ABSTRACT
SCY-078 (formerly MK-3118) is a novel orally active inhibitor of fungal β-(1,3)-glucan synthase (GS). SCY-078 is a derivative of enfumafungin and is structurally distinct from the echinocandin class of antifungal agents. We evaluated the in vitro activity of this compound against wild-type (WT) and echinocandin-resistant isolates containing mutations in the FKS genes of Candida spp. Against 36 Candida spp. FKS mutants tested, 30 (83.3%) were non-WT to 1 or more echinocandins, and only 9 (25.0%) were non-WT (MIC, >WT-upper limit) to SCY-078. Among C. glabrata isolates carrying FKS alterations, 84.0% were non-WT to the echinocandins versus only 24.0% for SCY-078. In contrast to the echinocandin comparators, the activity of SCY-078 was minimally affected by the presence of FKS mutations, suggesting that this agent is useful in the treatment of Candida infections due to echinocandin-resistant strains.
KEYWORDS: Candida, SCY, antifungal susceptibility testing, echinocandin resistance
INTRODUCTION
Candidemia and other forms of invasive candidiasis (IC; infection of normally sterile sites and tissues) are, without a doubt, important causes of morbidity, mortality, and excess hospital costs in seriously ill hospitalized individuals (1–3). It is also recognized that the present era is one of high non-albicans Candida (NAC) prevalence and antifungal resistance (4–12). NAC species are especially prominent in U.S. medical centers (6, 7, 10–12), and a recent publication from France demonstrates that exposure to fluconazole favors bloodstream infection (BSI) with Candida glabrata, C. krusei, and C. tropicalis, and exposure to an echinocandin is predictive of infection with C. glabrata, C. krusei, and C. parapsilosis (8). Notably, the emergence of multi-azole-resistant, echinocandin-resistant, and multidrug-resistant (resistant to 2 or more classes of antifungal agents) strains of these species has been reported in hospitals located in the United States, Europe, and Asia (4–6, 13–15). It is now clear that exposure to azoles and echinocandins sets the stage for IC due to antifungal-resistant NAC species and that infections with echinocandin-resistant and multidrug-resistant strains of Candida are positively associated with treatment failure and all-cause mortality rates (6–8, 16–20).
The emergence of NAC infections secondary to the use of azole antifungal agents for the prevention and treatment of IC has ushered in the echinocandin era of antifungal therapy (21–23). The members of the echinocandin class of antifungal agents (anidulafungin, caspofungin, and micafungin) are potent inhibitors of β-(1,3)-glucan synthase (GS), exhibit fungicidal activity against most species of Candida, and have been recommended as first-line agents to treat IC (22, 24–28). Although echinocandin-resistant isolates of Candida appear to be concentrated in a relatively small number of large tertiary-care hospitals (4, 14), increased use of both azoles and echinocandins may result in more widespread resistance, especially among NAC species (20). Measures to curb antifungal resistance development include antifungal stewardship, infection prevention measures (6, 29), and alternative treatment strategy development, including novel antifungal compounds (23, 30).
Resistance to echinocandin antifungal agents is acquired during therapy (4, 20), and the resulting mutations in the hot spot (HS) regions of genes encoding the catalytic subunits of GS (FKS1 and FKS2) significantly decrease the sensitivity of the target enzyme to the drug, resulting in higher MIC values and reduced pharmacodynamic and clinical responses (4, 31). Aside from emerging resistance, an important echinocandin limitation is that they must be administered daily by intravenous infusion, potentially prolonging hospital stays for patients undergoing echinocandin therapy and limiting them to inpatient settings in most instances. Given the importance of GS in the viability of Candida spp. (32), developing orally administered GS inhibitors would represent a major step forward by providing a simple therapy transition from the inpatient to the ambulatory setting.
Derivatives of the natural product enfumafungin, a fungal triterpenoid glycoside, are potent inhibitors of GS yet are structurally distinct from the echinocandins (33–36). SCY-078 (formerly MK-3118) is a semisynthetic orally bioavailable derivative of enfumafungin with in vitro and in vivo activity against several different species of Candida, Aspergillus, and select non-Aspergillus molds (33, 37–40). Importantly, in addition to being orally bioavailable with favorable pharmacodynamic properties (38), SCY-078 has been shown to retain in vitro activity against azole- and echinocandin-resistant strains of Candida (33, 36, 40). Mutations in FKS that result in resistance to the echinocandins are located in domains that may be distinct from those associated with decreased susceptibility to SCY-078, supporting the lack of complete cross-resistance between these agents that has been demonstrated in vitro (33, 38, 40).
The in vitro studies of SCY-078 activity against Candida spp. that have been published thus far demonstrate the excellent potency of SCY-078 against the most common species of Candida, including those with FKS mutations (33, 40). These studies are limited by the inclusion of a relatively small number of isolates of the most common species and the lack of comparison to echinocandins other than caspofungin.
In the present study, we determined the spectrum and potency of SCY-078, anidulafungin, caspofungin, and micafungin against a panel of 11 different species of Candida selected to represent both wild-type (WT) and echinocandin-resistant (ER) strains. All isolates were tested by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution (BMD) method that has been standardized for testing the echinocandins against Candida (41–43).
RESULTS AND DISCUSSION
Table 1 summarizes the in vitro susceptibilities of echinocandin WT and ER isolates of Candida spp. to SCY-078 and the 3 echinocandin comparators. Although a recent report highlighted the lack of reproducibility of caspofungin MIC results when tested using the CLSI reference broth microdilution methods (44), results were included in this study. Caspofungin is an important in-class comparator for the echinocandins; furthermore, the testing reported here was generated in a single laboratory, decreasing the issues with reproducibility of the results. In addition, the FKS mutation data included in this study support the categorization of ER as determined by caspofungin testing. The MIC values of SCY-078 ranged from 0.03 to 16 mg/liter depending on the species and echinocandin susceptibility. Whereas the activity of SCY-078 was comparable to that of the 3 echinocandins against WT strains of C. parapsilosis, C. lusitaniae, C. guilliermondii, and C. orthopsilosis, it was 4- to 66-fold less active against WT strains of the other species, reflecting the intrinsic WT susceptibilities of each species to this novel GS inhibitor.
TABLE 1.
MIC distributions of SCY-078 and 3 echinocandins against WT and ER Candida spp. isolates
| Species | Phenotype (no. of isolates) | Modal MICa (range [mg/liter]) |
|||
|---|---|---|---|---|---|
| SCY-078 | Anidulafungin | Caspofungin | Micafungin | ||
| C. albicans | WT (61) | 0.12 (0.03–0.25) | 0.015 (≤0.008–0.06) | 0.03 (0.015–0.06) | 0.015 (≤0.008–0.06) |
| ER (8) | NM (0.06–2) | 0.12 (≤0.008–1) | 2 (0.015–2) | 0.06 (0.015–2) | |
| C. glabrata | WT (39) | 0.5 (0.25–1) | 0.06 (0.015–0.25) | 0.06 (0.03–0.25) | 0.015 (≤0.008–0.03) |
| ER (28) | 1 (0.12–16) | 1 (0.015–4) | 0.5 (0.03–16) | 0.06 (≤0.008–4) | |
| C. tropicalis | WT (30) | 0.25 (0.06–2) | 0.015 (≤0.008–0.06) | 0.03 (0.015–0.12) | 0.03 (0.015–0.06) |
| ER (1) | 2 | 2 | 2 | 2 | |
| C. parapsilosis | WT (43) | 0.25 (0.12–4) | 2 (0.5–4) | 0.5 (0.25–2) | 2 (0.5–2) |
| C. krusei | WT (30) | 0.5–1 (0.25–2) | 0.03 (0.03–0.25) | 0.12 (0.06–0.25) | 0.12 (0.06–0.25) |
| ER (4) | 1 (1–4) | 0.06 (0.03–0.5) | NM (0.06–1) | 0.12 (0.06–0.25) | |
| C. dubliniensis | WT (19) | 0.12 (0.06–0.25) | 0.015 (≤0.008–0.06) | 0.03 (0.015–0.06) | 0.03 (≤0.008–0.06) |
| ER (1) | 0.25 | 0.5 | 2 | 1 | |
| C. lusitaniae | WT (22) | 2 (0.5–4) | 0.5 (0.015–1) | 0.5 (0.03–0.5) | 0.25 (0.03–0.5) |
| C. guilliermondii | WT (23) | 2 (1–4) | 2 (1–4) | 0.5 (0.25–1) | 1 (0.5–2) |
| C. orthopsilosis | WT (15) | 0.5 (0.06–1) | 1 (0.25–1) | 0.25 (0.06–0.5) | 0.5 (0.12–1) |
| C. pelliculosa | WT (14) | 0.25 (0.25–2) | 0.015 (≤0.008–2) | 0.03 (0.015–0.5) | 0.03–0.06 (0.015–1) |
| ER (1) | 1 | 0.06 | 0.5 | 0.5 | |
| C. kefyr | WT (11) | 0.5–1 (0.25–1) | 0.06 (0.03–0.06) | 0.015 (0.015–0.03) | 0.06 (0.03–0.12) |
| ER (1) | 0.06 | 0.5 | 0.06 | 0.25 | |
NM, no mode.
It is notable that whereas the increase in modal MIC values between WT and ER strains ranged from 2- to 133-fold for anidulafungin, 4- to 66-fold for caspofungin, and 1- to 66-fold for micafungin, modal MIC values for SCY-078 only increased by 2- to 8-fold across the different species (Table 1 and Fig. 1). Specifically, among the 36 FKS mutants tested, 30 (83.3%) were non-WT to 1 or more echinocandin, whereas only 9 (25.0%) were non-WT (MIC, >WT-upper limit[two 2-fold dilutions higher than the modal MIC value of each WT population]) to SCY-078 (Tables 2 and 3). Among the 25 FKS mutant strains of C. glabrata, 84.0% were non-WT to the echinocandins versus only 24.0% for SCY-078 (Table 3). Isolates of C. glabrata for which the SCY-078 MIC was >2 mg/liter (MIC, >WT-upper limit) all were non-WT and either intermediate or resistant to all three echinocandins (Tables 2 and 3). These isolates harbored a deletion at F659 or mutations L662 and S663 in the HS1 region of FKS2 (Table 3). Whereas FKS mutant strains of C. dubliniensis and C. kefyr were 2- to 8-fold more susceptible to SCY-078 than the echinocandins, 1 strain of C. tropicalis with a mutation in HS1 of FKS1 (F641S) was cross-resistant (MIC, 2 mg/liter) to all 3 echinocandins and SCY-078 (Table 3).
FIG 1.
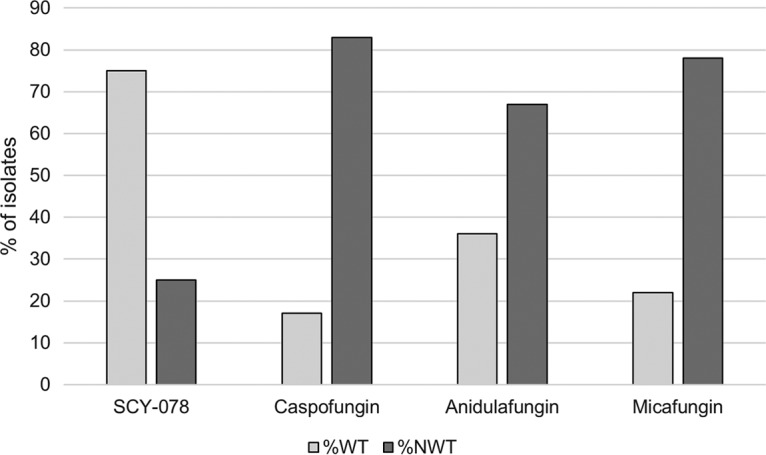
Activity of SCY-078, anidulafungin, caspofungin, and micafungin against strains displaying FKS mutations. %WT, percent wild type; %NWT, percent non-wild type (for SCY-078, %NWT is the percent exceeding the wild-type upper-limit value [WT-UL; two 2-fold dilutions higher than the modal MIC value of each WT population]).
TABLE 2.
In vitro susceptibilities of Candida spp. to SCY-078 and 3 echinocandin antifungal agentsa
| Species (no. of isolates tested) and antifungal agent | No. of isolates (no. of mutants) at MIC (mg/liter) of: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | >8 | |
| C. albicans (69) | ||||||||||||
| SCY-078 | 3 | 28 (2) | 29 | 5 (1) | 1 (1) | 1 | 2 (2) | |||||
| Anidulafungin | 27 (1) | 28 | 5 | 2 | 4 (3) | 1 | 2 (2) | |||||
| Caspofungin | 31 (1) | 30 | 1 | 1 | 2 (1) | 1 (1) | 3 (3) | |||||
| Micafungin | 3 | 32 (1) | 26 | 4 (1) | 2 (2) | 2 (2) | ||||||
| C. glabrata (67) | ||||||||||||
| SCY-078 | 1 (1) | 4 (2) | 29 (4) | 24 (10) | 2 (2) | 6 (5) | 1 (1) | |||||
| Anidulafungin | 2 (1) | 8 (1) | 23 | 8 | 4 (3) | 3 (3) | 7 (5) | 6 (6) | 6 (6) | |||
| Caspofungin | 14 (1) | 20 (2) | 7 (1) | 5 (3) | 7 (7) | 3 (1) | 6 (5) | 1 (1) | 2 (2) | 2 (2) | ||
| Micafungin | 2 (1) | 22 | 20 (4) | 5 (3) | 4 (3) | 3 (3) | 2 (2) | 4 (4) | 4 (4) | 1 (1) | ||
| C. parapsilosis (43) | ||||||||||||
| SCY-078 | 1 | 19 (1) | 12 | 8 | 2 | 1 | ||||||
| Anidulafungin | 1 | 15 | 22 | 5 (1) | ||||||||
| Caspofungin | 11 | 25 (1) | 6 | 1 | ||||||||
| Micafungin | 2 | 17 | 24 (1) | |||||||||
| C. tropicalis (31) | ||||||||||||
| SCY-078 | 1 | 9 | 15 | 4 | 2 (1) | |||||||
| Anidulafungin | 4 | 17 | 8 | 1 | 1 (1) | |||||||
| Caspofungin | 7 | 21 | 1 | 1 | 1 (1) | |||||||
| Micafungin | 8 | 16 | 6 | 1 (1) | ||||||||
| C. krusei (34) | ||||||||||||
| SCY-078 | 2 | 12 | 14 (1) | 5 | 1 (1) | |||||||
| Anidulafungin | 22 (1) | 9 | 1 | 1 | 1 (1) | |||||||
| Caspofungin | 7 (1) | 17 | 8 | 1 | 1 (1) | |||||||
| Micafungin | 12 | 19 (1) | 3 (1) | |||||||||
| C. dubliniensis (20) | ||||||||||||
| SCY-078 | 3 | 9 | 8 (1) | |||||||||
| Anidulafungin | 1 | 8 | 7 | 3 | 1 (1) | |||||||
| Caspofungin | 5 | 12 | 2 | 1 (1) | ||||||||
| Micafungin | 1 | 17 | 1 | 1 (1) | ||||||||
| C. lusitaniae (22) | ||||||||||||
| SCY-078 | 2 | 2 | 14 | 4 | ||||||||
| Anidulafungin | 2 | 3 | 3 | 4 | 9 | 1 | ||||||
| Caspofungin | 3 | 5 | 6 | 8 | ||||||||
| Micafungin | 1 | 1 | 4 | 5 | 11 | 1 | ||||||
| C. guilliermondii (23) | ||||||||||||
| SCY-078 | 2 | 12 | 9 | |||||||||
| Anidulafungin | 3 | 17 | 3 | |||||||||
| Caspofungin | 2 | 11 | 10 | |||||||||
| Micafungin | 1 | 21 | 1 | |||||||||
| C. orthopsilosis (15) | ||||||||||||
| SCY-078 | 1 | 6 | 7 | 1 | ||||||||
| Anidulafungin | 2 | 4 | 9 | |||||||||
| Caspofungin | 3 | 4 | 6 | 2 | ||||||||
| Micafungin | 1 | 4 | 7 | 3 | ||||||||
| C. pelliculosa (15) | ||||||||||||
| SCY-078 | 8 | 4 | 1 | 2 | ||||||||
| Anidulafungin | 3 | 7 | 2 | 2 | ||||||||
| Caspofungin | 4 | 5 | 2 | 1 | 1 | 2 | ||||||
| Micafungin | 2 | 5 | 5 | 2 | 1 | |||||||
| C. kefyr (12) | ||||||||||||
| SCY-078 | 1 (1) | 1 | 5 | 5 | ||||||||
| Anidulafungin | 5 | 6 | 1 (1) | |||||||||
| Caspofungin | 10 | 1 | 1 (1) | |||||||||
| Micafungin | 2 | 8 | 1 | 1 (1) | ||||||||
In vitro susceptibilities were determined by CLSI BMD method and read at a 24-h incubation time.
TABLE 3.
SCY-078 MIC results and those of anidulafungin, caspofungin, and micafungin against selected strains displaying FKS mutations
| Organism | β-(1,3)-d-glucan synthase mutation(s)a |
MIC (mg/liter) |
||||||
|---|---|---|---|---|---|---|---|---|
| FKS1 |
FKS2 |
|||||||
| HS1 | HS2 | HS1 | HS2 | SCY-078 | Anidulafungin | Caspofungin | Micafungin | |
| C. albicans | F641I | WT | 2 | 0.12 | 0.5 | 0.06 | ||
| C. albicans | F641S | WT | 2 | 0.12 | 1 | 0.5 | ||
| C. albicans | S629P | WT | 0.5 | 1 | 2 | 2 | ||
| C. albicans | L701 M | WT | 0.25 | 1 | 2 | 2 | ||
| C. albicans | ALT1929 | WT | 0.06 | ≤0.008 | 0.015 | 0.015 | ||
| C. albicans | S645P | WT | 0.06 | 0.12 | 2 | 0.5 | ||
| C. dubliniensis | S645P | WT | 0.25 | 0.5 | 2 | 1 | ||
| C. glabrata | WT | WT | F659 deletion | WT | 16 | 4 | 16 | 2 |
| C. glabrata | D632Y | WT | WT | WT | 4 | 2 | 1 | 0.12 |
| C. glabrata | WT | WT | F659S | WT | 4 | 2 | 2 | 0.25 |
| C. glabrata | WT | WT | L662W | WT | 4 | 4 | 2 | 1 |
| C. glabrata | WT | WT | S663P | WT | 4 | 4 | 2 | 1 |
| C. glabrata | WT | WT | S663P | WT | 4 | 4 | 4 | 2 |
| C. glabrata | WT | WT | F659V | WT | 2 | 1 | 0.5 | 0.12 |
| C. glabrata | WT | WT | S663P | WT | 2 | 1 | 0.5 | 1 |
| C. glabrata | F625S | WT | WT | WT | 1 | 0.25 | 0.06 | 0.03 |
| C. glabrata | R631S S629P | WT | WT | WT | 1 | 0.5 | 0.5 | 0.06 |
| C. glabrata | WT | WT | D648E | WT | 1 | 0.5 | 0.25 | 0.06 |
| C. glabrata | S645P | WT | 1 | 2 | 2 | 1 | ||
| C. glabrata | WT | WT | F659Y | WT | 1 | 2 | 2 | 0.25 |
| C. glabrata | WT | WT | S663P | WT | 1 | 1 | 0.5 | 0.25 |
| C. glabrata | WT | WT | S663P | WT | 1 | 1 | 0.5 | 0.5 |
| C. glabrata | S629P | WT | WT | WT | 1 | 2 | 8 | 2 |
| C. glabrata | S629P | WT | WT | WT | 1 | 4 | 8 | 2 |
| C. glabrata | WT | WT | S663P | WT | 1 | 4 | 16 | 4 |
| C. glabrata | L630I | WT | WT | WT | 0.5 | 0.03 | 0.12 | ≤0.008 |
| C. glabrata | F625Y | WT | WT | WT | 0.5 | 0.25 | 0.06 | 0.03 |
| C. glabrata | D632V | WT | WT | WT | 0.5 | 0.25 | 0.25 | 0.03 |
| C. glabrata | WT | WT | S663F | WT | 0.5 | 1 | 0.5 | 0.5 |
| C. glabrata | WT | WT | D666E K753Q | WT | 0.25 | 0.5 | 0.5 | 0.06 |
| C. glabrata | WT | WT | S663F | WT | 0.25 | 0.12 | 0.25 | 0.12 |
| C. glabrata | WT | WT | WT | P1371S | 0.12 | 0.015 | 0.03 | 0.03 |
| C. kefyr | WT | R1344S | 0.06 | 0.5 | 0.06 | 0.25 | ||
| C. krusei | NG2034 | 1 | 0.03 | 0.06 | 0.12 | |||
| C. krusei | L701 M | 4 | 0.5 | 1 | 0.25 | |||
| C. tropicalis | F641S | WT | 2 | 2 | 2 | 2 | ||
HS1/HS2, hot spots 1 and 2, respectively; WT, wild type.
These data demonstrate the in vitro activity of the novel GS inhibitor SCY-078 compared to the established echinocandin antifungal agents against 11 different species of Candida, including both WT and ER strains. As documented previously (33, 40), ER strains of Candida were included in the WT MIC distribution of SCY-078 (Table 2). This observation suggests that FKS mutations in GS, in general, have less of an effect on the in vitro activity of SCY-078 than the echinocandins. Indeed, Jimenez-Ortigosa et al. (33) demonstrated that kinetic inhibition of product-entrapped GS enzymes isolated from ER strains yielded lower 50% inhibitory concentrations for SCY-078 (MK-3118) than for caspofungin in C. albicans (3- to 5.5-fold) and C. glabrata (3.5- to 62-fold). Similar to Jimenez-Ortigosa et al. (33), we found that 84.0% of FKS mutant strains of C. glabrata were non-WT to the echinocandins versus only 24.0% for SCY-078. Given these findings, it is also notable that Lepak et al. (38) found in an in vivo murine IC model that the static and 1-log kill doses, as well as the total and free-drug area-under-the-curve/MIC pharmacodynamic targets for SCY-078, were numerically lower than those observed for echinocandins, suggesting that this agent will prove to be an oral option for the treatment of Candida infections, including IC.
The in vitro activity of SCY-078 against both WT and ER Candida spp. was assessed using CLSI BMD methods. The potency and spectrum of SCY-078 against this challenging collection of isolates was excellent and less affected by FKS mutations than the echinocandins. These data suggest that SCY-078 will be useful in the treatment of ER candidiasis.
MATERIALS AND METHODS
Organisms.
A total of 351 clinical isolates of Candida were tested, including 307 echinocandin WT strains (61 C. albicans, 39 C. glabrata, 30 C. tropicalis, 43 C. parapsilosis, 30 C. krusei, 19 C. dubliniensis, 22 C. lusitaniae, 23 C. guilliermondii, 15 C. orthopsilosis, 14 C. pelliculosa, and 11 C. kefyr) and 44 ER (non-WT or resistant to one or more echinocandin) strains (8 C. albicans, 28 C. glabrata, 1 C. tropicalis, 4 C. krusei, 1 C. dubliniensis, 1 C. pelliculosa, and 1 C. kefyr) (Table 1). All tested strains were from the SENTRY Antimicrobial Surveillance Program Collection (45). Isolates were identified to species level using a combination of conventional methods, matrix-assisted laser desorption ionization-time of flight mass spectrometry, and DNA sequence analysis as described previously (46, 47). Prior to testing, each isolate was passaged at least twice onto Sabouraud dextrose agar (Remel, Lenexa, Kansas, USA) and CHROMagar Candida medium (Becton Dickinson, Sparks, Maryland, USA) to ensure purity and viability.
Antifungal susceptibility testing.
All isolates were tested for in vitro susceptibility to SCY-078, anidulafungin, caspofungin, and micafungin using CLSI BMD methods (41, 42). The reference powder of SCY-078 was obtained from the manufacturer. Stock solutions were prepared in dimethyl sulfoxide (DMSO), and the final range of SCY-078 and comparator agent concentrations tested was 0.008 to 16 mg/liter.
CLSI BMD testing was performed exactly as outlined in documents M27-S4 (42) and M27-A3 (41) by using RPMI 1640 medium with 0.2% glucose, inocula of 0.5 × 103 to 2.5 × 103 cells/ml, and incubation at 35°C. MIC values for both SCY-078 and the echinocandins were determined visually following 24 h of incubation as the lowest concentration of drug that caused significant growth diminution levels (≥50% inhibition) relative to the growth control, as described by Pfaller et al. (40) and the CLSI (41, 42), respectively.
We used the revised CLSI clinical breakpoint (CBP) values to identify strains of C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei, and C. guilliermondii that were susceptible (S), intermediate (I), and resistant (R) to the echinocandins (48) (Table 4). CLSI has not established CBPs for the echinocandins and C. dubliniensis, C. lusitaniae, C. orthopsilosis, C. pelliculosa, and C. kefyr, and it recommends that the previously established epidemiological cutoff values (ECVs) be used to differentiate WT (no acquired resistance mechanisms) from non-WT (may harbor acquired resistance mechanisms) strains of these species (48) (Table 4). Neither CBPs nor ECVs have been determined for SCY-078 and Candida spp. For purposes of comparison, we have used a wild-type-upper-limit value as the susceptibility cutoff value for SCY-078 and each species (49, 50) (Tables 1 and 4).
TABLE 4.
Cutoff values and clinical breakpoints employed for comparison of SCY-078 to anidulafungin, caspofungin, and micafungin against Candida spp.c
| Species and antifungal agent | Cutoff value (mg/liter) |
CBPa (mg/liter) |
|||
|---|---|---|---|---|---|
| WT-UL | ECVb | S | I | R | |
| C. albicans | |||||
| SCY-078 | 0.5 | ||||
| Anidulafungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| Caspofungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| Micafungin | 0.03 | ≤0.25 | 0.5 | ≥1 | |
| C. glabrata | |||||
| SCY-078 | 2 | ||||
| Anidulafungin | 0.25 | ≤0.12 | 0.25 | ≥0.5 | |
| Caspofungin | 0.12 | ≤0.12 | 0.25 | ≥0.5 | |
| Micafungin | 0.03 | ≤0.06 | 0.12 | ≥0.25 | |
| C. tropicalis | |||||
| SCY-078 | 1 | ||||
| Anidulafungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| Caspofungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| Micafungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| C. parapsilosis | |||||
| SCY-078 | 1 | ||||
| Anidulafungin | 4 | ≤2 | 4 | ≥8 | |
| Caspofungin | 1 | ≤2 | 4 | ≥8 | |
| Micafungin | 4 | ≤2 | 4 | ≥8 | |
| C. krusei | |||||
| SCY-078 | 4 | ||||
| Anidulafungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| Caspofungin | 0.25 | ≤0.25 | 0.5 | ≥1 | |
| Micafungin | 0.12 | ≤0.25 | 0.5 | ≥1 | |
| C. dubliniensis | |||||
| SCY-078 | 0.5 | ||||
| Anidulafungin | 0.12 | ||||
| Caspofungin | 0.12 | ||||
| Micafungin | 0.12 | ||||
| C. lusitaniae | |||||
| SCY-078 | 8 | ||||
| Anidulafungin | 2 | ||||
| Caspofungin | 1 | ||||
| Micafungin | 0.5 | ||||
| C. guilliermondii | |||||
| SCY-078 | 8 | ||||
| Anidulafungin | 4 | ≤2 | 4 | ≥8 | |
| Caspofungin | 2 | ≤2 | 4 | ≥8 | |
| Micafungin | 2 | ≤2 | 4 | ≥8 | |
| C. orthopsilosis | |||||
| SCY-078 | 2 | ||||
| Anidulafungin | 2 | ||||
| Caspofungin | 0.5 | ||||
| Micafungin | 1 | ||||
| C. pelliculosa | |||||
| SCY-078 | 1 | ||||
| Anidulafungin | |||||
| Caspofungin | 0.12 | ||||
| Micafungin | |||||
| C. kefyr | |||||
| SCY-078 | 4 | ||||
| Anidulafungin | 0.25 | ||||
| Caspofungin | 0.03 | ||||
| Micafungin | 0.12 | ||||
CBP, clinical breakpoint; S, susceptible; I, intermediate; R, resistant. ECVs and CBP values are from Pfaller and Diekema (48).
ECV, epidemiologic cutoff value.
Cutoff values and clinical breakpoints were determined by 24-h CLSI broth microdilution methods.
Quality control was performed as recommended in CLSI document M27-A3 (41) using C. krusei ATCC 6258 and C. parapsilosis ATCC 22019. All ER isolates were further characterized regarding the presence or absence of a mutation in the HS regions of FKS1 and FKS2 (C. glabrata only) as described previously (45, 51, 52).
ACKNOWLEDGMENTS
We thank S. E. Costello for performing the sequencing of the FKS HS.
This study was sponsored by an investigational grant from Scynexis.
JMI Laboratories, Inc., has also received research and educational grants in 2014 to 2015 from Achaogen, Actavis, Actelion, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Durata, Enteris, Exela, Forest Research Institute, Furiex, Genentech, GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Seachaid, Shionogi, Tetraphase, The Medicines Co., Theravance, ThermoFisher, VenatoRX, Vertex, Wockhardt, Zavante, and some other corporations. In regard to speakers' bureaus and stock options, we have none to declare.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench MT, Bretagne S, Dromer F, Lortholary O. 2012. Candida spp. with acquired echinocandin resistance, France, 2004-2010. Emerg Infect Dis 18:86–90. doi: 10.3201/eid1801.110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. 2014. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis 20:1833–1840. doi: 10.3201/eid2011.140685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmakiotis D, Kyvernitakis A, Tarrand JJ, Kontoyiannis DP. 2015. Early initiation of appropriate treatment is associated with increased survival in cancer patients with Candida glabrata fungaemia: a potential benefit from infectious disease consultation. Clin Microbiol Infect 21:79–86. doi: 10.1016/j.cmi.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F, French Mycosis Study Group. 2014. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010). Intensive Care Med 40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller MA, Castanheira M, Lockhart SR, Jones RN. 2012. Candida glabrata: multidrug resistance and increased virulence in a major opportunistic fungal pathogen. Curr Fungal Infect Rep 6:154–164. doi: 10.1007/s12281-012-0091-0. [DOI] [Google Scholar]
- 10.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE. 2014. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One 9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller MA, Jones RN, Castanheira M. 2014. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006-2011. Mycoses 57:602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 12.Wang E, Farmakiotis D, Yang D, McCue DA, Kantarjian HM, Kontoyiannis DP, Mathisen MS. 2015. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother 70:2362–2368. doi: 10.1093/jac/dkv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, Chhina D, Rao R, Eshwara VK, Xess I, Kindo AJ, Umabala P, Savio J, Patel A, Ray U, Mohan S, Iyer R, Chander J, Arora A, Sardana R, Roy I, Appalaraju B, Sharma A, Shetty A, Khanna N, Marak R, Biswas S, Das S, Harish BN, Joshi S, Mendiratta D. 2015. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 14.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. The role of FKS mutations in C. glabrata: MIC values, echinocandin resistance and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. 2014. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 59:819–825. doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 18.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. 2013. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 57:3528–3535. doi: 10.1128/AAC.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shor E, Perlin DS. 2015. Coping with stress and the emergence of multidrug resistance in fungi. PLoS Pathog 11:e1004668. doi: 10.1371/journal.ppat.1004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguilar-Zapata D, Petraitiene R, Petraitis V. 2015. Echinocandins: the expanding antifungal armamentarium. Clin Infect Dis 61(Suppl 6):S604–S611. doi: 10.1093/cid/civ814. [DOI] [PubMed] [Google Scholar]
- 22.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ, Mycoses Study Group. 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 23.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. 2010. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 24.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Florl C, Richardson MD, Akova M, Bassetti M, Calandra T, Castagnola E, Cornely OA, Garbino J, Groll AH, Herbrecht R, Hope WW, Kullberg BJ, Lortholary O, Meersseman W, Petrikkos G, Roilides E, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 18(Suppl 7):S9–S18. doi: 10.1111/1469-0691.12038. [DOI] [PubMed] [Google Scholar]
- 25.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 26.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Florl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Suppl 7):S38–S52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 27.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Florl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal Infection Study Group. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18(Suppl 7):S53–S67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 28.Pappas PG, Kauffman CA, Andes D, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller MA, Castanheira M. 2016. Nosocomial candidiasis: antifungal stewardship and the importance of rapid diagnosis. Med Mycol 54:1–22. [DOI] [PubMed] [Google Scholar]
- 30.Tillotson J, Tillotson GS. 2015. The regulatory pathway for antifungal drugs: a US perspective. Clin Infect Dis 61(Suppl 6):S678–S683. doi: 10.1093/cid/civ819. [DOI] [PubMed] [Google Scholar]
- 31.Perlin DS. 2015. Echinocandin resistance in Candida. Clin Infect Dis 61(Suppl 6):S612–S617. doi: 10.1093/cid/civ791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas CM. 2001. Fungal beta(1,3)-D-glucan synthesis. Med Mycol 39(Suppl 1):S55–S66. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onishi J, Meinz M, Thompson J, Curotto J, Dreikorn S, Rosenbach M, Douglas C, Abruzzo G, Flattery A, Kong L, Cabello A, Vicente F, Pelaez F, Diez MT, Martin I, Bills G, Giacobbe R, Dombrowski A, Schwartz R, Morris S, Harris G, Tsipouras A, Wilson K, Kurtz MB. 2000. Discovery of novel antifungal (1,3)-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother 44:368–377. doi: 10.1128/AAC.44.2.368-377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peel M, Balkovec J, Fan W, Mamai A, Greenlee M, Liberator P, Hong J, Orr M, Ouvry G, Perry D, Liu H, Ogbu C, Lee S, Li K, Nelson K, Meng D, Parker D, Wildonger K, Abruzzo G, Flattery A, Galgoci A, Giacobbe R, Nielsen J, Misura A, Gill C, Sligar J, Powles M, Racine F, Dragovic J, Habulihaz B. 2010. Enfumafungin derivatives: orally active glucan synthase inhibitors, abstr. F1-845. Abstr 50th Intersci Conf Antimicrob Agents Chemother, Boston, MA American Society for Microbiology, Washington, DC. [Google Scholar]
- 36.Walker SS, Xu Y, Triantafyllou I, Waldman MF, Mendrick C, Brown N, Mann P, Chau A, Patel R, Bauman N, Norris C, Antonacci B, Gurnani M, Cacciapuoti A, McNicholas PM, Wainhaus S, Herr RJ, Kuang R, Aslanian RG, Ting PC, Black TA. 2011. Discovery of a novel class of orally active antifungal beta-1,3-D-glucan synthase inhibitors. Antimicrob Agents Chemother 55:5099–5106. doi: 10.1128/AAC.00432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamoth F, Alexander BD. 2015. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 59:4308–4311. doi: 10.1128/AAC.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lepak AJ, Marchillo K, Andes DR. 2015. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 59:1265–1272. doi: 10.1128/AAC.04445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 57:1065–1068. doi: 10.1128/AAC.01588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. Activity of MK-3118, a new oral glucan synthase inhibitor tested against Candida spp. by two international methods (CLSI and EUCAST). J Antimicrob Chemother 68:858–863. [DOI] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts: 4th informational supplement. M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: Integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles of opportunistic yeast and mould clinical isolates (2010-2011). Application of new CLSI clinical breakpoints and epidemiological cutoff values to characterize geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castanheira M, Woosley LN, Diekema DJ, Jones RN, Pfaller MA. 2013. Candida guilliermondii and other species of Candida misidentified as Candida famata: assessment by Vitek2, ITS sequence analysis and matrix-assisted laser desorption ionization–time of flight mass spectrometry in two global surveillance programs. J Clin Microbiol 51:117–124. doi: 10.1128/JCM.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfaller MA, Woosley LN, Messer SA, Jones RN, Castanheira M. 2012. Significance of molecular identification and antifungal susceptibility of clinically significant yeasts and moulds in a global antifungal surveillance program. Mycopathologia 174:259–271. doi: 10.1007/s11046-012-9551-x. [DOI] [PubMed] [Google Scholar]
- 48.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela JL, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and Iso-Sensitest media. Antimicrob Agents Chemother 54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canton E, Peman J, Iniguez C, Hervas D, Lopez-Hontangas JL, Pina-Vaz C, Camarena JJ, Campos-Herrero I, Garcia-Garcia I, Garcia-Tapia AM, Guna R, Merino P, Perez del Molino L, Rubio C, Suarez A, FUNGEMYCA Study Group. 2013. Epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole for six Candida species as determined by the colorimetric Sensititre YeastOne method. J Clin Microbiol 51:2691–2695. doi: 10.1128/JCM.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castanheira M, Woosley LN, Pfaller MA, Diekema DJ, Messer SA, Jones RN. 2010. Low prevalence of fks1 hotspot 1 mutations in a worldwide collection of Candida spp. Antimicrob Agents Chemother 54:2655–2659. doi: 10.1128/AAC.01711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, Pfaller MA. 2014. Frequency of fks mutations among Candida glabrata isolates from a 10-year global collection of bloodstream infection isolates. Antimicrob Agents Chemother 58:577–580. doi: 10.1128/AAC.01674-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


