ABSTRACT
In past years, several Chlamydia-related bacteria have been discovered, including Simkania negevensis, the founding member of the Simkaniaceae family. We evaluated the antimicrobial susceptibility patterns of this emerging intracellular bacterium and highlighted significant differences, compared with related Chlamydiales members. S. negevensis was susceptible to macrolides, clindamycin, cyclines, rifampin, and quinolones. Importantly, unlike other Chlamydiales members, treatment with β-lactams and vancomycin did not induce the formation of aberrant bodies, leading to a completely resistant phenotype.
KEYWORDS: Chlamydiales, Simkaniaceae, intracellular bacteria
TEXT
Rapid progress in diagnostic techniques has enabled the discovery of several novel Chlamydia-related bacteria, including Simkania negevensis. Mostly known for the pathogenic Chlamydia spp., the Chlamydiales order is now composed of at least 9 family-level lineages (1), each with specific biological characteristics. S. negevensis is the founding member of the Simkaniaceae family and represents an emerging pathogen previously associated with respiratory diseases, at least in the Middle East (2, 3). Infections were empirically treated with a macrolide-based regimen (4). Several differences regarding antimicrobial susceptibility have been highlighted among the different Chlamydiales family-level lineages (5, 6). Therefore, we investigated the antibiotic susceptibility of the Simkaniaceae family, which remains poorly studied, using S. negevensis as a model. We provide subsequent information on the evolution of antimicrobial resistance in this order, as well as potential therapeutic options.
Simkania negevensis strain Z was grown at 37°C in Vero cells in 25-cm2 cell culture flasks (Corning, USA), in Dulbecco's modified essential medium (DMEM) (PAN Biotech, Aidenbach, Germany) supplemented with 10% fetal calf serum (FCS), with 5% CO2. A 6- or 7-day-old coculture, diluted 1:1,000, was used to inoculate fresh A549 cells or Vero cells that had been seeded previously at 1.5 × 105 cells/ml on a 24-well plate (Corning), as described previously (7). At 2 h postinfection, the medium was changed for medium containing 2-fold serial dilutions of various antibiotics. Antibiotic-free wells served as growth controls, while uninfected cells served as negative controls. Twelve antibiotics from 8 different classes were used in this study. MICs were defined as the minimal concentrations that prevented bacterial growth at day 6, compared to a control infection performed in the absence of antibiotics. Growth at day 2 was also assessed for β-lactams, fosfomycin, and vancomycin, to ensure the absence of effects due to instability of the compounds after 48 h at 37°C. An in-house specific quantitative PCR targeting the 16S rRNA gene was used to quantify S. negevensis DNA, as described previously (7). The absence of antibiotic toxicity toward cells was determined by examining the microplates using an inverted microscope (Zeiss Axiovert 25; Carl Zeiss). When solvents other than distilled water (i.e., dimethyl sulfoxide [DMSO], 0.1 M HCl, and 1 M NaOH) were used to suspend antibiotic solutions, the absence of effects of these solvents on S. negevensis growth was assessed.
Like other Chlamydiales species, S. negevensis was susceptible to macrolides, clindamycin, cyclines, and rifampin (Table 1). Interestingly, S. negevensis was susceptible to quinolones; while Chlamydiaceae are sensitive, other Chlamydia-related bacteria, such as Waddlia chondrophila, Parachlamydia spp., and Estrella lausannensis, are resistant (5, 6, 8). Previous work suggested that S. negevensis was resistant to ciprofloxacin (9). In that study, MICs were determined in amoebae, as the minimal concentrations that prevented amoebal lysis. The observed results might have been due to the presence of an efflux pump in amoebae and decreasing quinolone bioavailability. Although several mutations in the gyrA and parC quinolone resistance-determining regions (QRDRs) were identified, they differed from those observed in resistant Chlamydia-related bacteria, which may explain the observed absence of resistance (6, 9).
TABLE 1.
Antibiotic susceptibility of Simkania negevensis, compared to others Chlamydialesa
| Drug | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| Simkaniaceae, S. negevensis (this study)b | Parachlamydiaceae, Parachlamydia acanthamoebae (8)c | Waddliaceae, W. chondrophila (5, 11)b | Criblamydiaceae, E. lausannensis (6)b |
Chlamydiaceae |
||
| C. trachomatis (10, 21–24)b | Chlamydia pneumoniae (11, 21)b | |||||
| Cyclines | ||||||
| Tetracycline | 2 | ND | ND | 0.25 | 0.25–0.5 | 0.125–0.5 |
| Doxycycline | 0.5 | 2–4 | 0.25 | 0.25 | 0.03–0.25 | 0.02–0.5 |
| Lincosamide | ||||||
| Clindamycin | 1 | ND | 2–4 | ND | 0.25–2 | ND |
| Macrolides | ||||||
| Erythromycin | ND | 0.06 | ND | ND | 0.02–2 | 0.02–0.25 |
| Clarithromycin | ND | <0.06 | ND | ND | 0.02–0.125 | 0.004–0.125 |
| Azithromycin | <0.06 | ND | 0.25 | 2 | 0.6–2 | 0.02–0.5 |
| β-Lactams | ||||||
| Penicillin derivatives | >1,000 | >32 | >32 | >32 | 0.25–2 | 5 |
| Ceftriaxone | >1,000 | >32 | >32 | >32 | 16–32 | ND |
| Phosphonic acid derivative | ||||||
| Fosfomycin | >1,000 | ND | 500 | NDd | 500–1,000 | >1,000 |
| Glycopeptide | ||||||
| Vancomycin | >1,000 | ND | ND | ND | 1,000 | 1,000 |
| Fluoroquinolones | ||||||
| Ciprofloxacin | 4 | >16 | >16 | 32 | 0.5–2 | 1–4 |
| Ofloxacin | 1 | >16 | >16 | 16 | 0.5–1 | 0.5–2 |
| Levofloxacin | 0.5 | ND | ND | ND | 0.12–0.5 | 0.25–1 |
| Rifamycin | ||||||
| Rifampin | <0.06 | 0.25–0.5 | ND | ND | <0.125 to 1 | <0.125 |
Shown are the MICs of various antibiotics against members of the Chlamydiales orders (5, 6, 8, 10, 11, 21–24). This table was adapted from reference 8 with permission. ND, not done.
Tested in mammalian cells.
Tested in amoebae.
Criblamydiaceae present the Cys115-to-Asp substitution in the active site of MurA, which is known to confer resistance to fosfomycin in Chlamydia spp.
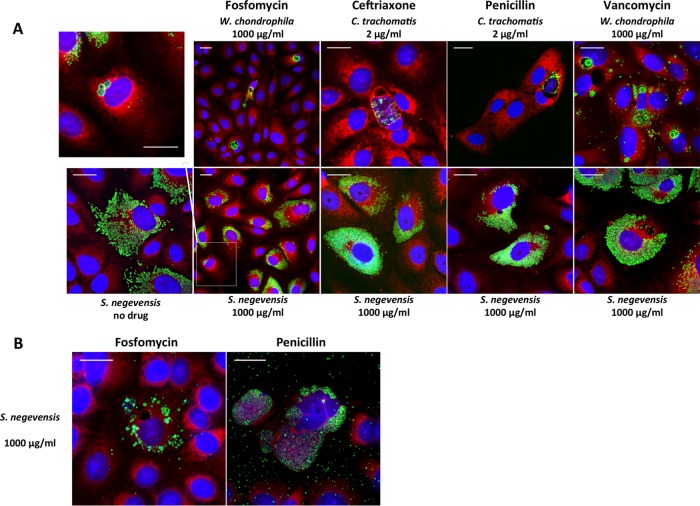
S. negevensis was resistant (MICs of >32 μg/ml) to three kinds of cell wall inhibitors, i.e., β-lactams, fosfomycin, and vancomycin. Chlamydiales members lack the traditional peptidoglycan (PG) layer. However, partial susceptibility to β-lactams is observed among Chlamydia spp., which are known to form aberrant bodies when treated with penicillin derivatives (10), while W. chondrophila is susceptible to high doses of fosfomycin (11). Aberrant bodies represent enlarged forms of the bacterium, due to abnormal division despite persisting DNA replication (11). Therefore, we evaluated the morphology of S. negevensis particles treated with β-lactams, fosfomycin, and vancomycin, in immunofluorescence assays using an in-house rabbit polyclonal anti-S. negevensis antibody, as described previously (7). As shown in Fig. 1A, no abnormal morphological aspects of S. negevensis could be observed with β-lactam treatment, even with concentrations as high as 1,000 μg/ml. This contrasted strikingly with the abnormal morphology of Chlamydia trachomatis observed with 2 μg/ml β-lactams, making S. negevensis unique among Chlamydiales members. Indeed, W. chondrophila (in the Waddliaceae family) and E. lausannensis (in the Criblamydiaceae family) form aberrant bodies with β-lactam treatment (500 μg/ml) (6, 12). Furthermore, unlike W. chondrophila (11), S. negevensis replication was not inhibited by high doses of β-lactams (1,000 μg/ml) (Table 1). This difference could not be explained by the slower replicative cycle, as similar observations were made at day 6 postinfection (Fig. 1B). Several β-lactamase motifs are included in the S. negevensis genome (13) and may contribute to the phenotype. However, W. chondrophila exhibits partial sensitivity to high doses of β-lactams despite having a class C β-lactamase encoded in its genome (14).
FIG 1.
Effects of cell wall inhibitors on Simkania negevensis infection and morphology. The growth of S. negevensis was observed by immunofluorescence, in the presence or absence of cell wall inhibitors. (A) Effects of β-lactam, fosfomycin, and vancomycin treatment in Vero cells at 48 h postinfection. S. negevensis, Chlamydia trachomatis strain UW-3/Cx, and Waddlia chondrophila strain WSU 86-1044 (ATCC VR-1470) were detected using a polyclonal anti-S. negevensis rabbit antibody (1:2,500), a mouse anti-major outer membrane porin (MOMP) antibody (1:50) (ab20881; Abcam, Cambridge, UK), or an anti-W. chondrophila rabbit antibody (1:2,000), respectively (green), followed by a secondary antibody (Alexa Fluor 488-conjugated goat anti-mouse or anti-rabbit antibody [1:500]; Molecular Probes, Thermo Fisher Scientific, Waltham, MA), mammalian cells were stained with Texas red-conjugated concanavalin A (1:50) (red), and nucleic acids were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000) (blue). (B) Effects of fosfomycin and penicillin treatment in Vero cells at day 6 postinfection.
Similarly to Chlamydia spp. (11), S. negevensis replication was not inhibited by high doses of fosfomycin, which targets the enzyme MurA (implicated in the early steps of PG biosynthesis). However, a small fraction of S. negevensis particles, which increased by day 6, showed abnormal morphological features consistent with aberrant bodies (Fig. 1A and B), although remaining significantly less important than observed for W. chondrophila (11). Chlamydia resistance to fosfomycin is suspected to be related to a single substitution (Cys115 to Asp) in the active site of MurA (11, 15). This mutation was not found in S. negevensis, supporting the observed partially sensitive phenotype. Finally, we did not observe aberrant bodies with vancomycin treatment, a drug that inhibits transpeptidation through high-affinity binding to the d-alanine precursor (Fig. 1A).
Recently, several works have demonstrated the presence of a modified version of PG, which is required for cell division (12, 16, 17), in Chlamydiales members, thus explaining their partial sensitivity to cell wall inhibitors. Interestingly, a recent study failed to isolate PG-like structures in S. negevensis (18), while such structures were identified in Protochlamydia amoebophila (18) and C. trachomatis (17). In the same work, incorporation of fluorescently labeled d-alanine could not be highlighted in S. negevensis (18), which correlates with the absence of vancomycin effects observed here. However, a previous work showed that, similarly to C. trachomatis, S. negevensis was susceptible to d-cycloserine, a molecule that inhibits the alanine racemase Alr and the alanine ligase Ddl, which are required for d-alanine formation (19). While a predicted Ddl enzyme is encoded in the S. negevensis genome, no Alr coding sequence is present, similarly to Chlamydiaceae (12). It is not known whether the serine hydroxymethyltransferase GlyA encoded in the S. negevensis genome could compensate for the absence of Alr, as described for Chlamydiaceae (20).
Despite the absence of PG-like structures, the activity of two PG-remodeling enzymes, NlpD and AmiA, was documented in S. negevensis (16), and enzymes implicated in PG biosynthesis are highly conserved among Chlamydiales members, including S. negevensis, which supports their crucial role (12). However, the different responses to different cell wall inhibitors, each targeting a specific step of PG biosynthesis, indicate that, despite the likely requirement for a modified form of PG for cell division, some significant differences exist in the PG biosynthesis pathway of S. negevensis, which might bring further insights into the mechanisms of Chlamydiales cell division.
In conclusion, in this work we highlighted several differences in the antimicrobial responses of S. negevensis, compared to other Chlamydiales members. Although the pathogenic role of Simkania spp. remains to be better defined, the precise knowledge of their antimicrobial susceptibility patterns provides significant information regarding the biology and evolution of the Chlamydiales order.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (SNSF) (MD-PhD grant 323530-158123 and grant 310030-162603).
We do not report any potential conflicts of interest.
REFERENCES
- 1.Pillonel T, Bertelli C, Salamin N, Greub G. 2015. Taxogenomics of the Chlamydiales. Int J Syst Evol Microbiol 65:1381–1393. doi: 10.1099/ijs.0.000090. [DOI] [PubMed] [Google Scholar]
- 2.Al-Younes HM, Paldanius M. 2014. High seroprevalence of Simkania negevensis in Jordan. Braz J Microbiol 45:1433–1437. doi: 10.1590/S1517-83822014000400038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahane S, Greenberg D, Friedman MG, Haikin H, Dagan R. 1998. High prevalence of “Simkania Z,” a novel Chlamydia-like bacterium, in infants with acute bronchiolitis. J Infect Dis 177:1425–1429. doi: 10.1086/517830. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman D, Kahane S, Lieberman D, Friedman MG. 1997. Pneumonia with serological evidence of acute infection with the Chlamydia-like microorganism “Z”. Am J Respir Crit Care Med 156:578–582. doi: 10.1164/ajrccm.156.2.9608081. [DOI] [PubMed] [Google Scholar]
- 5.Goy G, Greub G. 2009. Antibiotic susceptibility of Waddlia chondrophila in Acanthamoeba castellanii amoebae. Antimicrob Agents Chemother 53:2663–2666. doi: 10.1128/AAC.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Barsy M, Bottinelli L, Greub G. 2014. Antibiotic susceptibility of Estrella lausannensis, a potential emerging pathogen. Microbes Infect 16:746–754. doi: 10.1016/j.micinf.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Vouga M, Baud D, Greub G. 2017. Simkania negevensis may produce long-lasting infections in human pneumocytes and endometrial cells. Pathog Dis 75:ftw115. doi: 10.1093/femspd/ftw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vouga M, Diabi H, Boulos A, Baud D, Raoult D, Greub G. 2015. Antibiotic susceptibility of Neochlamydia hartmanellae and Parachlamydia acanthamoebae in amoebae. Microbes Infect 17:761–765. doi: 10.1016/j.micinf.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Casson N, Greub G. 2006. Resistance of different Chlamydia-like organisms to quinolones and mutations in the quinoline resistance-determining region of the DNA gyrase A- and topoisomerase-encoding genes. Int J Antimicrob Agents 27:541–544. doi: 10.1016/j.ijantimicag.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Hammerschlag MR, Gleyzer A. 1983. In vitro activity of a group of broad-spectrum cephalosporins and other beta-lactam antibiotics against Chlamydia trachomatis. Antimicrob Agents Chemother 23:493–494. doi: 10.1128/AAC.23.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacquier N, Frandi A, Pillonel T, Viollier P, Greub G. 2014. Cell wall precursors are required to organize the chlamydial division septum. Nat Commun 5:3578. doi: 10.1038/ncomms4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquier N, Viollier P, Greub G. 2015. The role of peptidoglycan in chlamydial cell division: towards resolving the chlamydial anomaly. FEMS Microbiol Rev 39:262–275. doi: 10.1093/femsre/fuv001. [DOI] [PubMed] [Google Scholar]
- 13.Collingro A, Tischler P, Weinmaier T, Penz T, Heinz E, Brunham RC, Read TD, Bavoil PM, Sachse K, Kahane S, Friedman MG, Rattei T, Myers GSA, Horn M. 2011. Unity in variety: the pan-genome of the Chlamydiae. Mol Biol Evol 28:3253–3270. doi: 10.1093/molbev/msr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertelli C, Collyn F, Croxatto A, Rückert C, Polkinghorne A, Kebbi-Beghdadi C, Goesmann A, Vaughan L, Greub G. 2010. The Waddlia genome: a window into chlamydial biology. PLoS One 5:e10890. doi: 10.1371/journal.pone.0010890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai K, Davies TA, Jacobs MR, Appelbaum PC. 2002. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob Agents Chemother 46:1273–1280. doi: 10.1128/AAC.46.5.1273-1280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frandi A, Jacquier N, Théraulaz L, Greub G, Viollier PH. 2014. FtsZ-independent septal recruitment and function of cell wall remodelling enzymes in chlamydial pathogens. Nat Commun 5:4200. doi: 10.1038/ncomms5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liechti GW, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT. 2014. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature 506:507–510. doi: 10.1038/nature12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilhofer M, Aistleitner K, Biboy J, Gray J, Kuru E, Hall E, Brun YV, VanNieuwenhze MS, Vollmer W, Horn M, Jensen GJ. 2013. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat Commun 4:2856. doi: 10.1038/ncomms3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahane S, Gonen R, Sayada C, Elion J, Friedman MG. 1993. Description and partial characterization of a new Chlamydia-like microorganism. FEMS Microbiol Lett 109:329–333. doi: 10.1111/j.1574-6968.1993.tb06189.x. [DOI] [PubMed] [Google Scholar]
- 20.De Benedetti S, Bühl H, Gaballah A, Klöckner A, Otten C, Schneider T, Sahl H-G, Henrichfreise B. 2014. Characterization of serine hydroxymethyltransferase GlyA as a potential source of D-alanine in Chlamydia pneumoniae. Front Cell Infect Microbiol 4:19. doi: 10.3389/fcimb.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlhoff SA, Hammerschlag MR. 2015. Treatment of chlamydial infections: 2014 update. Expert Opin Pharmacother 16:205–212. doi: 10.1517/14656566.2015.999041. [DOI] [PubMed] [Google Scholar]
- 22.Senn L, Hammerschlag MR, Greub G. 2005. Therapeutic approaches to Chlamydia infections. Expert Opin Pharmacother 6:2281–2290. doi: 10.1517/14656566.6.13.2281. [DOI] [PubMed] [Google Scholar]
- 23.Rice RJ, Bhullar V, Mitchell SH, Bullard J, Knapp JS. 1995. Susceptibilities of Chlamydia trachomatis isolates causing uncomplicated female genital tract infections and pelvic inflammatory disease. Antimicrob Agents Chemother 39:760–762. doi: 10.1128/AAC.39.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy AJ, Sandlin RC, Maurelli AT. 2003. In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J Bacteriol 185:1218–1228. doi: 10.1128/JB.185.4.1218-1228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]