ABSTRACT
Preliminary preclinical and observational studies suggest the potential utility of metformin as an adjunctive, host-directed agent for treatment of tuberculosis (TB). In this study, we sought to investigate the bactericidal and sterilizing activities of human-like exposures of metformin when given in combination with the first-line regimen against chronic tuberculosis in BALB/c mice. Mice receiving metformin adjunctive therapy had similar lung bacillary burdens with control mice during treatment, and the proportion of mice with microbiological relapse was similar between the two groups.
KEYWORDS: Mycobacterium tuberculosis, metformin, standard first-line regimen, BALB/c, bactericidal, sterilizing, host-directed therapy, relapse, cure, Denver regimen
TEXT
Host-directed therapy (HDT) may offer expanded therapeutic options for improving tuberculosis (TB) treatment (1–5). Metformin (MetF), an AMP-activated protein kinase (AMPK)-activating drug for type 2 diabetes (DM), was reported to inhibit intracellular growth of mycobacteria by inducing reactive oxygen species and to enhance the efficacy of conventional anti-TB drugs in mouse models of acute and chronic TB; its use was associated with decreased TB severity and improved clinical outcomes in a retrospective analysis of 220 patients with DM and TB (6). In another retrospective study, Srujitha et al. showed that MetF use was associated with a 3.9-fold reduction in TB incidence among patients with DM (7). Based on these findings, we hypothesized that MetF adjunctive therapy would enhance the bactericidal and sterilizing activities of the standard first-line treatment (8, 9) against chronic TB infection in mice and shorten the duration of curative treatment as assessed by microbiological relapse.
All animal-related procedures were approved by the Johns Hopkins University (JHU) School of Medicine Animal Care and Use Committee. A total of 170 female BALB/c mice aged 4 to 6 weeks (Charles River Labs, Wilmington, MA) were aerosol infected with Mycobacterium tuberculosis H37Rv (JHU) using the inhalation exposure system (Glas-Col, Terre Haute, IN) calibrated to deliver ∼102 CFU per mouse lung in two consecutive runs. After aerosol infection, the mice were randomized into the two treatment groups. Six weeks postinfection, the mice were treated daily (5 days/week) via esophageal cannulation with human-equivalent doses of rifampin (10 mg/kg), isoniazid (10 mg/kg), pyrazinamide (150 mg/kg), and ethambutol (100 mg/kg) (RHZE) with or without MetF (250 mg/kg) for up to 6 months (2, 10, 11). For the first 2 months of treatment, the mice received RHZE and, for the remaining 4 months, only rifampin and isoniazid (RH) to mirror the first-line regimen in humans. The rifampin dose preceded that of the other drugs by at least 1 h to prevent pharmacokinetic antagonism (12, 13). The dose of MetF selected for these studies was found to have anti-TB activity in C57BL/6 mice by Singhal et al. (6) and is estimated (14) to be equivalent to 25 mg/kg in humans (15), which is well tolerated (15, 16). Groups of 5 mice were sacrificed on the day after infection, on the day of treatment initiation, and at preselected time points after treatment to determine the numbers of CFU implanted in the lungs, baseline pretreatment CFU, and posttreatment CFU, respectively. The proportion of mice with culture-positive relapse was determined by holding cohorts of 15 mice for 3 additional months after completion of 3.5, 4.5, or 5.5 months of treatment. Relapse was defined as the presence of mycobacterial colonies upon plating entire undiluted lung homogenates. Animal body weights and lung and spleen weights were recorded at the time of sacrifice. The lungs of sacrificed animals were examined grossly for visible lesions, and small, randomly selected sections were formalin fixed for histopathology. The remainder of each lung was homogenized in 2.5 ml phosphate-buffered saline (PBS). Lung homogenates were plated on selective 7H11 plates (BD, Baltimore, MD) for CFU enumeration. CFU data were derived from five mice per group. Log-transformed CFU were used to calculate means and standard deviations (SDs). Comparisons of CFU data among experimental groups were performed by Student t test. Group relapse proportions were compared using Fisher's exact test. P values of <0.05 were considered to be statistically significant.
One day postinfection, the mean lung CFU counts (±SD) were 2.96 ± 0.01 log10 and 2.96 ± 0.04 log10 in aerosol runs 1 and 2, respectively. Four weeks later, on the day of treatment initiation (day 0), mean lung CFU counts were 6.56 ± 0.14 log10. During the initial phase of treatment, the standard regimen of RHZE reduced the lung CFU counts to 4.92 ± 0.28, 3.66 ± 0.21, and 1.43 ± 0.48 log10 after 0.5, 1, and 2 months of treatment, respectively (Fig. 1). Relative to the control regimen, the addition of MetF did not significantly alter lung CFU at 0.5 (P = 0.53), 1 (P = 0.87), or 2 months (P = 0.22) of treatment. After 3.5 months of treatment, the addition of MetF to standard therapy reduced mean lung CFU by 0.18 log10 relative to the control group (P = 0.039). At 4.5 months posttreatment, all mice treated with the control and experimental regimens were lung culture negative and remained so after 5.5 months of treatment.
FIG 1.
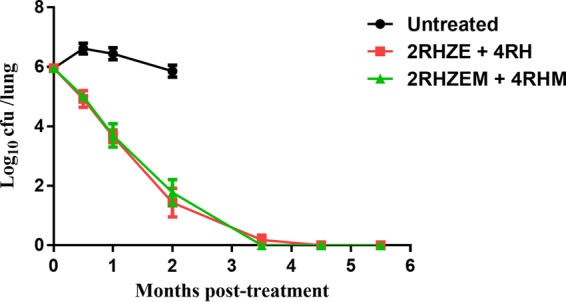
Adjunctive treatment with metformin (M) in Mycobacterium tuberculosis-infected BALB/c mice. RHZE, rifampin at 10 mg/kg, isoniazid at 10 mg/kg, pyrazinamide at 150 mg/kg, ethambutol at 100 mg/kg; M, metformin at 250 mg/kg.
Totals of 53.3%, 20%, and 6.6% of mice treated with the first-line regimen relapsed after treatment for 3.5, 4.5, and 5.5 months, respectively (Table 1). MetF adjunctive therapy did not significantly alter relapse proportions, as 46.6% (P = 0.52), 20% (P = 1.0), and 0% (P = 1.0) of mice relapsed after treatment for 3.5, 4.5, and 5.5 months, respectively.
TABLE 1.
Sterilizing activity of metformin in combination with the first-line regimen against chronic TB in mice
| Regimen | Percentage (proportion) relapse, assessed 12 wk after the completion of treatment for: |
||
|---|---|---|---|
| 3.5 mo | 4.5 mo | 5.5 mo | |
| 2 mo RHZE and 4 mo RH | 53.3 (8/15) | 20 (3/15) | 6.6 (1/15) |
| 2 mo RHZE and 4 mo RH + MetF | 46.6 (5/15) | 20 (3/15) | 0 (0/15) |
MetF-treated mice showed no overt signs of toxicity during the entire treatment period. There were no significant differences in total body weights, lung and spleen weights, gross lung pathology of mice lungs, or surface area of lung involved by inflammation at any of the treatment or relapse time points (data not shown).
To our knowledge, this is the first study to investigate the adjunctive sterilizing activity of MetF against TB in a preclinical model. Although there was a trend toward improved bactericidal activity in the MetF arm during the continuation phase of therapy, this effect did not consistently attain statistical significance. Our study and that of Singhal et al. (6) used the same MetF dose (250 mg/kg). One possible explanation for the discrepant findings between the two studies is the different mouse models used. Another potential explanation is that Singhal et al. used MetF as an adjunctive agent in combination with a single antitubercular drug (isoniazid or ethambutol), whereas we studied the activity in combination with the more potent first-line regimen RHZE, which may mask an adjunctive role for MetF. Alternatively, the inclusion of rifampin in our study may have altered the pharmacokinetics of MetF. Although concurrent use of rifampin and MetF may result in increased plasma concentrations of the latter in humans (17), it is possible that rifampin may accelerate the clearance of MetF and reduce drug exposures in BALB/c mice (18). Adjunctive therapy with host-modulating agents likely must strike a balance between an antimicrobial effect and excessive host inflammation, which may promote bacterial growth. Therefore, further preclinical pharmacokinetics studies of MetF coadministered with the first-line antitubercular regimen are urgently needed to guide the future study of this promising agent in clinical trials (19, 20). In addition, detailed pharmacodynamics studies are required to relate plasma drug exposures in mice to AMPK activation and anti-TB activity in cell culture systems (6, 21). Finally, it is important to recognize that observations made in the mouse model are not necessarily predictive of outcomes in clinical trials of TB treatment nor is early “sterilization” a predictor of cure in humans (22).
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases under award UH2AI122309 (to P.C.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
We declare no conflicts of interest.
REFERENCES
- 1.Hawn TR, Matheson AI, Maley SN, Vandal O. 2013. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol Rev 77:608–627. doi: 10.1128/MMBR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta NK, Bruiners N, Pinn ML, Zimmerman MD, Prideaux B, Dartois V, Gennaro ML, Karakousis PC. 2016. Statin adjunctive therapy shortens the duration of TB treatment in mice. J Antimicrob Chemother 71:1570–1577. doi: 10.1093/jac/dkw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A, Rao M, Wallis RS, Kaufmann SH, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G, Azhar E, Hoelscher M, Maeurer M, Host-Directed Therapies Network consortium. 2016. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis 16:e47–. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis RS, Hafner R. 2015. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 15:255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 5.Zumla A, Maeurer M, Host-Directed Therapies Network, Chakaya J, Hoelscher M, Ntoumi F, Rustomjee R, Vilaplana C, Yeboah-Manu D, Rasolof V, Munderi P, Singh N, Aklillu E, Padayatchi N, Macete E, Kapata N, Mulenga M, Kibiki G, Mfinanga S, Nyirenda T, Maboko L, Garcia-Basteiro A, Rakotosamimanana N, Bates M, Mwaba P, Reither K, Gagneux S, Edwards S, Mfinanga E, Abdulla S, Cardona PJ, Russell JB, Gant V, Noursadeghi M, Elkington P, Bonnet M, Menendez C, Dieye TN, Diarra B, Maiga A, Aseffa A, Parida S, Wejse C, Petersen E, Kaleebu P, Oliver M, Craig G, Corrah T, Tientcheu L, Antonio M, et al. 2015. Towards host-directed therapies for tuberculosis. Nat Rev Drug Discov 14:511–512. doi: 10.1038/nrd4696. [DOI] [PubMed] [Google Scholar]
- 6.Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B, Poidinger M, Chee C, Kaplan G, Wang YT, De Libero G. 2014. Metformin as adjunct antituberculosis therapy. Sci Transl Med 6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 7.Marupuru S, Senapati P, Pathadka S, Miraj SS, Unnikrishnan MK, Manu MK. 2017. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz J Infect Dis 21:312–316. doi: 10.1016/j.bjid.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro JA. 1990. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis. A twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med 112:407–415. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. 2010. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother 65:729–734. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta NK, Pinn ML, Karakousis PC. 2014. Sterilizing activity of thioridazine in combination with the first-line regimen against acute murine tuberculosis. Antimicrob Agents Chemother 58:5567–5569. doi: 10.1128/AAC.03408-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta NK, Pinn ML, Karakousis PC. 2014. Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis. Antimicrob Agents Chemother 58:4048–4053. doi: 10.1128/AAC.02981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon J, Dickinson JM, Sole K, Mitchison DA. 1996. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother 40:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J 22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y, Zhang H, Lu L, Li C, Huang S, Xing Y, Zhou D, Meng A. 2015. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med 87:15–25. doi: 10.1016/j.freeradbiomed.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. 2008. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 17.Cho SK, Yoon JS, Lee MG, Lee DH, Lim LA, Park K, Park MS, Chung JY. 2011. Rifampin enhances the glucose-lowering effect of metformin and increases OCT1 mRNA levels in healthy participants. Clin Pharmacol Ther 89:416–421. doi: 10.1038/clpt.2010.266. [DOI] [PubMed] [Google Scholar]
- 18.Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, van de Vijver S, Panduru NM, Hill PC, Ruslami R, Moore D, Aarnoutse R, Critchley JA, van Crevel R. 2014. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol 2:740–753. doi: 10.1016/S2213-8587(14)70110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restrepo BI. 2016. Metformin: Candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients. Tuberculosis (Edinb) 101S:S69–S72. doi: 10.1016/j.tube.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Te Brake LH, van den Heuvel JJ, Buaben AO, van Crevel R, Bilos A, Russel FG, Aarnoutse RE, Koenderink JB. 2016. Moxifloxacin is a potent in vitro inhibitor of OCT- and MATE-mediated transport of metformin and ethambutol. Antimicrob Agents Chemother 60:7105–7114. doi: 10.1128/AAC.01471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowling RJ, Lam S, Bassi C, Mouaaz S, Aman A, Kiyota T, Al-Awar R, Goodwin PJ, Stambolic V. 2016. Metformin pharmacokinetics in mouse tumors: implications for human therapy. Cell Metab 23:567–568. doi: 10.1016/j.cmet.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, Pappas F, Phillips PP, Nunn AJ, REMoxTB Consortium. 2014. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]


