ABSTRACT
β-Lactam/β-lactamase inhibitors (BLBLIs) were compared to carbapenems in two cohorts of hematological neutropenic patients with extended-spectrum-β-lactamase (ESBL) bloodstream infection (BSI): the empirical therapy cohort (174 patients) and the definitive therapy cohort (251 patients). The 30-day case fatality rates and other secondary outcomes were similar in the two therapy groups of the two cohorts and also in the propensity-matched cohorts. BLBLIs might be carbapenem-sparing alternatives for the treatment of BSI due to ESBLs in these patients.
KEYWORDS: ESBLs, bloodstream infection, neutropenia, beta-lactam/beta-lactamase inhibitors, mortality
TEXT
Bloodstream infection (BSI) due to extended-spectrum-β-lactamases (ESBLs) is increasingly being identified among patients with hematological malignancies (1–5). This is of special concern because a delay in initiating adequate antibiotic therapy may impair their outcomes (1, 6). Carbapenems are increasingly used for treating severe infections caused by ESBL producers. This is particularly worrisome in a scenario in which carbapenem-resistant Enterobacteriaceae (CRE) are spreading rapidly and are adversely compromising patients' outcomes (7–12). While recent investigations have suggested that β-lactam/β-lactamase inhibitors (BLBLIs) may be reliable options for the treatment of BSI due to ESBL-producing Gram-negative bacilli (ESBL-GNB), especially in nonimmunocompromised patients (13–19), other studies have found contrasting data (20, 21). To date, the efficacy of BLBLIs for the treatment of BSI due to ESBL-GNB in high-risk hematological patients has not been established. In this regard, assessing the usefulness of BLBLIs as carbapenem-sparing antibiotic regimens may be a key step in the efforts to minimize the spread of CRE. This study aimed to assess the efficacy of BLBLIs compared with carbapenems for the treatment of hematological neutropenic patients with BSI due to ESBL-GNB.
The study comprised hematological neutropenic patients, including hematopoietic stem cell transplant (HSCT) recipients, who had had at least one episode of BSI due to an ESBL-GNB and who had received carbapenems or BLBLIs for >24 h as the empirical or definitive antibiotic therapy. Patients were recruited retrospectively at 22 centers from nine different countries (Argentina, Australia, Brazil, Canada, Germany, Italy, Spain, Turkey, and the United States) from 1 January 2006 to 31 March 2015.
The primary endpoint was the case fatality rate at 30 days from onset of BSI. Secondary outcomes included the following: (i) 7-day and (ii) 14-day case fatality rates; (iii) persistent BSI; (iv) relapse of BSI; (v) colonization/infection by bacteria resistant to the antibiotics under study; and (vi) superinfection due to any bacteria. The protocol of the study has been published elsewhere (22). Antimicrobial therapy administered before susceptibility results were available was considered empirical therapy, and antibiotic therapy administered afterward was considered definitive therapy. Initial empirical antibiotic therapy was considered inadequate if the treatment regimen did not include at least one antibiotic active in vitro against the infecting microorganism. (See additional information regarding methodology in Text S1 in the supplemental material).
The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The protocol of the study was approved by all appropriate regulatory agencies and the local Research Ethics Committees, with the following local reference number: EPA020/15. The need for informed consent and information sheets was waived by the ethics committees because of the retrospective nature of the study.
Clinical samples were processed at the microbiology laboratories of each participating center in accordance with standard operating procedures. Enterobacteriaceae species were identified using standard microbiological techniques at each center. ESBL production was screened in all isolates with diminished susceptibility to cephalosporins and confirmed using standard procedures and phenotypic methods, such as disk-diffusion or Etest, or molecular characterization by PCR, depending on the participating center. The following β-lactams were used for confirmation by testing their synergistic effect with amoxicillin-clavulanate: cefepime, ceftazidime, cefotaxime, cefuroxime, and aztreonam. In vitro susceptibility was determined according to CLSI recommendations (23).
Patients who were given BLBLIs were compared with those treated with carbapenems as empirical and/or definitive therapy. Two non-mutually exclusive cohorts were constructed and analyzed separately. The empirical therapy cohort (ETC) included patients who received empirical therapy with BLBLI or carbapenem and whose isolates were susceptible to the empirical antimicrobial administered. The definitive therapy cohort (DTC) comprised patients who received definitive therapy with an active BLBLI or carbapenem. We used an uncorrected chi-square statistic to evaluate the primary endpoint under the null hypothesis of the 30-day case fatality rate between study groups. Survival functions were estimated using Kaplan-Meier curves and compared using the log rank test. The adjusted mortality odds ratio (OR) at 30 days was estimated in both cohorts by a multivariate logistic regression model. A propensity score (PS)—the probability of receiving BLBLIs as empirical and/or targeted therapy—was calculated in both cohorts (ETC and DTC), using a nonparsimonious multivariate logistic regression model in which the outcome variable was the use of BLBLI as empirical and/or definitive therapy. Each patient of each therapy group in the two cohorts was later matched to another patient using the nearest-matching method. This analysis allowed the identification of pairs of patients who had very close PS values but differed in terms of the therapy received. The final data set matched only pairs of patients on carbapenems and BLBLIs. The analysis was performed using R software (R v. 3.2.5).
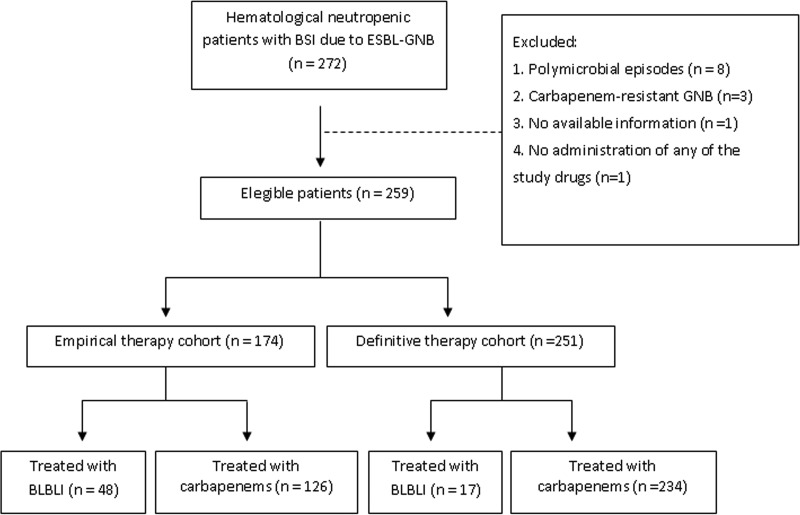
Figure 1 shows the flow chart of patients included in the study. A total of 259 episodes of BSI due to ESBL-GNB in 253 patients were eligible for analysis. The ETC comprised 174 patients and the DTC 251. Characteristics of patients are detailed in Table 1. The most frequent source of BSI was an endogenous source (52.8%), followed by catheter-related BSI (18.1%), neutropenic enterocolitis (8.1%), and urinary tract and perianal infections (6.9% each). The most frequently isolated ESBL-GNB species was Escherichia coli (73.7%), followed by Klebsiella pneumoniae (23.1%) and Klebsiella oxytoca and Enterobacter cloacae (1.5% each). (See additional information regarding the results in Text S1).
FIG 1.
Flow chart of patients included in the study.
TABLE 1.
Characteristics of patients with bloodstream infection caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae according to therapy
| Patient characteristic | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Empirical therapy cohort (n = 174) |
Definitive therapy cohort (n = 251) |
|||||
| Carbapenem; n = 126 | BLBLI; n = 48 | P | Carbapenem; n = 234 | BLBLI; n = 17 | P | |
| No. (%) of males | 76 (60.3) | 32 (66.6) | 0.55 | 154 (65.8) | 7 (41.1) | 0.07 |
| Median age in yrs (IQRa) | 50 (34–61) | 55 (40–63) | 0.10 | 52 (36–63) | 57 (37–60) | 0.71 |
| No. (%) of patients with: | ||||||
| Acute leukemia | 70 (55.5) | 25 (52) | 0.99 | 137 (58.5) | 9 (52.9) | 0.69 |
| Comorbidities | 18 (14.2) | 17 (35.4) | 0.003 | 49 (20.9) | 5 (29.4) | 0.37 |
| Hematopoietic stem cell transplant | 49 (38.8) | 15 (31.25) | 0.44 | 77 (32.9) | 6 (35.2) | 0.99 |
| Graft vs host disease | 13 (10.3) | 2 (4.1) | 0.72 | 17 (7.2) | 2 (11.76) | 0.87 |
| Corticosteroid therapy | 73 (57.9) | 25 (52) | 0.58 | 115 (49.1) | 11 (64.7) | 0.35 |
| Profound neutropenia (<100 neutrophils/mm3) | 17 (13.4) | 9 (18.7) | 0.48 | 37 (15.8) | 3 (17.6) | 0.72 |
| Median no. of days of previous neutropenia (IQR) | 6 (3–12) | 5.5 (4–9) | 0.65 | 6 (3–12) | 7.5 (5.7–9) | 0.68 |
| No. (%) of patients with: | ||||||
| Previous antibiotics (1 mo) | 107 (84.9) | 33 (68.7) | 0.01 | 187 (79.9) | 12 (70.5) | 0.61 |
| Previous hospital admission (1 mo) | 90 (71.4) | 36 (75) | 0.67 | 167 (71.3) | 9 (52.9) | 0.09 |
| Previous episode of BSI | 19 (15) | 6 (12.5) | 0.84 | 34 (14.5) | 2 (11.7) | 0.99 |
| Concomitant infection | 25 (19.8) | 7 (14.5) | 0.51 | 51 (21.7) | 4 (23.5) | 0.99 |
| MASCC index score < 21 | 57 (45.2) | 22 (45.8) | 0.99 | 102 (43.5) | 7 (41.1) | 0.63 |
| Complex bloodstream infection | 25 (19.8) | 14 (29.1) | 0.26 | 48 (20.5) | 7 (41.1) | 0.064 |
| Septic shock at presentation | 36 (28.5) | 10 (20.8) | 0.38 | 53 (22.6) | 2 (11.7) | 0.7 |
| Granulocyte colony-stimulating factor | 79 (62.7) | 23 (47.9) | 0.075 | 129 (55.1) | 6 (35.2) | 0.14 |
| Active combination antibiotic therapy | 42 (33.3) | 12 (25) | 0.37 | 22 (9.4) | 1 (5.8) | 0.99 |
| Adequate initial empirical antibiotic therapy | 179 (76.5) | 15 (88.2) | 0.37 | |||
| Enterobacteriaceae species infection | ||||||
| Escherichia coli | 91 (72.2) | 37 (77) | 0.77 | 174 (74.3) | 14 (82.3) | 0.84 |
| Klebsiella pneumoniae | 31 (24.6) | 11 (22.9) | 54 (23) | 3 (17.6) | ||
| Enterobacter cloacae | 1 (0.79) | 0 | 3 (1.2) | 0 | ||
| Klebsiella oxytoca | 3 (2.38) | 0 | 3 (1.2) | 0 | ||
IQR, interquartile range.
Of the 174 patients included in the ETC, 126 received a carbapenem (93 received meropenem, 31 imipenem, and 2 ertapenem) and 48 a BLBLI (44 received PTZ, 2 amoxicillin-clavulanate [AMC], and 2 cefoperazone-sulbactam). In the DTC, 234 patients received carbapenems (130 received meropenem, 79 imipenem, and 25 ertapenem) and 17 were given BLBLIs (12 received PTZ and 5 AMC).
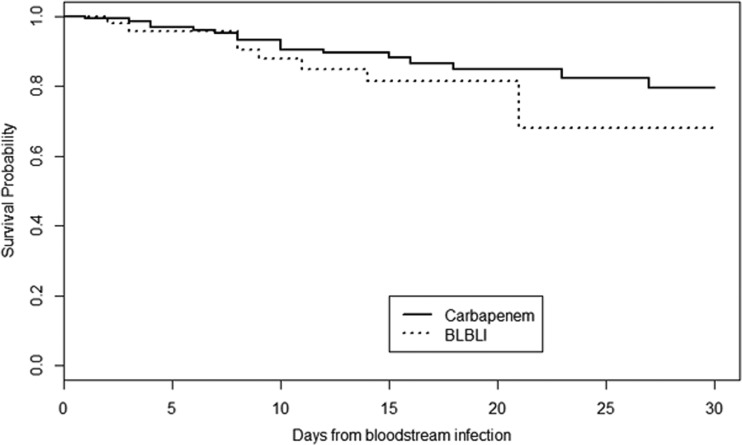
As shown in Table 2, in the ETC there were no significant differences in the 30-day case fatality rate between the patients treated with carbapenems and those who received BLBLIs. Similarly, there were no significant differences between any of the secondary endpoints, including 7-day and 14-day case fatality rates. Figure 2 shows the Kaplan-Meier survival curves in the ETC. After applying the PS-based matched analysis, we were able to match 35 pairs of patients treated empirically with BLBLIs or carbapenems according to the PS. In this PS-matched cohort, the 30-day case fatality rates did not differ significantly between groups (11.4% versus 20%, P = 0.32). Similar results were obtained in analyzing the 14-day case fatality rates (11.4% versus 14.2%, P = 0.99) and the 7-day case fatality rates (2.8% versus 2.8%, P = 0.99). The only independent risk factor for 30-day case fatality rate was a Multinational Association for Supportive Care in Cancer (MASCC) score of <21 (OR, 3.61; 95% confidence interval [95% CI], 1.19 to 12.72; P = 0.03). Neither therapy with carbapenems nor therapy with BLBLIs was found to be an independent risk factor for 30-day case fatality.
TABLE 2.
Outcomes of patients with bloodstream infection caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae according to therapya
| Characteristic | No. (%) of patients with indicated treatment |
|||||
|---|---|---|---|---|---|---|
| Empirical therapy cohort (n = 174) |
Definitive therapy cohort (n = 251) |
|||||
| Carbapenem; n = 126 | BLBLI; n = 48 | P | Carbapenem; n = 234 | BLBLI; n = 17 | P | |
| ICU admission | 30 (23.8) | 7 (14.5) | 0.25 | 44 (18.8) | 2 (11.7) | 0.74 |
| 30-day case fatality rate | 17 (13.4) | 10 (20.8) | 0.33 | 37 (15.8) | 1 (5.8) | 0.99 |
| 14-day case fatality rate | 12 (9.5) | 7 (14.5) | 0.49 | 23 (9.8) | 1 (5.8) | 0.99 |
| 7-day case fatality rate | 6 (4.7) | 2 (4.1) | 0.99 | 11 (4.7) | 0 | NA |
| Relapse of bloodstream infection | 0 | 2 (4.1) | NA | 4 (1.7) | 0 | NA |
| Persistent bloodstream infection | 5 (3.9) | 5 (10.4) | 0.13 | 11 (4.7) | 3 (17.6) | 0.059 |
| Colonization/infection by resistant bacteria | 8 (6.3) | 1 (2) | 0.28 | 15 (6.4) | 1 (5.8) | 0.99 |
| Superinfection | 11 (8.7) | 2 (4.1) | 0.51 | 21 (8.9) | 2 (11.1) | 0.68 |
ICU, intensive care unit; NA, not applicable.
FIG 2.
Kaplan-Meier survival curves in the empirical therapy cohort.
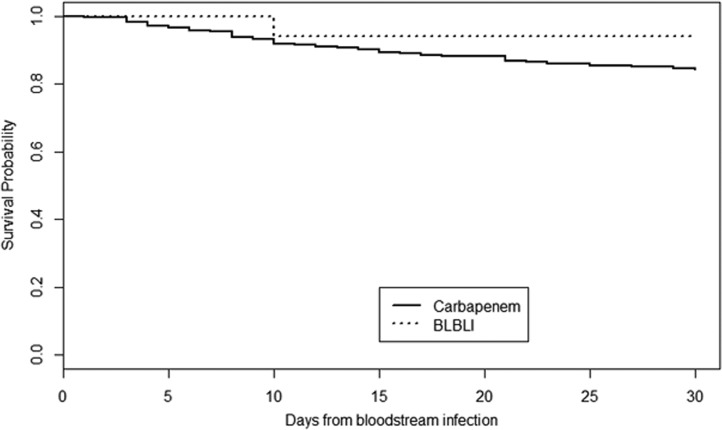
As shown in Table 2, in the DTC there were no significant differences in the 30-day case fatality rates between the patients treated with carbapenems and those who received BLBLIs. Similar results were obtained regarding all the secondary endpoints. Figure 3 shows the Kaplan-Meier curves of survival in the DTC. After applying the nearest-matching method, we were able to match 15 pairs of patients treated definitively with BLBLIs or carbapenems according to the PS. In this cohort, there were no significant differences regarding 30-day and 14-day case fatality rates between the patients treated with carbapenems and those who received BLBLIs (6.5% versus 12.5% [P = 0.99] and 6.25% versus 12.5% [P = 0.99], respectively). No patient died from BSI within the first 7 days in any group. Neither therapy with carbapenems nor BLBLIs was found to be an independent risk factor for 30-day case fatality.
FIG 3.
Kaplan-Meier survival curves in the definitive therapy cohort.
In our study, there were no significant differences in the 30-day case fatality rates between the patients treated with carbapenems and those who received BLBLIs. Interestingly, the 30-day case fatality rates in the two groups were also similar in the propensity-matched cohorts. Furthermore, neither therapy with a carbapenem nor therapy with a BLBLI was found to be an independent risk factor for 30-day case fatality in either therapy cohort. It should also be stressed that the nonsignificant differences in the 30-day case fatality rates found in the ETC were not observed in the analysis of the 7-day case fatality rates. The impact of an empirical antibiotic regimen on outcomes is probably better assessed by taking early mortality into consideration. Also, the nonsignificantly higher 30-day mortality rate in the DTC in patients receiving carbapenems was probably due to the larger number of patients treated with this regimen. We found that secondary outcomes differed little between therapy groups in both cohorts. However, persistent BSI was more frequent in patients who received BLBLIs as a definitive therapy, although the difference did not reach statistical significance. Nevertheless, given the low number of patients receiving targeted BLBLI therapy, we cannot draw any firm conclusion. To date, the published data on the use of BLBLIs for the treatment of infections caused by ESBL-GNB are conflicting and mainly refer to nonimmunocompromised patients (13–21). Our study was the first to assess the usefulness of BLBLIs for the treatment of ESBL-BSI in a severely immunosuppressed cohort of hematological patients with neutropenia. This issue is of great concern due to the fact that hematological patients often receive repeated cycles of broad-spectrum antibiotics and are at a high risk of developing infections due to resistant bacteria, including carbapenem-resistant organisms. In this regard, the use of BLBLIs as carbapenem-sparing alternatives to treat suspected or documented ESBL infections may be a useful strategy to avoid the spread of CRE.
Our multicenter study has some limitations. First, this was a retrospective study, and so the typical limitations of this design applied. However, randomized trials comparing empirical and definitive antibiotic regimens are difficult to perform in severely immunosuppressed patients. Second, as carbapenems are the drugs recommended for the treatment of severe infections by ESBL-GNB, it was difficult to enroll a large number of patients treated with BLBLIs in both cohorts, but especially in the DTC. This led to a lack of the power required to assess mortality and detect true differences. Finally, the BLBLI MICs were not available for all the strains, and data regarding molecular characterization of ESBLs could not be obtained.
We conclude that BLBLIs, if active in vitro, might be carbapenem-sparing alternatives for the treatment of BSI due to ESBLs in high-risk hematological patients. Physicians dealing with these patients should consider using BLBLIs for the treatment of infections due to ESBL-producing Enterobacteriaceae. This strategy may prove useful in limiting the spread of carbapenem resistance in this high-risk population.
Supplementary Material
ACKNOWLEDGMENTS
We thank the ESGBIS and the ESGICH study groups for supporting the study.
This study was supported by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III—cofinanced by European Development Regional Fund “A way to achieve Europe” EDRF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015).
The BICAR Study investigators are as follows: Fe Tubau, Hospital Universitari de Bellvitge, Universitat de Barcelona, l'Hospitalet de Llobregat, Barcelona, Spain; Isabel Sánchez-Ortega, Hospital Duran y Reynals—ICO, Barcelona, Spain; Nieves Larrosa and Pere Barba, Vall d'Hebron University Hospital, Barcelona, Spain; Beatriz Mirelis, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; Esther Calbo, University Hospital Mútua de Terrassa, Barcelona, Spain; Irene Gracia-Ahufinger and Julián Torre-Cisneros, Reina Sofía University Hospital-IMIBIC-UCO, Córdoba, Spain; José Antonio Lepe, Ildefonso Espigado, José Miguel Cisneros, María D. Navarro, and Marina de Cueto, Virgen del Rocío and Virgen Macarena University Hospitals, Seville, Spain; Miriam Vara and Leire López-Soria, Cruces University Hosptial, Bilbao, Spain; Manuel Lizasoain and José María Aguado, 12 de Octubre University Hospital, Madrid Spain; Rosa Escudero, Javier López-Jiménez, and Jesús Fortún, Ramón y Cajal Hospital, Madrid, Spain; Matteo Bassetti, Santa Maria Misericordia University Hospital, Udine, Italy; Emrah Seyhoglu, Hacettepe University School of Medicine, Ankara, Turkey; Helena Duani and Paulo Henrique Orlandi, Hospital das Clínicas, UFMG Belo Horizonte, Brazil; Lígia Câmera Pierrotti and Karim Yaqub Ibrahim, Instituto do Câncer do Estado de São Paulo, Brazil; Renata Neto Pires and Denusa Wiltgen, Hospital Santa Casa de Misericordia de Porto Alegre, Brazil; Dina Kabbani, University Hospital of Alberta, Canada.
J.R.-B. has been a scientific consultant for Merck, AstraZeneca, Achaogen, and InfectoPharm, a speaker in accredited educational activities for Merck and AstraZeneca, and a recipient of research grants from COMBACTE-NET, COMBACTE-CARE, and COMBACTE-MAGNET, funded by the Innovative Medicines Initiative (European Union and EFPIA in kind). A.F. has received research fees from Merck and from Astellas for data and safety monitoring. J.C. has received lecture fees from Novartis, Astellas, Pfizer, MSD, Janssen, and Astra-Zeneca.
None of the other authors have any potential conflicts of interest to declare.
Footnotes
For a commentary on this article, see https://doi.org/10.1128/AAC.01094-17.
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00164-17.
REFERENCES
- 1.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, Leone G, Cauda R, Pagano L. 2009. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect 58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R, Arnan M, Marin M, Carratalà J, Gudiol F. 2010. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 65:333–341. doi: 10.1093/jac/dkp411. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kwon JC, Choi SM, Lee DG, Park SH, Choi JH, Yoo JH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. 2013. Escherichia coli and Klebsiella pneumoniae bacteremia in patients with neutropenic fever: factors associated with extended-spectrum β-lactamase production and its impact on outcome. Ann Hematol 92:533–541. doi: 10.1007/s00277-012-1631-y. [DOI] [PubMed] [Google Scholar]
- 4.Ha YE, Kang CI, Cha MK, Park SY, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. 2013. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents 42:403–409. doi: 10.1016/j.ijantimicag.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I, Duarte R, Calvo M, Carratalà J. 2011. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother 66:657–663. doi: 10.1093/jac/dkq494. [DOI] [PubMed] [Google Scholar]
- 6.Lin MY, Weinstein RA, Hota B. 2008. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother 52:3188–3194. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL; European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group. 2015. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 20:pii=30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 8.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauck C, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D; Antibacterial Resistance Leadership Group. 2016. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumonia infections. Clin Microbiol Infect 22:513–519. doi: 10.1016/j.cmi.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 11.Satlin MJ, Cohen N, Ma KC, Gedrimaite Z, Soave R, Askin G, Chen L, Kreiswirth BN, Walsh TJ, Seo SK. 2016. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect 73:336–345. doi: 10.1016/j.jinf.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satlin MJ, Calfee DP, Chen L, Fauntleroy KA, Wilson SJ, Jenkins SG, Feldman EJ, Roboz GJ, Shore TB, Helfgott DC, Soave R, Kreiswirth BN, Walsh TJ. 2013. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma 54:799–806. doi: 10.3109/10428194.2012.723210. [DOI] [PubMed] [Google Scholar]
- 13.Shiber S, Yahav D, Avni T, Leibovici L, Paul M. 2015. β-Lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 70:41–47. doi: 10.1093/jac/dku351. [DOI] [PubMed] [Google Scholar]
- 14.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á; Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 16.Harris PN, Yin M, Jureen R, Chew J, Ali J, Paynter S, Paterson DL, Tambyah PA. 2015. Comparable outcomes for β-lactam/β-lactamase inhibitor combinations and carbapenems in definitive treatment of bloodstream infections caused by cefotaxime-resistant Escherichia coli or Klebsiella pneumoniae. Antimicrob Resist Infect Control 4:14. doi: 10.1186/s13756-015-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng TM, Khong WX, Harris PN, De PP, Chow A, Tambyah PA, Lye DC. 2016. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One 11:e0153696. doi: 10.1371/journal.pone.0153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai HY, Chen YH, Tang HJ, Huang CC, Liao CH, Chu FY, Chuang YC, Sheng WH, Ko WC, Hsueh PR. 2014. Carbapenems and piperacillin/tazobactam for the treatment of bacteremia caused by extended-spectrum β-lactamase-producing Proteus mirabilis. Diagn Microbiol Infect Dis 80:222–226. doi: 10.1016/j.diagmicrobio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, the REIPI/ESGBIS/INCREMENT Group. 2016. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:4159–4169. doi: 10.1128/AAC.00365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE; Antibacterial Resistance Leadership Group. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ofer-Friedman H, Shefler C, Sharma S, Tirosh A, Tal-Jasper R, Kandipalli D, Sharma S, Bathina P, Kaplansky T, Maskit M, Azouri T, Lazarovitch T, Zaidenstein R, Kaye KS, Marchaim D. 2015. Carbapenems versus piperacillin-tazobactam for bloodstream infections of nonurinary source caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol 36:981–985. doi: 10.1017/ice.2015.101. [DOI] [PubMed] [Google Scholar]
- 22.Gudiol C, Royo-Cebrecos C, Tebe C, Abdala E, Akova M, Álvarez R, Maestro-de la Calle G, Cano A, Cervera C, Clemente WT, Martín-Dávila P, Freifeld A, Gómez L, Gottlieb T, Gurguí M, Herrera F, Manzur A, Maschmeyer G, Meije Y, Montejo M, Peghin M, Rodríguez-Baño J, Ruiz-Camps I, Sukiennik TC, Carratalà J; for the BICAR study group. Clinical efficacy of β-lactam/β-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum β-lactamase-producing Enterobacteriaceae in hematological patients with neutropenia: study protocol for a retrospective observational study (BICAR). BMJ Open, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute (CLSI). 2016. Performance standards for antimicrobial susceptibility testing; approved standard, 26th ed CLSI supplement M100S. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.