
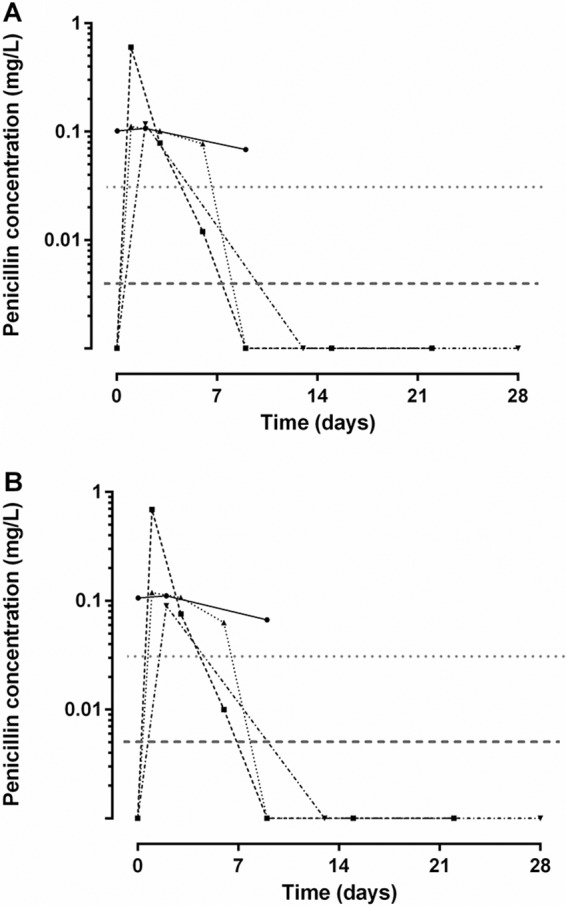
Time-concentration profiles of penicillin G in patients receiving intramuscular benzathine penicillin injections (group 1). Data show plasma concentrations (panel A) and dried blood spot concentrations (panel B). MIC for group A streptococcus (0.02 mg/liter; dotted gray line) and the limit of quantification for the assay (dashed gray line) are shown.
In group 3 patients, the correlation between plasma and finger prick DBS concentrations was poor (rs = 0.41 [−0.12 to 0.78], P = 0.11) and the slope (CI95) of the linear regression line for unadjusted DBS versus plasma concentrations collected directly from the finger was well below unity at 0.33 (0.19 to 0.47). The Bland-Altman plot also showed marked systematic bias for group 3 penicillin concentrations of >10 mg/liter. Whole-blood samples that were collected from the peripherally inserted central catheter (PICC) line at the same time as plasma samples and spotted onto the DBS filter paper correlated well with the measured plasma penicillin concentration (rs = 0.96 [0.87 to 0.99], P < 0.0001).
DISCUSSION
In the present study, we validated a novel penicillin G DBS assay against plasma concentrations in three common clinical situations in which parenteral penicillin is administered. The DBS assay proved sensitive and accurate relative to simultaneous plasma concentrations in group 1 and group 2 patients. There was poor correlation between plasma and DBS concentrations from finger prick samples in group 3 patients. It is likely that the latter finding was the result of falsely elevated plasma concentrations. In order to minimize discomfort, the members of this group were sampled through the PICC line. Although the PICC line was flushed with normal saline solution and ∼5 ml of blood was withdrawn and discarded prior to sampling, it is likely that residual penicillin G remained within the lumen of the PICC line or the access port. Overestimation of plasma concentrations in samples drawn from central venous access lines is well recognized for a range of drug assays, including antibiotics assays (15, 16).
The present DBS assay is sensitive enough to be used in PK and PK/PD studies of BPG, where concentrations are likely to be low. Indeed, the highest DBS penicillin G concentration in the four group 1 patients was 0.6 mg/liter. The LOQ for the assay was 0.005 mg/liter. It is reassuring that at least one patient had a concentration between the LOQ and the reported GAS threshold drug MIC of 0.02 mg/liter, because it demonstrates that the DBS assay covers the very low concentrations required for valid PK modeling (17). This is important where small differences in the times between BPG injections may have important clinical and programmatic implications for ARF control (18).
Thermal stability of penicillin G in DBS is a practical consideration for planning further PK and PK/PD studies because patients with RHD often live in tropical, rural, and remote settings where cold storage is not available. Our data indicate that penicillin G is not stable at or above 35°C for more than 2 h. A 1-h drying period followed by refrigeration would be recommended in very hot environments. At conventional room temperature and under refrigerated conditions, penicillin G in DBS is stable for approximately 12 h and 6 days, respectively. In these situations, samples could be collected and dried at the patient's residence, transported in an insulated vessel with ice bricks, and stored in a conventional freezer before transfer to a storage laboratory for analysis.
Although penicillin G was not detectable beyond 14 days in the patients who were treated with BPG, the remaining data cannot be used to inform a specific population PK model due to the small sample size. Unfortunately, the heaviest patient (who weighed 198 kg) had only one detectable penicillin G concentration, on day 2 after i.m. injection. Due to logistical challenges, there were no further samples taken until day 13, when penicillin concentrations were not detectable. The fact that the lightest patient still had a measurable penicillin G level at day 9 suggests that he had a more prolonged detectable drug profile, consistent with published data (19). Logistical challenges also meant that further samples were not available after that time in that patient. Taken together, the concentrations obtained in our study accord with recent population PK models of BPG in healthy young adults (19) and provide evidence of the utility of this method for further PK studies of BPG using the DBS platform. At present, further validation is required to provide confidence that the DBS platform can be used in situations where penicillin concentrations are likely to be higher than 10 mg/liter, including ambulatory care programs for patients receiving penicillin via continuous infusion.
MATERIALS AND METHODS
Ethics.
This study was approved by the Western Australian South Metropolitan Human Research Ethics Committee (15-143) and the Curtin University Human Research Ethics Committee (HR64/2016).
Study sample.
Patients were recruited from the general wards, sexual health outpatient clinics, and the outpatient parenteral antibiotic treatment program at Fiona Stanley Hospital, Western Australia. They were eligible to participate if they were receiving any formulation of penicillin G as part of infection management. This included patients receiving i.m. BPG for syphilis or as prophylaxis to prevent lower leg cellulitis and parenteral penicillin G given as a bolus dose every 4 h or by 24-h continuous infusion as part of an ambulatory care management plan for severe infection. Participants were excluded if they had a history of anaphylaxis, allergy, or adverse reaction to penicillin or were currently pregnant or breastfeeding.
After written informed consent had been obtained, baseline characteristics, including age, gender, weight, serum creatinine level, and hematocrit level and the presence of comorbidities, were recorded. The sampling schedule for each patient varied according to the penicillin preparation, dosing interval, and patient and study team availability. Paired venous and DBS samples were collected predose and 1, 2, 3, 4, 5, 7, 14, 21, and 28 days after i.m. BPG (group 1). In patients treated with regular bolus doses of intravenous benzylpenicillin (group 2) administered every 4 h, samples were collected at 0, 15, and 30 min and 1, 2, 2.5, 3.5, and 4 h (predose); 5, 6, 7, and 8 h (predose); 12 h (predose); 20 h (predose); and 24 h (predose). In patients receiving continuous infusions (group 3), samples were taken weekly at the time of routine blood sampling, at the end of the infusion, and at a number of time points after cessation of the infusion. For group 1 and group 2 patients, venous samples for drug assay were collected via venipuncture or through a peripheral cannula. To minimize discomfort, venous samples for drug assay in group 3 were collected from the peripherally inserted central catheter (PICC) being utilized for the continuous penicillin infusion. A venous hematocrit level value and serum creatinine concentration value were obtained from the clinical record at a time point as close as possible to the time of administration of the first dose in each patient.
After collection, venous blood samples were centrifuged and plasma samples was separated and placed on ice before storage at −80°C. At each sampling time point, duplicate DBSs were collected on filter paper cards (Whatman 903 Protein Saver cards; GE Healthcare Australia Pty Ltd., Parramatta, New South Wales, Australia). Mixed capillary blood from a finger prick was taken at the same time as the venous blood was drawn. Each finger prick sample was spotted onto filter paper directly from the finger with the blood drawn by capillary action and distributed evenly over the filter paper. A 50-μl sample of the venous whole blood was also spotted onto the DBS card. The DBS cards were air-dried at room temperature for 1 to 2 h, placed in an airtight foil envelope with a single sachet of desiccant, and transported on ice before storage at −80°C.
Analytical materials and reagents.
Penicillin G sodium (molecular weight [MW], 356.4) was purchased from Sigma-Aldrich Chemicals, St. Louis, MO, USA. Deuterated penicillin G-d7 potassium salt (MW, 379.5; internal standard) was purchased from Toronto Research Chemicals Inc., Toronto, Ontario, Canada. LC-MS-grade formic acid, acetonitrile, methanol, and water were purchased from Fisher Scientific, Fair Lawn, NJ, USA. All other chemicals were of analytical grade.
Instrumentation and chromatographic conditions.
A triple-quadrupole LC-MS system (model 8040; Shimadzu, Kyoto, Japan) was used for all assays. The instrument comprised a Nexera Ultra High Performance Liquid Chromatograph (UHPLC) pump (LC-30A), a degasser (DGU-20A5), an autosampler (SIL-30A), and a column oven (CTO-30A). Interface sources, electrospray ionization (ESI), and atmospheric pressure chemical ionization (APCI) were included in the system. Authentic standards of penicillin G and penicillin G-d7 were scanned and optimized for parent and product ions. The specific precursor-product ion pair values for penicillin G and penicillin G-d7 were m/z 335.1→160.05 and m/z 341.95→160.05, respectively, and the collision energy values for penicillin G and penicillin G-d7 were −11.1 V and −11.8 V, respectively. Quantitation was performed by multiple reaction monitoring (MRM) in ESI-positive (ESI+) mode. The optimized mass spectra were acquired with an interface voltage of 4.5 kV, a detector voltage of 1.0 kV, a heat block temperature of 400°C, and a desolvation temperature of 210°C. Nitrogen was used as the nebulizer gas at a flow rate of 3 liters/min and drying gas at a flow rate of 6 liters/min. Argon was used as the collision gas at 230 kPa.
Chromatographic separation was performed on a Kinetex XB-C18 column (Phenomenex Inc., LaneCove, New South Wales, Australia) (2.1 by 50 mm, 2.6-μm pore size) at a column temperature of 40°C. The mobile phase comprised an isocratic combination of 50% solvent A (water–0.1% [wt/vol] formic acid) and 50% solvent B (methanol–0.1% [wt/vol] formic acid), pumped at a flow rate of 0.4 ml/min. The sample injection volume was 5 μl. The retention time of both penicillin G and penicillin G-d7 was 1.1 min.
Sample preparation.
Penicillin G and penicillin G-d7 stock solutions were prepared separately at 10 mg/ml in methanol. All stock solutions were stored at −80°C. The working standards were prepared by serial dilution from the primary stock. Standard curve and quality control samples (QCS) in plasma were prepared using 10 μl of relevant working standards, spiked into 1 ml of each matrix, in order to maintain the consistency of added volumes. QCS were prepared in blank plasma at concentrations of 0.1, 1, 10, and 100 mg/liter. All QCS were stored at −80°C prior to use. The standard curve range was 0.01 to 100 mg/liter.
Plasma extraction.
Plasma (10 μl) was extracted using a protein precipitation method. The extraction solution comprised methanol and acetonitrile (50:50) and contained 5 mg/liter of internal standard. Extraction solution (300 μl) was added to the sample and then subjected to vortex mixing for 1 min, and the reaction mixture was centrifuged at 1,500 × g for 10 min. The supernatant (100 μl) was mixed with 100 μl of water. Subsequent sample preparations were adapted to a deep-well plate method using 10 μl plasma and the steps described above (with the same volumes). The plate was shaken on a Thermomixer C instrument (Eppendorf AG-22331, Hamburg, Germany) at 1,000 rpm for 30 min. The plate was centrifuged in a Heraeus Megafuge 16R centrifuge, using a plate bucket (model M-20; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 2,000 × g for 10 min, and then processed as described above.
Plasma method validation.
Standard curves (0.01 to 100 mg/liter) were linear (r2, ≥0.999). Plasma intraday and interday coefficients of variation (CV; percent) were determined at 0.1, 1, 10, and 100 mg/liter. Chromatographic data were processed using LAB Solution (Version 5.56; Shimadzu, Japan). Matrix effects (ion suppression/enhancement), absolute recovery, and process efficiency were determined at 0.1, 1, 10, and 100 mg/liter using our established method (13, 14). The lower limit of quantification (LOQ) and lower limit of detection (LOD) were determined at signal-to-noise ratios of 10:1 and 3:1, respectively. A sample chromatogram of penicillin G in plasma is shown in Fig. 4.
FIG 4.
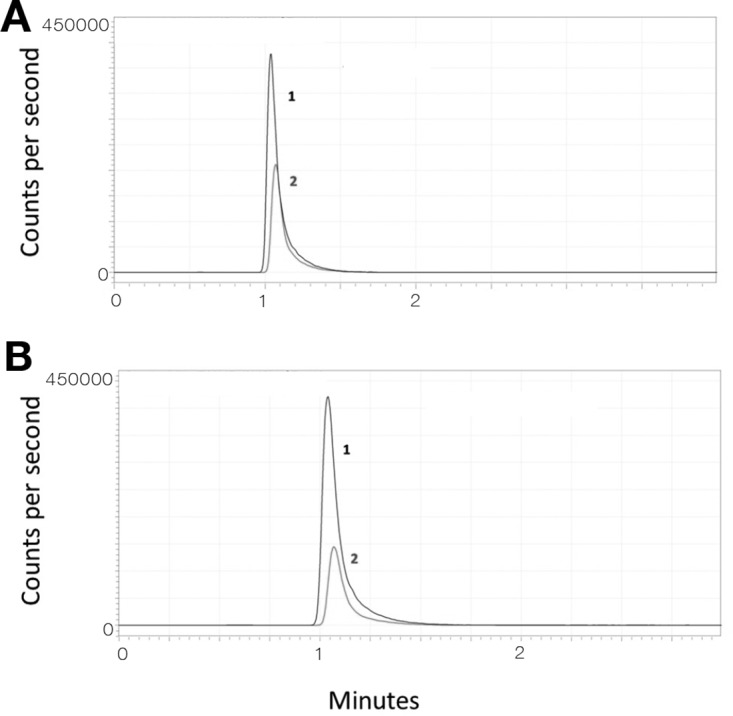
Chromatograms showing penicillin G (peak 2; gray) in plasma (panel A; 6.4 mg/liter) and dried blood spot (panel B; 5.9 mg/liter) samples from an adult patient. Peak 1 (black) represents the internal standard (penicillin G d7). The intensity of background noise in blank plasma and DBS was generally <200 cps (y axis).
Dried blood spot standard and quality control preparation.
Fresh venous human blood was collected from healthy volunteers in lithium heparin tubes, and the hematocrit levels were determined using a hemocentrifuge (Kendro Laboratory Products GmbH, Hanau, Germany; 5,000 rpm for 5 min). DBS standards and QCS were prepared using 10 μl of relevant working standards, spiked into 1 ml of each matrix, in order to maintain consistency of added volumes. QCS were prepared in blank blood at concentrations of 0.1, 1, 10, and 100 mg/liter and stored at −80°C prior to use. The standard curve range was 0.01 to 100 mg/liter. After 30 min of equilibration, the blood standards (50 μl) were spotted on the Whatman filter paper card, air-dried at room temperature (22°) for approximately 2 h, and stored as described above.
Extraction of penicillin from DBS, method validation, and blood-to-plasma partition ratio.
A single 6-mm-diameter chad was manually punched and placed into a borosilicate tube. The extraction solution for DBS comprised water, methanol, and acetonitrile (20:40:40) with 5 mg/liter of internal standard. Extraction solution (300 μl) was added to the tube, and the tube was sonicated in a water bath at room temperature for 30 min. The sample was then centrifuged at 1,500 × g for 10 min. Supernatant (100 μl) was separated and mixed with 100 μl of water for LC-MS assays. This method was also adapted to a deep-well plate, by extracting analytes from the 6-mm-diameter chads with 300 μl of extraction solution. The plate was then shaken at 1,000 rpm for 1 h and centrifuged at 2,000 × g for 10 min, and 100 μl of supernatant was mixed with 100 μl of water prior to LC-MS assay. Standard curves (0.01 to 100 mg/liter) were linear (r2, ≥0.998). DBS intraday and interday CVs were determined at 0.1, 1, 10, and 100 mg/liter. A sample chromatogram of penicillin G in DBS is shown in Fig. 4.
A range of samples with low to high hematocrit levels (0.24, 0.32, 0.50, and 0.59) were artificially prepared by adding plasma or red cells to whole blood. The matrix effect and process efficiency and recovery were assessed as described above for plasma. The blood-to-plasma partition ratio was determined at 0.1, 1, 10, and 100 mg/liter using a method established in our laboratory (13).
Penicillin G stability in dried blood spots.
Fresh whole blood was spiked with penicillin G at 1 and 30 mg/liter. Aliquots (50 μl) were spotted onto blood collection cards and air-dried at room temperature for 2 h. The DBS cards were stored at 35°C (incubator), 22°C (room temperature; monitored), 4°C (laboratory refrigerator), and −20°C until analyzed. The extraction and processing of samples were performed as described above, using triplicate DBSs at each time point. Samples were analyzed at predetermined times over a 4-week period.
A first-order degradation reaction was quantified by fitting a single exponential equation to the concentration-time data for samples stored at 4°C and −20°C. A biexponential equation provided the best model and goodness of fit for samples stored at 22°C and 35°C (confirmed with the Akaike information criterion), suggesting a consecutive first-order degradation reaction. Using these curves, the time required to reach 95% of the initial concentration (t95) was calculated.
Statistical methods.
Correlations between plasma and DBS concentrations were assessed using Spearman's rank correlation coefficient (rs) with 95% confidence intervals (CI95). Bland-Altman plots were constructed using plasma concentrations as the reference standard (GraphPad Prism version 6.05; GraphPad Software Inc., La Jolla, USA).
ACKNOWLEDGMENTS
We thank the nursing staff at Fiona Stanley Hospital for assistance in identifying patients and Bruce Sunderland for advice on drug degradation processes. We also thank the participants enrolled in this study.
This work was supported by the Telethon-Perth Children's Hospital Research Fund, a joint initiative of the Channel 7 Telethon Trust and the Western Australian Department of Health, by an NHMRC project grant (1047105), and by a Fremantle Hospital Medical Research Foundation Research grant. T.M.E.D. is supported by an NHMRC Practitioner Fellowship.
REFERENCES
- 1.Carapetis JR, McDonald M, Wilson NJ. 2005. Acute rheumatic fever. Lancet 366:155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 2.Carapetis JR. 2015. The stark reality of rheumatic heart disease. Eur Heart J 36:1070–1073. doi: 10.1093/eurheartj/ehu507. [DOI] [PubMed] [Google Scholar]
- 3.de Dassel JL, Ralph AP, Carapetis JR. 2015. Controlling acute rheumatic fever and rheumatic heart disease in developing countries: are we getting closer? Curr Opin Pediatr 27:116–123. doi: 10.1097/MOP.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 4.Roberts KV, Maguire GP, Brown A, Atkinson DN, Remenyi B, Wheaton G, Ilton M, Carapetis J. 2015. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust 203:e221–. doi: 10.5694/mja15.00139. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis JR, Brown A, Wilson NJ, Edwards KN; Rheumatic Fever Guidelines Writing Group. 2007. An Australian guideline for rheumatic fever and rheumatic heart disease: an abridged outline. Med J Aust 186:581–586. [DOI] [PubMed] [Google Scholar]
- 6.Wyber R, Zuhlke L, Carapetis J. 2014. The case for global investment in rheumatic heart-disease control. Bull World Health Organ 92:768–770. doi: 10.2471/BLT.13.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyber R, Boyd BJ, Colquhoun S, Currie BJ, Engel M, Kado J, Karthikeyan G, Sullivan M, Saxena A, Sheel M, Steer A, Mucumbitsi J, Zuhlke L, Carapetis J. 2016. Preliminary consultation on preferred product characteristics of benzathine penicillin G for secondary prophylaxis of rheumatic fever. Drug Deliv Transl Res 6:572–578. doi: 10.1007/s13346-016-0313-z. [DOI] [PubMed] [Google Scholar]
- 8.Davis PS, Copeman WS. 1957. Rheumatic diseases. Br J Clin Pract 11:936–939. [PubMed] [Google Scholar]
- 9.Stollerman GH, Rusoff JH. 1952. Prophylaxis against group A streptococcal infections in rheumatic fever patients; use of new repository penicillin preparation. JAMA 150:1571–1575. doi: 10.1001/jama.1952.03680160021005. [DOI] [PubMed] [Google Scholar]
- 10.Denny FW, Wannamaker LW, Brink WR, Rammelkamp CH Jr, Custer EA. 1950. Prevention of rheumatic fever; treatment of the preceding streptococcic infection. JAMA 143:151–153. doi: 10.1001/jama.1950.02910370001001. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein AR, Wood HF, Epstein JA, Taranta A, Simpson R, Tursky E. 1959. A controlled study of three methods of prophylaxis against streptococcal infection in a population of rheumatic children. II. Results of the first three years of the study, including methods for evaluating the maintenance of oral prophylaxis. N Engl J Med 260:697–702. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein AR, Spagnuolo M, Jonas S, Kloth H, Tursky E, Levitt M. 1968. Prophylaxis of recurrent rheumatic fever. Therapeutic-continuous oral penicillin vs monthly injections. JAMA 206:565–568. [PubMed] [Google Scholar]
- 13.Knippenberg B, Page-Sharp M, Salman S, Clark B, Dyer J, Batty KT, Davis TM, Manning L. 2016. Validation and application of a dried blood spot assay for biofilm-active antibiotics commonly used for treatment of prosthetic implant infections. Antimicrob Agents Chemother 60:4940–4955. doi: 10.1128/AAC.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page-Sharp M, Nunn T, Salman S, Moore BR, Batty KT, Davis TM, Manning L. 2015. Validation and application of a dried blood spot ceftriaxone assay. Antimicrob Agents Chemother 60:14–23. doi: 10.1128/AAC.01740-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naschitz JE, Gagarin A, Schor RG. 2008. Spurious toxic vancomycin levels. Eur J Intern Med 19:e36–. doi: 10.1016/j.ejim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Shulman RJ, Ou C, Reed T, Gardner P. 1998. Central venous catheters versus peripheral veins for sampling blood levels of commonly used drugs. JPEN J Parenter Enteral Nutr 22:234–237. doi: 10.1177/0148607198022004234. [DOI] [PubMed] [Google Scholar]
- 17.Broderick MP, Hansen CJ, Faix DJ. 2012. Factors associated with loss of penicillin G concentrations in serum after intramuscular benzathine penicillin G injection: a meta-analysis. Pediatr Infect Dis J 31:722–725. doi: 10.1097/INF.0b013e31825051d4. [DOI] [PubMed] [Google Scholar]
- 18.Lue HC, Wu MH, Hsieh KH, Lin GJ, Hsieh RP, Chiou JF. 1986. Rheumatic fever recurrences: controlled study of 3-week versus 4-week benzathine penicillin prevention programs. J Pediatr 108:299–304. doi: 10.1016/S0022-3476(86)81009-5. [DOI] [PubMed] [Google Scholar]
- 19.Broderick MP, Hansen CJ, Russell KL, Kaplan EL, Blumer JL, Faix DJ. 2011. Serum penicillin G levels are lower than expected in adults within two weeks of administration of 1.2 million units. PLoS One 6:e25308. doi: 10.1371/journal.pone.0025308. [DOI] [PMC free article] [PubMed] [Google Scholar]