ABSTRACT
Escherichia coli sequence type 131 (ST131) predominates globally among multidrug-resistant (MDR) E. coli strains. We used whole-genome sequencing (WGS) to investigate 63 MDR E. coli isolates from 7 North Carolina community hospitals (2010 to 2015). Of these, 39 (62%) represented ST131, including 37 (95%) from the ST131-H30R subclone: 10 (27%) from its H30R1 subset and 27 (69%) from its H30Rx subset. ST131 core genomes differed by a median of 15 (range, 0 to 490) single-nucleotide variants (SNVs) overall versus only 7 within H30R1 (range, 3 to 12 SNVs) and 11 within H30Rx (range, 0 to 21). The four isolates with identical core genomes were all H30Rx. Epidemiological and clinical characteristics did not vary significantly by strain type, but many patients with MDR E. coli or H30Rx infection were critically ill and had poor outcomes. H30Rx isolates characteristically exhibited fluoroquinolone resistance and CTX-M-15 production, had a high prevalence of trimethoprim-sulfamethoxazole resistance (89%), sul1 (89%), and dfrA17 (85%), and were enriched for specific virulence traits, and all qualified as extraintestinal pathogenic E. coli. The high overall prevalence of CTX-M-15 appeared to be possibly attributable to its association with the ST131-H30Rx subclone and IncF[F2:A1:B−] plasmids. Some phylogenetically clustered non-ST131 MDR E. coli isolates also had distinctive serotypes/fimH types, fluoroquinolone mutations, CTX-M variants, and IncF types. Thus, WGS analysis of our community hospital source MDR E. coli isolates suggested ongoing circulation and differentiation of E. coli ST131 subclones, with clonal segregation of CTX-M variants, other resistance genes, Inc-type plasmids, and virulence genes.
KEYWORDS: extended-spectrum β-lactamase (ESBL), CTX-M, multidrug-resistant (MDR) Escherichia coli, whole-genome sequencing, community hospitals
INTRODUCTION
Multidrug-resistant (MDR) Escherichia coli, including strains that produce extended-spectrum β-lactamases (ESBLs), are a growing clinical and public health challenge. Since 2000, CTX-M enzymes have become the most prevalent ESBL type in Gram-negative species, particularly among E. coli and other Enterobacteriaceae (1).
Currently, E. coli sequence type (ST) 131 (ST131), associated with CTX-M-15-type ESBLs, predominates among MDR E. coli strains in the United States and worldwide (1–3). Its H30 subclone underlies the ST131 expansion (2). We showed previously that CTX-M-producing E. coli ST131 was prevalent among ESBL-producing isolates from community hospitals (4). Few other reports address ESBL-producing E. coli from U.S. community settings (5, 6).
Whole-genome sequencing (WGS), an emerging tool for advanced molecular epidemiological investigations involving MDR organisms, allows identification of extended resistance and virulence genotypes and elucidation of transmission routes (7–13). Recently, WGS-based phylogenetic analyses of E. coli ST131 strains identified a CTX-M-15-associated sublineage, designated H30Rx, within ST131-H30R, which is the fluoroquinolone-resistant subset within ST131-H30 (11). Such findings suggest that H30Rx contributes importantly to extensive antimicrobial resistance.
Here, we used WGS to investigate the clinical and molecular epidemiology of recent MDR E. coli isolates, especially CTX-M-producing E. coli ST131, from patients with clinically significant infections in North Carolina community hospitals. We also analyzed resistance genes, virulence genes, and plasmid types in relation to phylogeny.
(This work was presented in part at IDWeek2016, New Orleans, LA [poster presentation no. 200].)
RESULTS
WGS of the 63 E. coli study isolates generated an average of 3,560,352 read pairs per isolate. Metagenomic sequence classification confirmed all the isolates as E. coli. After reference-guided assembly, all samples had ≥10× coverage over ≥75% of the genome, and 62/63 (98%) had ≥25× coverage over ≥75% of the genome (see Fig. S1 in the supplemental material). Therefore, nearly all the genome assemblies were suitable for single-nucleotide variant (SNV) calling.
ST distribution.
According to in silico multilocus sequence typing (MLST), ST131 was the most prevalent ST, accounting for 39 (62%) of the 63 study isolates. Among the remaining 24 isolates (38%), the most prevalent ST was ST405 (6; 25% of the non-ST131 isolates; 10% overall). The fimH30 allele was present in 36 (92%) of the 39 ST131 isolates versus only 2 (8%) of the 24 non-ST131 isolates (P < 0.001). By phylogroup, whereas all the ST131 isolates were from group B2 (by definition), the non-ST131 isolates were from, in descending frequency, groups D (n = 10: 2 ST38, 2 ST69, and 6 ST405), A (n = 5: 1 ST44, 1 ST167, 2 ST1284, and 1 ST1294), F (n = 3: 3 ST648), B2 (n = 3: 2 ST12 and 1 ST1193), B1 (n = 2: 2 ST224), and undefined (n = 1: 1 ST3057). By serotype, the 39 ST131 isolates were either O25:H4 (n = 38 [97%]: 36 fimH30 and 2 fimH35) or O16:H5 (n = 1 [3%]: fimH41), whereas the non-ST131 isolates exhibited other serotypes, including the 6 ST405 isolates (all O102:H6).
SNV-based phylogeny.
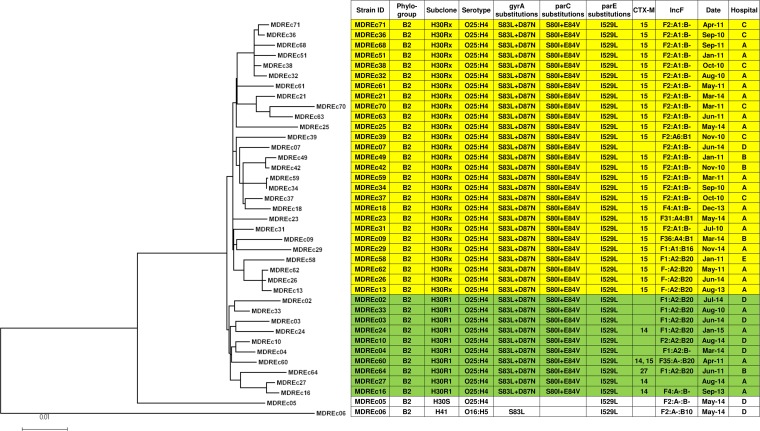
In the core genome phylogeny, the H30R1 and H30Rx clades were placed within a larger H30R clade (Fig. 1), and other STs (i.e., ST12, ST38, ST69, ST224, ST405, ST648, and ST1284) demonstrated similar sub-ST differentiation (Fig. 2). Of the 39 ST131 isolates, 37 (95%) were H30R; 10 (27%) of these were H30R1 and 27 (73%) were H30Rx, including 2 (MDREc63 and MDREc70) that were classified as H30Rx despite having fimH35, since both were placed with the other H30Rx isolates in the WGS phylogeny. In pairwise comparisons, the median number of SNV differences between different core genomes was 15 within ST131 overall (range, 0 to 490), including only 7 within H30R1 (range, 3 to 12 SNVs) and 11 within H30Rx (range, 0 to 21), compared with 6,627 (range, 12 to 15,128) for the non-ST131 genomes overall and 24 (range, 13 to 36) for the ST405 genomes.
FIG 1.
Maximum-likelihood phylogram with metadata for 39 multidrug-resistant E. coli ST131 strains. The tree with the highest log likelihood (−14,477.1472) is shown. The tree is drawn to scale, with branch lengths measured in numbers of substitutions per site. The analysis involved 39 nucleotide sequences. All positions containing gaps and missing data were eliminated. The final data set contained 4,015 total positions. Yellow shading, H30Rx isolates; green shading, H30R1 isolates.
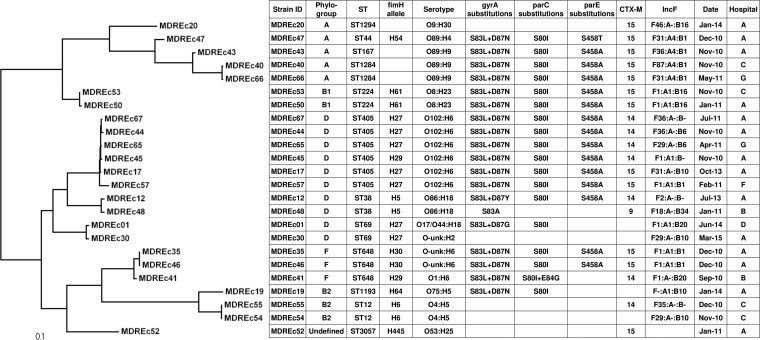
FIG 2.
Maximum-likelihood phylogram with metadata for 24 multidrug-resistant E. coli non-ST131 strains. The tree with the highest log likelihood (−120,371.7695) is shown. The tree is drawn to scale, with branch lengths measured in numbers of substitutions per site. The analysis involved 24 nucleotide sequences. All positions containing gaps and missing data were eliminated. The final data set contained 17,336 total positions.
The four instances in which different isolates exhibited identical core genomes (i.e., no SNVs) involved H30Rx isolates: (i) MDREc07 and MDREc42 (hospitals D, 2013, and B, 2010); (ii) MDREc13, MDREc26, and MDR62 (hospital A, 2013, 2014, and 2011); (iii) MDREc32, MDREc36, and MDREc38 (hospital A and hospital C, all 2010); and (iv) MDREc34 and MDREc59 (hospital A, 2010 and 2011). Some of these indistinguishable isolates shared the same hospital location at almost the same time (MDREc36 and MDREc38), suggesting either nosocomial transmission or multiple introductions of the same clone from our community, or at different times (MDREc13, MDREc26, and MDREc62; MDREc34 and MDREc59), suggesting local endemicity for specific clones.
Patient characteristics.
Of the 63 source patients for the study isolates (Table 1), 37 (59%) were admitted from home and 29 (46%) had a urinary tract infection. Community onset health care-associated infections were the most common (30 [48%]), followed by community-acquired infections (22 [35%]); hospital-acquired infections were uncommon (6 [10%]). Forty-nine (78%) patients survived, while 5 (8%) died during hospitalization. Clinical and epidemiological characteristics did not vary significantly in relation to strain type, except for an association of non-H30Rx ST131 isolates with hospital D.
TABLE 1.
Clinical and epidemiological characteristics of 63 community hospital patients with multidrug-resistant E. coli infections
| Categorya | Specific variableb | No. of isolates (column %) |
|||||
|---|---|---|---|---|---|---|---|
| Total (n = 63) | ST131 (n = 39) | Non-ST131 (n = 24) | ST131 H30Rx (n = 27) | ST131 non-H30Rx (n = 12) | All non-H30Rx (n = 36) | ||
| Age >65 | 38 (60) | 24 (62) | 14 (58) | 16 (59) | 8 (67) | 22 (61) | |
| Sex | Male | 30 (48) | 19 (49) | 11 (46) | 14 (52) | 5 (42) | 16 (44) |
| Female | 26 (41) | 18 (46) | 8 (33) | 11 (41) | 7 (58) | 15 (42) | |
| Unknown | 7 (11) | 2 (5) | 5 (21) | 2 (7) | 0 | 5 (14) | |
| Race | African American | 19 (30) | 12 (31) | 7 (29) | 8 (30) | 4 (33) | 11 (31) |
| American Indian | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | |
| Asian | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | |
| Caucasian | 33 (52) | 23 (59) | 10 (42) | 15 (56) | 8 (67) | 18 (50) | |
| Hispanic | 1 (2) | 1 (3) | 0 | 1 (4) | 0 | 0 | |
| Unknown | 8 (13) | 3 (8) | 5 (21) | 3 (11) | 0 | 5 (14) | |
| Community hospital | A | 35 (56) | 21 (54) | 14 (58) | 16 (59) | 5 (42) | 19 (53) |
| B | 6 (10) | 4 (10) | 2 (8) | 3 (11) | 1 (8) | 3 (8) | |
| C | 10 (16) | 6 (15) | 4 (17) | 6 (22) | 0 | 4 (11) | |
| D | 8 (13) | 7 (18) | 1 (4) | 1 (4) | 6 (50) | 7 (19) | |
| E | 1 (2) | 1 (3) | 0 | 1 (4) | 0 | 0 | |
| F | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | |
| G | 2 (3) | 0 | 2 (8) | 0 | 0 | 2 (6) | |
| Admission source | Home | 37 (59) | 21 (54) | 16 (67) | 12 (44) | 9 (75) | 25 (69) |
| Nursing home | 13 (21) | 10 (26) | 3 (13) | 8 (30) | 2 (17) | 5 (14) | |
| Transfer from hospital | 4 (6) | 3 (8) | 1 (4) | 2 (7) | 1 (8) | 2 (6) | |
| Other extended-care facility | 3 (5) | 3 (8) | 1 (4) | 3 (11) | 0 | 1 (3) | |
| Unknown | 5 (8) | 2 (5) | 3 (13) | 2 (7) | 0 | 3 (8) | |
| Type of infection | Urinary tract infection | 29 (46) | 19 (49) | 10 (42) | 13 (48) | 6 (50) | 16 (44) |
| Bloodstream infection | 21 (33) | 14 (36) | 7 (29) | 9 (33) | 5 (42) | 12 (33) | |
| Pneumonia | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | |
| Other | 7 (11) | 4 (10) | 3 (13) | 3 (11) | 1 (8) | 4 (11) | |
| Unknown | 5 (8) | 2 (5) | 3 (13) | 2 (7) | 0 | 3 (8) | |
| Acquisition | Community acquired | 22 (35) | 14 (36) | 8 (33) | 8 (30) | 6 (50) | 14 (39) |
| Community onset, health care associated | 30 (48) | 20 (51) | 10 (42) | 15 (56) | 5 (42) | 15 (42) | |
| Hospital onset, health care associated | 6 (10) | 3 (8) | 3 (13) | 2 (7) | 1 (8) | 4 (11) | |
| Unknown | 5 (8) | 2 (5) | 3 (13) | 2 (7) | 0 | 3 (8) | |
| ICU stay | Before infection | 4 (6) | 2 (5) | 2 (8) | 1 (4) | 1 (8) | 3 (8) |
| After infection | 46 (73) | 30 (77) | 16 (67) | 20 (74) | 10 (83) | 26 (72) | |
| Unknown | 13 (21) | 7 (18) | 6 (25) | 6 (22) | 1 (8) | 7 (19) | |
| Dialysis | Yes | 4 (6) | 3 (8) | 1 (4) | 3 (11) | 0 | 1 (3) |
| No | 51 (81) | 32 (82) | 19 (79) | 20 (74) | 12 (100) | 31 (86) | |
| Unknown | 8 (13) | 4 (10) | 4 (17) | 4 (15) | 0 | 4 (11) | |
| Outcome | Died | 5 (8) | 4 (10) | 1 (4) | 3 (11) | 1 (8) | 2 (6) |
| Survived | 49 (78) | 30 (77) | 19 (79) | 20 (74) | 10 (83) | 29 (81) | |
| Discharged home | 22 (35) | 15 (38) | 7 (29) | 9 (33) | 6 (50) | 13 (36) | |
| Discharged to hospice | 5 (8) | 2 (5) | 3 (13) | 2 (7) | 0 | 3 (8) | |
| Discharged to LTCF | 16 (25) | 11 (28) | 5 (21) | 8 (30) | 3 (25) | 8 (22) | |
| Discharged to other facility | 6 (10) | 2 (5) | 4 (17) | 1 (4) | 1 (8) | 5 (14) | |
| Unknown | 9 (14) | 5 (13) | 4 (17) | 4 (15) | 1 (8) | 5 (14) | |
Category of clinical or epidemiological characteristic.
LTCF, long-term-care facility. All comparisons (ST131 versus non-ST131, H30Rx versus non-H30Rx ST131, or H30Rx versus non-H30Rx) yielded P values of >0.05, except for community hospital category D (for H30Rx versus non-H30Rx ST131, P = 0.002).
Resistance phenotypes and genotypes.
Although most or all isolates were susceptible to imipenem, ertapenem, amikacin, and minocycline, most (80 to 90%) were resistant to trimethoprim-sulfamethoxazole and fluoroquinolones (ciprofloxacin and levofloxacin) (Table 2 and Fig. 3), drugs that are commonly used orally to treat urinary tract infections in community hospitals (4). Compared to non-H30Rx ST131 isolates, or all non-H30Rx isolates, H30Rx isolates had higher resistance prevalences for most antibiotics (Table 2). Accordingly, they had significantly higher aggregate resistance scores (median score [interquartile range], 6 [5 to 6] for H30Rx versus 3.5 [3 to 4] for non-H30Rx ST131 isolates [P < 0.001] and 4 [3 to 6] for all non-H30Rx isolates [P = 0.02]).
TABLE 2.
Antimicrobial resistance characteristics among 63 multidrug-resistant E. coli isolates from community hospitals
| Antimicrobial category | Resistance gene/antimicrobial agent | No. (column %) of isolates |
P valuec |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 63) | ST131 (n = 39) | Non-ST131 (n = 24) | ST131 H30Rx (n = 27) | ST131 non-H30Rx (n = 12) | All non-H30Rx (n = 36) | ST131 vs non-ST131 | H30Rx vs non-H30Rx ST131 | H30Rx vs all non-H30Rx | ||
| Beta-lactam | blaCTX-M-9 | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | |||
| blaCTX-M-14 | 11 (17) | 4 (10) | 7 (29) | 0 | 4 (33) | 11 (31) | 0.006 | 0.002 | ||
| blaCTX-M-15 | 39 (62) | 27 (69) | 12 (50) | 26 (96) | 1 (8) | 13 (36) | <0.001 | <0.001 | ||
| blaCTX-M-27 | 1 (2) | 1 (3) | 0 | 0 | 1 (8) | 1 (3) | ||||
| Any CTX-M | 51 (81) | 31 (79) | 20 (83) | 26 (96) | 5 (42) | 25 (69) | <0.001 | 0.009 | ||
| Aminoglycoside | aadA1 | 4 (6) | 4 (10) | 0 | 4 (15) | 0 | 0 | 0.03 | ||
| aadA5 | 45 (71) | 30 (77) | 15 (63) | 23 (85) | 7 (58) | 22 (61) | 0.05 | |||
| strA | 28 (44) | 13 (33) | 15 (63) | 7 (26) | 6 (50) | 21 (58) | 0.04 | 0.01 | ||
| strB | 27 (43) | 12 (31) | 15 (63) | 6 (22) | 6 (50) | 21 (58) | 0.02 | 0.005 | ||
| PMQRa | aac(6′)-Ib-cr | 25 (40) | 16 (41) | 9 (38) | 15 (56) | 1 (8) | 10 (28) | 0.01 | 0.04 | |
| qnrS1 | 1 (2) | 0 | 1 (4) | 0 | 0 | 1 (3) | ||||
| QRDRb | 1A1B1, gyrA | 37 (59) | 37 (95) | 0 | 27 (100) | 10 (83) | 10 (28) | <0.001 | <0.001 | |
| 20A1B1, gyrA | 8 (13) | 0 | 8 (33) | 0 | 0 | 8 (22) | <0.001 | 0.008 | ||
| 1a1A1B1, parC | 37 (59) | 37 (95) | 0 | 27 (100) | 10 (83) | 10 (28) | <0.001 | <0.001 | ||
| 5A1, parC | 11 (17) | 0 | 11 (46) | 0 | 0 | 11 (31) | <0.001 | 0.002 | ||
| 3L, parE | 39 (62) | 39 (100) | 0 | 27 (100) | 12 (100) | 12 (33) | <0.001 | <0.001 | ||
| 1A1, parE | 9 (14) | 0 | 9 (38) | 0 | 0 | 9 (25) | <0.001 | 0.008 | ||
| Sulfonamide | sul1 | 49 (78) | 32 (82) | 17 (71) | 24 (89) | 8 (67) | 25 (69) | |||
| sul2 | 28 (44) | 13 (33) | 15 (63) | 7 (26) | 6 (50) | 21 (58) | 0.04 | 0.01 | ||
| Trimethoprim | dfrA17 | 45 (71) | 30 (77) | 15 (63) | 23 (85) | 7 (58) | 22 (61) | 0.05 | ||
| Phenicol | catA1 | 5 (8) | 0 | 5 (21) | 0 | 0 | 5 (14) | 0.006 | ||
| catB3 | 25 (40) | 17 (44) | 8 (33) | 16 (59) | 1 (8) | 9 (25) | 0.005 | 0.009 | ||
| Tetracycline | tet(A) | 31 (49) | 23 (59) | 8 (33) | 17 (63) | 6 (50) | 14 (39) | |||
| tet(B) | 10 (16) | 0 | 10 (42) | 0 | 0 | 10 (28) | <0.001 | 0.003 | ||
| Phenotype | Gentamicin | 21 (33) | 12 (31) | 9 (38) | 8 (30) | 4 (33) | 13 (36) | |||
| Amikacin | 9 (14) | 7 (18) | 2 (8) | 7 (26) | 0 | 2 (6) | 0.03 | |||
| Ciprofloxacin | 56 (89) | 37 (95) | 19 (79) | 27 (100) | 10 (83) | 29 (81) | 0.02 | |||
| Levofloxacin | 55 (87) | 37 (95) | 18 (75) | 27 (100) | 10 (83) | 28 (78) | 0.05 | 0.008 | ||
| Trimethoprim-sulfamethoxazole | 49 (78) | 32 (82) | 17 (71) | 24 (89) | 8 (67) | 25 (69) | ||||
| Ampicillin-sulbactam | 55 (87) | 34 (87) | 21 (88) | 25 (93) | 9 (75) | 30 (83) | ||||
| Piperacillin-tazobactam | 24 (38) | 16 (41) | 8 (33) | 16 (59) | 0 | 8 (22) | <0.001 | 0.004 | ||
| Ceftriaxone | 52 (83) | 31 (79) | 21 (88) | 26 (96) | 5 (42) | 26 (72) | <0.001 | 0.02 | ||
| Cefepime | 46 (73) | 26 (67) | 20 (83) | 22 (81) | 4 (33) | 24 (67) | 0.008 | |||
| Ertapenem | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Imipenem | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Minocycline | 13 (21) | 2 (5) | 11 (46) | 1 (4) | 1 (8) | 12 (33) | <0.001 | 0.004 | ||
PMQR, plasmid-mediated quinolone resistance.
1A1B1, S83L/D87N; 20A1B1, S83L/D87N; 1a1A1B1, c321g/S80I/E84V; 5A1, S80I; 3L, I529L; 1A1, S458A.
Only P values of <0.05 are shown.
FIG 3.
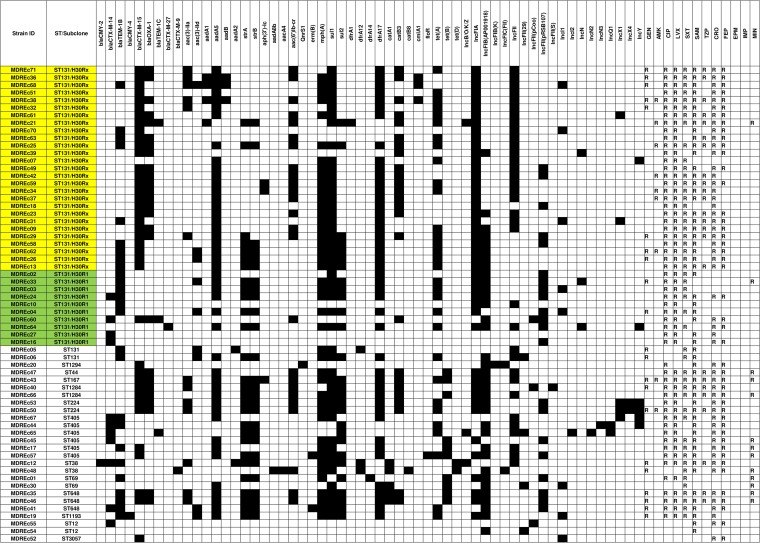
Distribution of acquired resistance genes and plasmids within 63 multidrug-resistant E. coli strains. Antimicrobial susceptibility profiles (R, resistant; blank, susceptible) and presence (black)/absence (white) of resistance genes and plasmids for each isolate are shown. SAM, ampicillin-sulbactam; TZP, piperacillin-tazobactam; CRO, ceftriaxone; FEP, cefepime; IMP, imipenem; EPM, ertapenem; GEN, gentamicin; AMK, amikacin; CIP, ciprofloxacin; LVX, levofloxacin; SXT, trimethoprim-sulfamethoxazole; MIN, minocycline. Yellow shading, H30Rx isolates; green shading, H30R1 isolates.
WGS analysis identified multiple resistance genes and plasmids, plus associations of blaCTX-M with diverse classes of antimicrobial resistance (Fig. 3; see Table S1 in the supplemental material). Whereas CTX-M-15 was closely associated with H30Rx isolates (P < 0.001), CTX-M-14 was associated with non-H30Rx ST131 isolates, both overall (P = 0.002) and within ST131 (P = 0.006) (Table 2). All 37 H30R isolates contained characteristic H30-associated quinolone resistance-determining region (QRDR) mutations, including gyrA 1A1B1 (S83L/D87N), parC 1a1A1B1 (S80I/E84V), and parE 3L (I529L) (P < 0.001 for H30Rx versus all non-H30Rx isolates and for ST131 versus non-ST131). Additionally, 15 (56%) of the 27 H30Rx isolates, but only 1 (8%) of the 12 non-H30Rx ST131 isolates, possessed the plasmid-mediated quinolone resistance determinant aac(6′)-Ib-cr (P = 0.01). H30Rx isolates also had a significantly higher frequency than did all non-H30Rx isolates of certain non-quinolone resistance genes (i.e., aadA1, aadA5, catB3, and dfrA17).
Virulence genes.
ST131 (versus non-ST131) and H30Rx (versus non-H30Rx) isolates were significantly enriched with specific virulence genes (i.e., pap, sfa, yfcV, iss, kps, iha, iuc, iutA, sat, and malX) (Table 3; see Table S2 in the supplemental material). Accordingly, they had significantly higher aggregate virulence scores (median score [interquartile range], 22 [21 to 23] for ST131 versus 14 [12.25 to 17.5] for non-ST131, P < 0.001, and 22 [21 to 23] for H30Rx versus 15.5 [13 to 21] for all non-H30Rx, P < 0.001). Likewise, a much greater proportion qualified as extraintestinal pathogenic E. coli (ExPEC), i.e., 37 (95%) of 39 ST131 isolates, including all 27 H30Rx isolates, versus 8 (33%) of 24 non-ST131 isolates (P < 0.001 for all comparisons). However, despite their differences in virulence gene content, these strain groups did not differ significantly in associated clinical outcomes (Table 1). Regardless of the strain type, 46 (73%) of 63 patients were admitted to an intensive care unit (ICU) after infection, and 10 (16%) died or were discharged to hospice.
TABLE 3.
Putative virulence genes among 63 multidrug-resistant E. coli isolates from community hospitals
| Category | Virulence gene | No. (column %) of isolates |
P valuea |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 63) | ST131 (n = 39) | Non-ST131 (n = 24) | ST131 H30Rx (n = 27) | ST131 non-H30Rx (n = 12) | All non-H30Rx (n = 36) | ST131 vs non-ST131 | H30Rx vs all non-H30Rx | ||
| Adhesin | dra | 13 (21) | 12 (31) | 1 (4) | 8 (30) | 4 (33) | 5 (14) | 0.01 | |
| fim | 59 (94) | 39 (100) | 20 (83) | 27 (100) | 12 (100) | 32 (89) | 0.02 | ||
| nfaE | 13 (21) | 12 (31) | 1 (4) | 8 (30) | 4 (33) | 5 (14) | 0.01 | ||
| pap | 44 (70) | 37 (95) | 7 (29) | 27 (100) | 10 (83) | 17 (47) | <0.001 | <0.001 | |
| sfa | 44 (70) | 37 (95) | 7 (29) | 27 (100) | 10 (83) | 17 (47) | <0.001 | <0.001 | |
| yfcV | 45 (71) | 39 (100) | 6 (25) | 27 (100) | 12 (100) | 18 (50) | <0.001 | <0.001 | |
| Protectin | iss | 48 (76) | 38 (97) | 10 (42) | 27 (100) | 11 (92) | 21 (58) | <0.001 | <0.001 |
| kfiC | 12 (19) | 11 (28) | 1 (4) | 5 (19) | 6 (50) | 7 (19) | 0.02 | ||
| kps | 47 (75) | 38 (97) | 9 (38) | 27 (100) | 11 (92) | 20 (56) | <0.001 | <0.001 | |
| Siderophore | chu | 55 (87) | 38 (97) | 17 (71) | 26 (96) | 12 (100) | 29 (81) | 0.004 | |
| fyuA | 58 (92) | 39 (100) | 19 (79) | 27 (100) | 12 (100) | 31 (86) | 0.006 | ||
| iha | 39 (62) | 36 (92) | 3 (13) | 26 (96) | 10 (83) | 13 (36) | <0.001 | <0.001 | |
| irp | 58 (92) | 39 (100) | 19 (79) | 27 (100) | 12 (100) | 31 (86) | 0.006 | ||
| iuc | 47 (75) | 35 (90) | 12 (50) | 25 (93) | 10 (83) | 22 (61) | <0.001 | 0.007 | |
| iutA | 47 (75) | 35 (90) | 12 (50) | 25 (93) | 10 (83) | 22 (61) | <0.001 | 0.007 | |
| Toxin | sat | 38 (60) | 35 (90) | 3 (13) | 25 (93) | 10 (83) | 13 (36) | <0.001 | <0.001 |
| Miscellaneous | malX | 53 (84) | 39 (100) | 14 (58) | 27 (100) | 12 (100) | 26 (72) | <0.001 | 0.003 |
| ExPEC | NAb | 45 (71) | 37 (95) | 8 (33) | 27 (100) | 10 (83) | 18 (50) | <0.001 | <0.001 |
Only P values of <0.05 are shown.
NA, not applicable.
Plasmid replicon families/types.
H30Rx isolates contained IncFIA plasmid types more frequently, and IncFIB plasmid types less frequently, than other isolates (P < 0.001) (Table 4). In silico pMLST associated IncF[F2:A1:B−] with H30Rx and IncF[F1:A2:B20] with H30R1, whereas neither of these IncF types appeared in non-ST131 isolates.
TABLE 4.
Plasmid replicon families and plasmid MLST (pMLST) among 63 multidrug-resistant E. coli isolates from community hospitals
| Plasmid replicon family/pMLST | No. (column %) of isolates |
P valuea |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 63) | ST131 (n = 39) | Non-ST131 (n = 24) | ST131 H30Rx (n = 27) | ST131 non-H30Rx (n = 12) | All non-H30Rx (n = 36) | ST131 vs non-ST131 | H30Rx vs non-H30Rx ST131 | H30Rx vs all non-H30Rx | |
| IncB/O/K/Z | 3 (5) | 1 (3) | 2 (8) | 1 (4) | 0 | 2 (6) | |||
| IncFIA | 46 (73) | 34 (87) | 12 (50) | 27 (100) | 7 (58) | 19 (53) | 0.003 | 0.001 | <0.001 |
| IncFIB | 35 (56) | 16 (41) | 19 (79) | 8 (30) | 8 (67) | 27 (75) | 0.004 | 0.04 | <0.001 |
| IncFIC | 2 (3) | 0 | 2 (8) | 0 | 0 | 2 (6) | |||
| IncFII | 55 (87) | 35 (90) | 20 (83) | 24 (89) | 11 (92) | 31 (86) | |||
| IncI | 9 (14) | 5 (13) | 4 (17) | 3 (11) | 2 (17) | 6 (17) | |||
| IncN | 5 (8) | 3 (8) | 2 (8) | 1 (4) | 2 (17) | 4 (11) | |||
| IncQ | 2 (3) | 0 | 2 (8) | 0 | 0 | 2 (6) | |||
| IncX | 5 (8) | 2 (5) | 3 (13) | 2 (7) | 0 | 3 (8) | |||
| IncY | 6 (10) | 2 (5) | 4 (17) | 1 (4) | 1 (8) | 5 (14) | |||
| pSL483 | 1 (2) | 1 (3) | 0 | 0 | 1 (8) | 1 (3) | |||
| Col | 49 (78) | 30 (77) | 19 (79) | 21 (78) | 9 (75) | 28 (78) | |||
| F2:A1:B− | 18 (29) | 18 (46) | 0 | 18 (67) | 0 | 0 | <0.001 | <0.001 | <0.001 |
| F1:A2:B20 | 6 (10) | 6 (15) | 0 | 1 (4) | 5 (42) | 5 (14) | 0.007 | ||
Only P values of <0.05 are shown.
Other results for non-ST131 isolates.
The non-ST131 isolates exhibited associations analogous to those noted among the ST131 isolates between specific STs and distinctive serotypes/fimH types (for ST12, ST38, ST69, ST224, ST405, ST648, and ST1284), QRDR mutations (for ST12, ST224, ST405, ST648, and ST1284), CTX-M variants (for ST1284, ST224, ST405, and ST648), and IncF types (for ST224 and ST648) (Fig. 2). All ST405 isolates had identical QRDR mutations (gyrA 20A1B1 [S83L/D87N], parC 5A1 [S80I], and parE 1A1 [S458A]), which were distinct from those associated with ST131.
DISCUSSION
We used WGS to analyze MDR E. coli isolates collected over 6 years from community hospitals in North Carolina to identify molecular markers of resistance, virulence, and transmission. To our knowledge, this is the first WGS study of clinical MDR E. coli isolates from community hospital inpatients.
We found that ST131 was by far the most prevalent lineage (62% overall) and was represented mainly by H30Rx (69% of ST131 isolates), followed by H30R1 (26% of ST131 isolates). This dominance of H30Rx within ST131 is likely due to our focus on MDR E. coli and the typically more extensive resistance profiles of H30Rx isolates compared with other ST131 isolates, including even members of the fluoroquinolone-resistance-associated H30R1 subclone (11).
We also documented clonal segregation of CTX-M variants, other resistance genes, and Inc-type plasmids, both within ST131 and among non-ST131 isolates. With finer resolution than MLST, WGS-based genotyping identified a variety of SNVs among isolates from the same ST or even sub-ST clade, demonstrating mainly dissemination of diverse H30R1/H30Rx strains throughout the study community rather than focal nosocomial or community outbreaks affecting a single community hospital. The endemicity documented here suggested that H30R1/H30Rx strains were circulating prior to the study period. Based on previous epidemiological data, the introduction of these strains likely occurred from 2006 through 2007, when the rate of ESBL-producing E. coli infection in our network of community hospitals rose from 0 to 12 patients per 100,000 patient-days (14).
The SNV-based phylogeny showed differentiation of our ST131 strains into the previously described H30R1 and H30Rx clades (11–13). Corresponding to this clonal structure, we identified the IncFIA and IncFII (e.g., IncF[F2:A1:B−]) replicons in most H30Rx isolates that produced CTX-M-15. While ST131 can harbor ESBL-encoding plasmids of different incompatibility groups, blaCTX-M-15 has been associated primarily with IncF, especially FIA-FII fusion or FII replicons (3). A recent WGS study of ST131 isolates from the United States likewise described associations of IncF[F2:A1:B−] with H30Rx and IncF[F1:A2:B20] with H30R1, demonstrating fixation and ongoing evolution of specific plasmids within these separate but closely related sublineages (15). Our data extend these findings to community hospital source ST131 isolates.
All H30R study isolates had distinctive H30R-associated double nonsynonymous mutations in gyrA and parC (16–18), which confer high-level quinolone resistance (13). The H30Rx strains additionally harbored aac(6′)Ib-cr (encoding a dual-function aminoglycoside- and fluoroquinolone-modifying enzyme) significantly more frequently than other strains, supporting an association of the gene specifically with H30Rx (18). They also exhibited a high prevalence of trimethoprim-sulfamethoxazole resistance (89%), sul1 (89%), and dfrA17 (85%) and had higher aggregate resistance scores than other strains, consistent with other reports from the United States and elsewhere (9, 19, 20). These features may give H30Rx strains a competitive advantage over other ESBL-producing strains, especially with use of fluoroquinolones and extended-spectrum cephalosporins (21, 22), thereby contributing to their epidemiological success.
As observed previously (23, 24), most ST131 study isolates and all H30Rx isolates qualified as ExPEC, with an extensive array of putative virulence genes and higher virulence scores, suggesting high virulence potential. However, higher virulence scores were not associated with worse clinical outcomes. Among ST131/H30Rx strains, host characteristics may confound associations of virulence genes with clinical severity and/or mortality (21). Nonetheless, in a recent analysis, the H30 subclone was significantly associated with persistent infections and adverse outcomes despite multivariable adjustment for underlying host factors and resistance to the prescribed antibiotic (21). In our study, a majority of patients with H30Rx were critically ill and had poor outcomes (i.e., 74% frequency of ICU admission after infection and 11% all-cause mortality), although this did not differ significantly from other strain groups.
It remains unclear whether the most extensively resistant E. coli ST131 strains are from the community or health care setting (2). In a recent study of unselected E. coli clinical isolates from the U.S. Midwest, ST131 was associated with young children and the elderly, antimicrobial resistance, and health care-associated infections (25). In contrast, in our North Carolina community hospital-based study of MDR E. coli isolates, we observed no significant difference in patient age or site of infection acquisition between ST131 and non-ST131 strains or between H30Rx and non-H30Rx ST131 strains. Our results establish that health care personnel in community hospitals may encounter MDR E. coli ST131 in patients with community-acquired infection, community onset health care-associated infections, or hospital onset health care-associated infections. This indicates a need to facilitate appropriate antimicrobial therapy and infection control measures against MDR E. coli ST131 in community hospitals.
In that regard, approximately 80% of the present community hospital MDR E. coli infections were present on admission with or without prior health care exposure, similar to our previous finding (4). Additionally, various movements of patients with MDR E. coli ST131-H30Rx occurred among different health care facilities, both before admission to and after discharge from our community hospitals (e.g., admission from home to community hospital and then discharge to home, nursing home, or hospice) (see Table S3 in the supplemental material). This emphasizes potential routes for strain dissemination and the important role of community hospitals and transferring/receiving health care facilities as reservoirs of MDR E. coli.
Although previous WGS studies have investigated E. coli ST131 (11–13), to our knowledge, none has addressed the molecular epidemiology of non-ST131 MDR E. coli. Here, although most non-ST131 isolates, like the ST131 isolates, produced CTX-M variants (e.g., CTX-M-15 or CTX-M-14) and exhibited quinolone resistance, they had different QRDR mutations and IncF types than did the ST131 strains. Although ST131 is currently the most prevalent MDR E. coli strain in our community and globally, attention to resistance in non-ST131 E. coli also is clearly warranted.
This study has several limitations. First, limited clinical and epidemiological data were available from our surveillance databases, although data entry and definitions were standardized across participating hospitals. Second, our sample was derived from multiple centers over a 6-year period, adding breadth to our analysis, but at the expense of depth. While this reduced our power to detect meaningful associations, it may increase the generalizability of our findings. Third, our small sample size limited the power to confirm statistical associations, particularly for non-ST131 isolates.
In summary, our WGS analysis of MDR E. coli isolates from community hospitals in North Carolina (2010 to 2015) provides insights into the diversity of their resistance and virulence genes and their complex transmission dynamics in our community, including at the level of STs, subclones, and plasmids, especially for the CTX-M-producing ST131-H30Rx subclone. Our findings both confirm those of previous similar studies and uniquely extend them to community hospitals (11–13). The striking dominance of H30Rx strains carrying IncF[F2:A1:B−] plasmids suggests that this lineage represents an especially successful combination of extensive resistance and virulence. Further genomic-epidemiological studies of E. coli ST131 and its H30R1 and H30Rx subclones are needed in community hospitals to inform antimicrobial stewardship programs, empirical therapy approaches, and infection control strategies rooted in the community.
MATERIALS AND METHODS
Bacterial isolates.
The study sites were 7 geographically dispersed community hospitals in or near North Carolina affiliated with the Duke Infection Control Outreach Network (DICON). Between 2010 and 2015, local infection preventionists prospectively used Centers for Disease Control and Prevention surveillance definitions (26) to identify all hospitalized patients with MDR E. coli infections. Isolates were classified as MDR if they were nonsusceptible to ≥1 agent in ≥3 antimicrobial classes (27). The hospital laboratories confirmed ESBL production phenotypically by using Clinical and Laboratory Standards Institute (CLSI) criteria. Duke University's Institutional Review Board approved the study.
During 2010 to 2015, the study hospitals reported 100 total MDR E. coli infections. Sixty-three nonduplicate MDR E. coli isolates (54 were ESBL producers), including 61 from 6 North Carolina hospitals and 2 from 1 Virginia hospital, were saved in the DICON Biorepository and subsequently sent to the University of North Carolina at Chapel Hill (UNC) for further analysis.
Clinical and epidemiological data.
Clinical and epidemiological data for the source patients were obtained through the DICON database. Variables included age, sex, race, admission source, type of infection, setting for infection acquisition, presence/absence of ICU stay, dialysis use, and discharge outcome.
Antimicrobial susceptibility testing.
The UNC Clinical Laboratories reassessed isolates' susceptibility to 12 antibiotics by disk diffusion according to the 2015 CLSI guideline (28). Isolates classified as intermediate or resistant were regarded as resistant.
Whole-genome sequencing.
Isolates were grown overnight in LB broth at 37°C. Total DNA was extracted and purified using an UltraClean microbial DNA isolation kit (Mo Bio, Carlsbad, CA). Sequencing libraries were prepared using the NEBNext Ultra DNA Library Prep kit for Illumina (New England BioLabs, Ipswich, MA) with NEBNext Multiplex oligonucleotides (New England BioLabs). Size-fractionated DNA libraries were labeled, pooled, and sequenced at the UNC High-Throughput Sequencing Facility in a single Illumina HiSeq2500 run using 125-base paired-end V4 chemistry.
Sequence assembly and mapping.
De novo and reference-guided assembly methods were used for gene identification and comparative genetic analysis, respectively, unless otherwise noted. De novo assemblies were compiled using the A5-miseq pipeline with default parameter settings (29). Reference-guided assemblies against E. coli strain EC958 (GenBank accession no. HG941718.1) were compiled using bwa mem (30) (see Fig. S1 in the supplemental material). Assemblies were deduplicated and subjected to local realignment through high-entropic regions using Picard (http://broadinstitute.github.io/picard/) and the Genome Analysis Toolkit (GATK) v3.3 (31).
Variant calling.
SNVs were identified by applying the GATK UnifiedGenotyper across all reference-aligned isolates simultaneously. The SNVs were filtered stringently using cutoffs responsive to the underlying distribution of quality scores. For filtering, the parameters included the following: quality by depth, ≥ 25; mapping quality, ≥59; Fisher score, ≤4; map quality rank sum, ≥−4.0; and read position rank sum, ≥−4.0.
Genetic analysis.
Species identification was confirmed by k-mer-based classification based on short-read sequences using Kraken (32). Phylogenetic groups were inferred from known ST assignments (33). Specific genes and alleles thereof were identified via the Center for Genomic Epidemiology's bioinformatic pipeline (http://www.genomicepidemiology.org/), using default settings except where otherwise stated. Specifically, serotypeFinder 1.1 (34) was used for serotypes, FimTyper 1.0 (35) for fimH types, MLST v1.7 (36) and the Achtman MLST scheme (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli) (37) for in silico MLST, ResFinder v2.1 (38) for acquired resistance genes (threshold, 98% identity; minimum length, 60%), PlasmidFinder 1.3 for plasmid incompatibility groups (threshold, 95% identity), and pMLST 1.4 (39) for plasmid STs. The resistance score was the number of antimicrobial classes for which an isolate was resistant to ≥1 representative agent (40).
For E. coli ST131 isolates, subclones H30R (ciprofloxacin resistant; fimH allele 30), H30Rx (a CTX-M-15-associated clonal subset within H30R), and H30R1 (a sister clade to H30Rx within H30R) were classified as described previously (11, 15). H30Rx was defined based on a specific SNV, ybbW G723A, as identified by BLASTn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (19).
To investigate QRDRs, established alleles of gyrA, parC, and parE were identified using a gyrA, parC, parE allele database generously provided by E. Sokurenko, University of Washington. Stepwise mutational derivatives of each numbered allele in gyrA, parC, and parE were identified.
Virulence genes were identified using a previously described list of 119 such sequences (10). Gene presence/absence was determined by BLASTn based on >80% sequence identity and >80% coverage (10). Genes were classified functionally as adhesin, bacteriocin/microcin, invasin, motility, protectin, siderophore, toxin, and miscellaneous (41). The virulence score was the number of unique virulence operons detected, irrespective of the number of constituent genes (23). Isolates were defined as ExPEC if positive for ≥2 of (i) any pap, (ii) any sfa or foc, (iii) any afa or dra, (iv) any kps, and (v) iutA, as described previously (42), with modification.
For phylogenetic analysis, recombinant genomic regions were identified using Gubbins (43) and were excluded. A maximum-likelihood (ML) tree was inferred using the Tamura-Nei model (44) within MEGA7 (45) (use of the general time-reversible substitution model yielded a similar tree). The ML phylogeny was constructed from genetic variation in nonrecombinant genomic regions. The initial tree(s) for the heuristic search was obtained automatically by applying the neighbor-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with superior log likelihood value. Pairwise SNV differences between core genomes were calculated using bedtools (https://bedtools.readthedocs.io/en/latest/).
Statistical analysis.
Using JMP 11 (Statistical Analysis System, Cary, NC, USA), categorical variables were compared by the two-tailed Fisher test or the chi-square test, and continuous variables were evaluated by the Wilcoxon test. The significance threshold was a P value of <0.05.
Accession number(s).
Sequence data have been deposited in the DDBJ/EMBL/GenBank Sequence Read Archive (SRA) under accession numbers DRX055674 to DRX055737.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Veronika L. Tchesnokova and Evgeni V. Sokurenko for their assistance with assignment of gyrA, parC, and parE alleles. We also acknowledge Nicole Stoesser for providing her list of virulence sequences.
The opinions expressed here are strictly ours and do not necessarily reflect those of our respective institutions or the Department of Veterans Affairs.
H.K. received financial support from a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad. C.M.P. was supported by NIH training grant AI109979. This material is also based in part on work supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs grant 1 I01 CX000920-01 (J.R.J.). D.J.A. received support from the NIH/NIAID (K23AI095357).
J.R.J. has ST131-related research grants from Actavis/Allergan, Merck, and Tetraphase; a consultancy with Janssen/Crucell; and patent applications for tests related to specific E. coli strains. We report no other conflicts of interest relevant to this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00912-17.
REFERENCES
- 1.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee R, Johnson JR. 2014. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother 58:4997–5004. doi: 10.1128/AAC.02824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolas-Chanoine MH, Bertrand X, Madec JY. 2014. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LF, Freeman JT, Nicholson B, Keiger A, Lancaster S, Joyce M, Woods CW, Cook E, Adcock L, Louis S, Cromer AL, Sexton DJ, Anderson DJ. 2014. Widespread dissemination of CTX-M-15 genotype extended-spectrum-β-lactamase-producing Enterobacteriaceae among patients presenting to community hospitals in the southeastern United States. Antimicrob Agents Chemother 58:1200–1202. doi: 10.1128/AAC.01099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee R, Strahilevitz J, Johnson JR, Nagwekar PP, Schora DM, Shevrin I, Du H, Peterson LR, Robicsek A. 2013. Predictors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli infection in a Midwestern community. Infect Control Hosp Epidemiol 34:947–953. doi: 10.1086/671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS II, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 56:641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanamori H, Parobek CM, Weber DJ, van Duin D, Rutala WA, Cairns BA, Juliano JJ. 2015. Next-generation sequencing and comparative analysis of sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii at a large academic burn center. Antimicrob Agents Chemother 60:1249–1257. doi: 10.1128/AAC.02014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salipante SJ, SenGupta DJ, Cummings LA, Land TA, Hoogestraat DR, Cookson BT. 2015. Application of whole-genome sequencing for bacterial strain typing in molecular epidemiology. J Clin Microbiol 53:1072–1079. doi: 10.1128/JCM.03385-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherchan JB, Hayakawa K, Miyoshi-Akiyama T, Ohmagari N, Kirikae T, Nagamatsu M, Tojo M, Ohara H, Sherchand JB, Tandukar S. 2015. Clinical epidemiology and molecular analysis of extended-spectrum-β-lactamase-producing Escherichia coli in Nepal: characteristics of sequence types 131 and 648. Antimicrob Agents Chemother 59:3424–3432. doi: 10.1128/AAC.00270-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoesser N, Sheppard AE, Moore CE, Golubchik T, Parry CM, Nget P, Saroeun M, Day NP, Giess A, Johnson JR, Peto TE, Crook DW, Walker AS, Modernizing Medical Microbiology Informatics Group. 2015. Extensive within-host diversity in fecally carried extended-spectrum-β-lactamase-producing Escherichia coli isolates: implications for transmission analyses. J Clin Microbiol 53:2122–2131. doi: 10.1128/JCM.00378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan MD, Gomes Moriel D, Peters KM, Davies M, Rogers BA, Dougan G, Rodriguez-Bano J, Pascual A, Pitout JD, Upton M, Paterson DL, Walsh TR, Schembri MA, Beatson SA. 2014. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, Turner P, Anson LW, Kasarskis A, Batty EM, Kos V, Wilson DJ, Phetsouvanh R, Wyllie D, Sokurenko E, Manges AR, Johnson TJ, Price LB, Peto TE, Johnson JR, Didelot X, Walker AS, Crook DW. 2016. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio 7:e02162-15. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman JT, Sexton DJ, Anderson DJ. 2009. Emergence of extended-spectrum β-lactamase-producing Escherichia coli in community hospitals throughout North Carolina: a harbinger of a wider problem in the United States? Clin Infect Dis 49:e30–e32. doi: 10.1086/600046. [DOI] [PubMed] [Google Scholar]
- 15.Johnson TJ, Danzeisen JL, Youmans B, Case K, Llop K, Munoz-Aguayo J, Flores-Figueroa C, Aziz M, Stoesser N, Sokurenko E, Price LB, Johnson JR, Castanheira M. 2016. Separate F-type plasmids have shaped the evolution of the H30 subclone of Escherichia coli sequence type 131. mSphere 1:e00121-16. doi: 10.1128/mSphere.00121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colpan A, Johnston B, Porter S, Clabots C, Anway R, Thao L, Kuskowski MA, Tchesnokova V, Sokurenko EV, Johnson JR, VICTORY (Veterans Influence of Clonal Types on Resistance: Year 2011) Investigators. 2013. Escherichia coli sequence type 131 (ST131) subclone H30 as an emergent multidrug-resistant pathogen among US veterans. Clin Infect Dis 57:1256–1265. doi: 10.1093/cid/cit503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine MH, Debroy C, Robicsek A, Hansen G, Urban C, Platell J, Trott DJ, Zhanel G, Weissman SJ, Cookson BT, Fang FC, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko EV. 2013. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J Infect Dis 207:919–928. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peirano G, van der Bij AK, Freeman JL, Poirel L, Nordmann P, Costello M, Tchesnokova VL, Pitout JD. 2014. Characteristics of Escherichia coli sequence type 131 isolates that produce extended-spectrum β-lactamases: global distribution of the H30-Rx sublineage. Antimicrob Agents Chemother 58:3762–3767. doi: 10.1128/AAC.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee R, Robicsek A, Kuskowski MA, Porter S, Johnston BD, Sokurenko E, Tchesnokova V, Price LB, Johnson JR. 2013. Molecular epidemiology of Escherichia coli sequence type 131 and Its H30 and H30-Rx subclones among extended-spectrum-β-lactamase-positive and -negative E. coli clinical isolates from the Chicago region, 2007 to 2010. Antimicrob Agents Chemother 57:6385–6388. doi: 10.1128/AAC.01604-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura Y, Johnson JR, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S, Kyoto-Shiga Clinical Microbiology Study Group. 2015. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J Antimicrob Chemother 70:1639–1649. doi: 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JR, Thuras P, Johnston BD, Weissman SJ, Limaye AP, Riddell K, Scholes D, Tchesnokova V, Sokurenko E. 2016. The pandemic H30 subclone of Escherichia coli sequence type 131 is associated with persistent infections and adverse outcomes independent from its multidrug resistance and associations with compromised hosts. Clin Infect Dis 62:1529–1536. doi: 10.1093/cid/ciw193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JR, Menard M, Johnston B, Kuskowski MA, Nichol K, Zhanel GG. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002 to 2004. Antimicrob Agents Chemother 53:2733–2739. doi: 10.1128/AAC.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen B, Frimodt-Moller J, Leihof RF, Struve C, Johnston B, Hansen DS, Scheutz F, Krogfelt KA, Kuskowski MA, Clabots C, Johnson JR. 2014. Temporal trends in antimicrobial resistance and virulence-associated traits within the Escherichia coli sequence type 131 clonal group and its H30 and H30-Rx subclones, 1968 to 2012. Antimicrob Agents Chemother 58:6886–6895. doi: 10.1128/AAC.03679-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee R, Johnston B, Lohse C, Chattopadhyay S, Tchesnokova V, Sokurenko EV, Johnson JR. 2013. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob Agents Chemother 57:5912–5917. doi: 10.1128/AAC.01065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention, National Healthcare Safety Network. January 2017. CDC/NHSN surveillance definitions for specific types of infections. https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed 24 April 2017.
- 27.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 20th informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Coil DJG, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 30.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997 [q-bio.GN] https://arxiv.org/abs/1303.3997. [Google Scholar]
- 31.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clermont O, Gordon D, Denamur E. 2015. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology 161:980–988. doi: 10.1099/mic.0.000063. [DOI] [PubMed] [Google Scholar]
- 34.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. 2015. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokurenko E, Tchesnokova V, Muradova M, Chattophadhay S, Ahrenfeldt J, Allesøe R, Hansen F, Hammerum A, Hasman H. 2016. Fimtyper; a Web tool for E. coli fimH genotyping, abstr PO8 Abstr 11th Int Meet Microb Epidemiol Markers (IMMEM XI), Estoril, Portugal https://www.escmid.org/fileadmin/src/media/PDFs/3Research_Projects/Conferences/IMMEM_11_Abstracts_Book.pdf. [Google Scholar]
- 36.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS II, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, Paterson D, Quinn J, Hanson ND, Johnston BD, Clabots C, Kuskowski MA, AMERECUS Investigators. 2012. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob Agents Chemother 56:2364–2370. doi: 10.1128/AAC.05824-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JR, Russo TA. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int J Med Microbiol 295:383–404. doi: 10.1016/j.ijmm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA, Smith KE. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob Agents Chemother 47:2161–2168. doi: 10.1128/AAC.47.7.2161-2168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.