ABSTRACT
More than 5 years after a United Nations peacekeeping battalion introduced cholera to Haiti, over 150,000 peacekeepers continue to be deployed annually from countries where cholera is endemic. The United Nations has thus far declined to provide antimicrobial chemoprophylaxis to peacekeepers, a policy based largely on concerns that the risks of drug resistance generation and spread would outweigh the potential benefits of preventing future cholera importations. In this study, we sought to better understand the relative benefits and risks of cholera chemoprophylaxis for peacekeepers in terms of antibiotic resistance. Using a stochastic model to quantify the potential impact of chemoprophylaxis on importation and transmission of drug-resistant and drug-sensitive Vibrio cholerae, we found that chemoprophylaxis would decrease the probability of cholera importation but would increase the expected number of drug-resistant infections if an importation event were to occur. Despite this potential increase, we found that at least 10 drug-sensitive infections would likely be averted per excess drug-resistant infection under a wide range of assumptions about the underlying prevalence of drug resistance and risk of acquired resistance. Given these findings, policymakers should reconsider whether the potential resistance risks of providing antimicrobial chemoprophylaxis to peacekeepers are sufficient to outweigh the anticipated benefits.
KEYWORDS: cholera, prophylaxis, antibiotic resistance, competition, mathematical model, transmission dynamics
INTRODUCTION
The inadvertent introduction of cholera to Haiti by a deployment of Nepalese peacekeepers in October 2010 sparked one of the most severe cholera epidemics in modern history. Reports of more than 9,000 deaths and 700,000 cases likely represent a considerable underestimate of true disease burden (1). Recognition of the role of United Nations (UN) peacekeepers in precipitating the outbreak has prompted the consideration of biomedical interventions to prevent Vibrio cholerae importation during future deployments of peacekeepers from countries where cholera is endemic (2). The need for such interventions is considerable, as approximately 150,000 peacekeepers are deployed from such countries each year (3).
Although oral cholera vaccines (OCVs) were added to predeployment immunization schedules for all peacekeepers in November 2015 (4, 5), the inefficacy of such vaccines against asymptomatic cholera shedding (6, 7) suggests that immunization is a suboptimal strategy for preventing importations such as occurred in Haiti. A recent modeling study concluded that antimicrobial chemoprophylaxis of peacekeepers departing countries where cholera is endemic would be more effective than vaccination in preventing Haiti's outbreak, reducing the probability of an epidemic by 91% if implemented alone and 98% if coupled with immunization (3). Chemoprophylaxis was also previously recommended by a UN-commissioned independent panel of experts investigating Haiti's cholera outbreak (2).
Despite the recommendations from the independent panel of experts and the results of the prior modeling study (3), concerns about selection for drug-resistant (DR) V. cholerae strains have forestalled the UN′s implementation of this recommendation (8–10). The decision to withhold chemoprophylaxis reflects two suppositions: first, that providing chemoprophylaxis to peacekeepers would increase the expected burden of DR cholera, and second, that this risk outweighs the potential benefit of preventing drug-sensitive (DS) infections. Previous modeling work has demonstrated a complexity of potential relationships between prophylaxis and the burden of drug resistance in general (11). In this scenario, chemoprophylaxis could select for drug resistance among arriving peacekeepers. However, by preventing the importation and epidemic take-off of DS pathogens, it could also reduce subsequent opportunities for resistance acquisition and spread during a cholera epidemic.
The potential impacts of chemoprophylaxis for UN peacekeepers on importation and outbreaks of DS and DR cholera cannot easily be addressed through empirical studies. Because there are no existing data on the potential population impact of similarly targeted, short-term prophylaxis to prevent cholera transmission, mathematical modeling is an appealing approach that allows us to provide projections based on our understandings of V. cholerae transmission and the mechanisms of chemoprophylaxis. Therefore, we developed a mathematical model to examine the risks and benefits of chemoprophylaxis for UN peacekeepers in the context of drug resistance. Our model builds on previous work (3) by considering both DS and DR V. cholerae strains; DR strains may preexist in the peacekeepers' country of origin, or resistance may be acquired through chemoprophylaxis or antibiotic treatment. Although motivated by the events in Haiti, here we present a general model intended to have broad future applicability and display the sensitivity of our results across a wide range of parameter values.
RESULTS
We used a stochastic modeling framework to simulate cholera importation and transmission. We adopted a parsimonious approach focused on elucidating the causes and consequences of drug resistance across a wide range of assumptions. Briefly, we simulated the arrival of 500 UN peacekeepers to a cholera-susceptible host population of 1 million under both chemoprophylaxis and no-chemoprophylaxis scenarios to explore the predicted impact of predeparture chemoprophylaxis among peacekeepers on DS and DR cholera infections within the host population. Due to the implementation of oral cholera vaccination (OCV) among peacekeepers, our baseline assumption was that peacekeepers also received OCV, with alternative results assuming no OCV use presented in the supplemental material. We report results based on 10,000 randomly generated parameter sets intended to represent variability or uncertainty in key measures of cholera natural history and epidemiology. The model structure and parameters are described in greater detail in Materials and Methods.
For each parameter set, we recorded data from repeated stochastic runs. Each run resulted in either no cholera cases among the arriving peacekeepers, cholera cases occurring among the arriving peacekeepers but never transmitted to the host population, a small number of transmitted cases to the host population but no large epidemic, or a large epidemic in the host population. Simulations in which large DS epidemics occurred also frequently resulted in smaller outbreaks of DR cholera through acquisition of resistance.
Figure 1 shows the range of potential outcomes based on 5,000 model runs at the midpoint of each parameter range. Of these runs, no epidemics appear to have been seeded by both DS and DR infections arriving simultaneously, consistent with the low importation probability. The most obvious impact of prophylaxis is to reduce the number of DS cases; this sample of 5,000 stochastic runs resulted in 37 DS importations under the no-prophylaxis scenario but only one (which did not take off) under the prophylaxis scenario. The impact of prophylaxis on DR epidemics is less obvious and therefore is explored in greater detail below for the full range of parameter sets, with runs for each parameter set repeated until 2,000 importation events had occurred.
FIG 1.
Epidemic curves for 5,000 model runs at parameter midpoints. Importation occurred in 59 model runs for the no-prophylaxis scenario and 26 runs for the prophylaxis scenario; only these runs are displayed. (A) Thirty-seven DS importations occurred under the no-prophylaxis scenario. (B) Log scale of DS infections under the no-prophylaxis scenario, revealing additional importations that failed to take off. (C) Only one DS importation, and no DS epidemics, occurred under the prophylaxis scenario for these 5,000 runs. (D) DR infections under the no-prophylaxis scenario. (E) Log scale of DR infections under the no-prophylaxis scenario, revealing small outbreaks of DR infections resulting from acquired resistance following treatment. (F) DR infections under the prophylaxis scenario appear fairly similar to those under the no-prophylaxis scenario.
Chemoprophylaxis of UN peacekeepers has the potential to increase or decrease drug-resistant infections in the host population.
Figure 2A displays the predicted impacts of providing chemoprophylaxis to UN peacekeepers on the expected number of DR infections in the host country across the explored parameter range. To produce this figure, we averaged across all model runs for a single parameter set, including zeros for all cases in which importation did not occur. These results cluster around 0, with prophylaxis either increasing or decreasing the expected number of DR infections depending on the particular epidemic scenario. However, across all 10,000 sampled parameter sets, the use of chemoprophylaxis among peacekeepers never increased the expected number of DR cholera infections in the host country by more than 0.2% of the population size over the course of the epidemic.
FIG 2.
Simulation mean effects of peacekeeper prophylaxis versus no prophylaxis. Blue indicates results favoring prophylaxis, and red indicates results favoring no prophylaxis. Results are based on 10,000 random parameter sets assuming 500 peacekeepers in a host population of 1 million susceptible individuals. For each parameter set, results were obtained by subtracting the mean value from repeated stochastic runs assuming no prophylaxis from the mean value given prophylaxis. Subplot interpretations are as follows. (A) Prophylaxis may increase or decrease the overall expected number of DR cholera infections; these values cluster around 0 and never reach ≥2,000 (≥0.2% of the population). (B) Prophylaxis always decreases the probability of cholera importation. (C) Prophylaxis always increases the expected number of DR cholera infections conditional on cholera importation. (D) Prophylaxis is likely to prevent ≥10 DS cholera infections per excess DR cholera infection.
To understand why chemoprophylaxis could either increase or decrease the expected number of drug-resistant infections in the host country, we separated this measure into two components: first, the probability of cholera importation, and second, the expected number of DR infections conditional on importation.
First, we estimated the effects of chemoprophylaxis on the probability of cholera importation, i.e., the chances of at least one infection occurring in the general population of the host country. Across model runs, estimated importation probabilities ranged from approximately 0.05% to 6% without chemoprophylaxis and 0.003% to 3% with chemoprophylaxis. As shown in Fig. 2B, chemoprophylaxis among peacekeepers consistently decreased the probability of a V. cholerae importation event across all of the scenarios that we explored. These results are based on the assumption that as many as 50% of V. cholerae strains in the peacekeeper country of origin are resistant to the applied drug, indicating that chemoprophylaxis could be a robust means of preventing cholera importation even when the drug is not universally effective.
Next, we considered the effects of chemoprophylaxis among peacekeepers on the predicted average number of DR cholera infections in the community, conditional upon V. cholerae importation. Chemoprophylaxis among peacekeepers consistently increased the expected number of DR cholera infections when importation did occur throughout the explored parameter ranges (Fig. 2C). Furthermore, these values were frequently large, with the excess expected number of DR infections conditional on importation exceeding 10% of the total population for 92% of parameter sets. This finding reflects the intuitive concern about cholera prophylaxis: because prophylaxis exclusively targets DS phenotypes, any importation events that do occur are more likely to be triggered by a DR strain. However, this is a rare event and is made even rarer by the use of chemoprophylaxis.
Because cholera chemoprophylaxis for UN peacekeepers decreases the probability of cholera importation to the host country but increases the expected number of DR infections conditional on importation, its impact on the overall expected number of DR infections in the host country is more modest and may occur in either direction.
The number of averted DS cholera infections is expected to overwhelm any increase in DR infections as a result of chemoprophylaxis.
It is clearly desirable to provide chemoprophylaxis to UN peacekeepers under scenarios for which this approach decreases the expected number of DR infections. For the remaining scenarios, we estimated the expected number of DS infections that could be averted through chemoprophylaxis for each additional DR infection (Fig. 2D). Providing chemoprophylaxis to UN peacekeepers resulted in at least 10 DS infections averted per excess DR infection for 99% of parameter sets. A total of 206 of 10,000 parameter sets produced a number of DS infections averted per excess DR infection in excess of 1,000, while only 5 resulted in values below 1. These results suggest that even if chemoprophylaxis increases the risk of DR cholera infections, its preventive impact on the number of DS cases is likely to be much greater.
Factors influencing the risk-benefit profile.
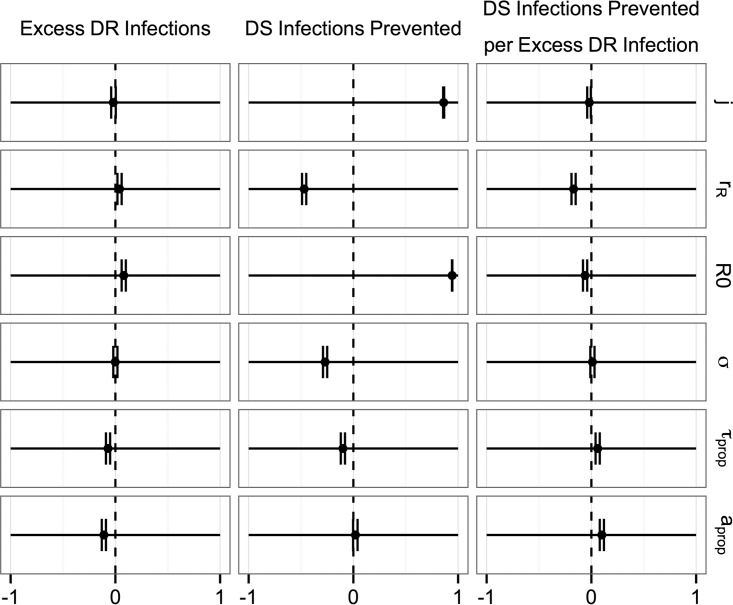
Taken individually, the parameter ranges explored primarily had only small effects on the number of excess DR infections resulting from prophylaxis as well as the ratio of DS infections prevented per excess DR infection (Fig. 3). The excess number of DR infections resulting from prophylaxis was slightly decreased when we assumed a higher probability of receiving treatment and higher probability of acquired resistance, both of which could increase the opportunities for DR cholera to emerge out of a DS epidemic in the absence of prophylaxis, and slightly increased for higher values for the basic reproductive number in the absence of treatment (R0). The number of DS infections prevented per excess DR infection was most negatively associated with DR prevalence in the country of origin, reflecting the intuition that chemoprophylaxis would be less effective in settings with a high prevalence of drug resistance and most positively associated with the probabilities of treatment and of acquired resistance.
FIG 3.
Partial rank correlation coefficients between variable parameters and the impact of prophylaxis. Point estimates and 95% confidence intervals are shown. The six variable parameters are as shown in Table 1 (j, cholera incidence in peacekeeper country of origin; rR, proportion of DR cholera in the country of origin; R0, basic reproductive number in the absence of treatment; σ, proportion of cholera infections that are symptomatic; τprop, proportion of symptomatic individuals who receive treatment; aprop, proportion of individuals receiving treatment or prophylaxis who acquire resistance).
Several parameters were moderately or strongly associated with the number of DS infections prevented by prophylaxis. Most notably, the incidence of cholera in the peacekeepers' country of origin and the basic reproductive number displayed a positive association with the number of DS infections prevented by prophylaxis, and the proportion of DR cholera cases in the peacekeepers' country of origin displayed a negative association.
Sensitivity analyses.
In addition to exploring the impact of these variable parameters, we ran sensitivity analyses to assess the potential impact of a smaller population size, less or more frequent transmission from asymptomatic infections, higher or lower efficacy of prophylaxis (in terms of prevention of infection), and either no vaccination or more effective vaccination than assumed at baseline. The detailed results of these analyses are shown in the supplemental material. Briefly, changing our assumptions in ways that allowed for more transmission (i.e., assuming no vaccination or greater transmission from asymptomatic infections) resulted in higher importation probabilities and prophylaxis having a greater range of impacts, both positive and negative, on the excess number of DR cases. For example, whereas the excess number of DR infections resulting from prophylaxis clustered primarily between −1,000 and 1,000 for our baseline scenario, the results similarly clustered between −15 and 15 when we assumed more effective vaccination and −3,000 and 3,000 when we assumed no vaccination. However, these ranges are all small relative to the population size of 1 million, and all sensitivity analyses resulted in similar distributions for the number of DS infections prevented per excess DR case. The degrees of impact of our variable parameters on the number of DS infections prevented per excess DR case resulting from prophylaxis were similar across all of the scenarios, with the most influential parameter consistently being the proportion of DR cholera cases in the peacekeepers' country of origin.
DISCUSSION
We have presented a mathematical model intended to estimate the effects of administering chemoprophylaxis to UN peacekeepers on the acquisition and spread of drug-resistant cholera. While administering chemoprophylaxis to UN peacekeepers could under some circumstances increase the expected number of DR cholera infections, our findings suggest that the expected increase in DR infections is likely to remain fairly small relative to the size of the population and expected number of DS infections averted. For each excess DR infection caused by chemoprophylaxis, we predict that this approach is likely to prevent at least 10 DS infections.
The benefits of chemoprophylaxis would likely be strengthened by the use of a drug for which background resistance was rare in the peacekeepers' country of origin. Although surveillance systems to monitor DR cholera are poor, resistance to first-line therapies such as tetracycline derivatives, ciprofloxacin, and chloramphenicol is prevalent in some geographical regions (12–14). Lower rates of resistance to azithromycin, potentially contributing to the drug's superior efficacy against cholera diarrhea in clinical trials, have been reported (15, 16). Local resistance profiles should inform the choice of antimicrobial drugs used for chemoprophylaxis.
For this analysis, we have focused on the potential benefits and risks of chemoprophylaxis in terms of the number of DS and DR cholera infections. The impacts of chemoprophylaxis could extend beyond these to other metrics, such as cost-effectiveness and risk of infection with other diseases. Chemoprophylaxis was previously found to be the lowest-cost option available to prevent cholera importation by UN peacekeepers, though this analysis did not consider its potential impacts on drug resistance (3). The resource requirements for DR cases could potentially be higher, for example, if antibiotic treatments were more expensive or the duration of hospital stays increased. However, because we predict the impacts of prophylaxis on the number of DR infections to be small relative to the impact on DS infections, and because most individuals with cholera infections do not receive antibiotics, we do not believe that costs should be a major barrier to implementation. Another limitation of this analysis is our inability to directly estimate the potential impact of providing cholera chemoprophylaxis to UN peacekeepers on antibiotic resistance for diseases besides cholera. These effects are more difficult to predict. As a secondary analysis, we attempted to address the issue of resistance selection for other bacteria indirectly, by comparing the number of antibiotics used for chemoprophylaxis to the number that would be used for treatment of symptomatic cases in the event of an epidemic (see the supplemental material). Although in some cases these results suggest that chemoprophylaxis could reduce the total expected number of antibiotic doses by preventing the occurrence of DS epidemics, this outcome is highly sensitive to the size of the susceptible population and the likelihood of importation. Comparing the number of peacekeepers deployed each year (around 150,000) to the global burden of antibiotic consumption (estimated at over 73 billion standard units in 2010 [17]) suggests that providing chemoprophylaxis to peacekeepers is unlikely to be a major global driver of antibiotic resistance.
Our analysis is additionally limited by the lack of data surrounding several model parameters. While we incorporated data from empirical studies whenever possible, our results are dependent on the parameter values and distributions that we have assumed, and any inaccuracies in these assumptions may be propagated throughout our model. To mitigate this impact, we have assigned wide ranges to the most uncertain parameters and have also conducted sensitivity analyses around the size of the susceptible population, the relative infectiousness of asymptomatic cases, the efficacy of prophylaxis, and the impacts of vaccination (see the supplemental material), all of which appear to have minimal impact on our principal findings. We also made simplifying assumptions surrounding births, deaths, and immunity to allow for discrete SIR (susceptible-infectious-recovered)-type epidemics. If we were to relax those assumptions, allowing cholera to become endemic, our results could change. However, we predict that the impact of changing these assumptions on the number of DS infections prevented per excess DR case would be small, given the minor impact that we observed when changing the size of the susceptible population in the sensitivity analysis.
Mathematical modeling, though a useful first step in risk analyses such as these, ideally should not substitute for the collection of real-world data on the impact of targeting prophylaxis to populations at risk of transmission. We suggest that if chemoprophylaxis among UN peacekeepers is implemented, it should be accompanied by surveillance and monitoring of drug-resistant infections as well as potential importations of cholera and other bacteria. Widespread use of chemoprophylaxis in other scenarios, e.g., among patient contacts, is a separate issue and should not be interpreted as being justified by our analysis. Indiscriminate use of chemoprophylaxis has been associated with local emergence of resistance in V. cholerae and may thus do more harm than good (18–22).
Mathematical modeling of DS and DR strains has previously been used to inform use of chemoprophylaxis or preventive treatment for other diseases, including tuberculosis, HIV infection, and malaria (23–25). These studies have typically used deterministic models and focused on endemic diseases. Stochastic models such as the one presented here allow modelers to account for randomness in transmission when case counts are low, and they have previously been used to inform policy in areas such as hospital-associated infections, disease emergence and importation, and disease elimination (26–28). This study is unique in its use of a stochastic model to analyze the impact of prophylaxis on importation and epidemic spread of both DS and DR pathogens in an area where they are not endemic. As such, the modeling framework and results presented here may also apply to similar scenarios of nonendemic diseases when targeted prophylaxis is proposed for a small group of at-risk individuals, for example, in the case of an emerging infectious disease.
Our results suggest that the average number of DS cholera infections prevented through targeted use of predeployment chemoprophylaxis among UN peacekeepers could greatly exceed any potential increase in the number of DR cholera infections. Therefore, we urge UN policymakers to reassess the risks and benefits surrounding the judicious use of targeted antimicrobial chemoprophylaxis to reduce the risks of future outbreaks of cholera associated with peacekeeper deployments.
MATERIALS AND METHODS
Below, we provide a detailed overview of the model structure and parameters used in our analysis. Parameter definitions and values are provided in Table 1 and described in greater detail within this text.
TABLE 1.
Parameter definitions and values
| Parameter | Definition | Value or distributiona | References or additional information |
|---|---|---|---|
| j | Incidence of (symptomatic) cholera in peacekeepers' country of origin | Unif(0.1,8) per 1,000 people per year | 3, 30; see supplemental material for details |
| rR | Proportion of DR cholera in country of origin | Unif(0,0.5) | 12–14 |
| R0 | Basic reproductive number in the absence of treatment or vaccination | Unif(1.25,5) | 31, 32 |
| σ | Proportion of cholera infections that are symptomatic | Beta(242,706) | Symptom probability in Haiti survey 0.255 (0.228, 0.284) (33); similar point estimate (0.242) in meta-analysis of reference 3 |
| τprop (rate = τ) | Proportion symptomatic who receive treatment | Unif(0.05,0.5) | 18, 34–37; see supplemental material for details |
| aprop (rate = a) | Proportion receiving treatment or prophylaxis who acquire resistance | 10^Unif(−5,−2) | 19, 20, 38–40; see supplemental material for details |
| Nimp | Number of peacekeepers | 500 | Assumed; similar to number in Haiti (3) |
| N | Population size | 1,000,000 | Assumed; 10,000 also tested (see supplemental material) |
| 1/γ | Average duration of infection (untreated) | 5 days | 3 (we made the simplifying assumption that this was the same for asymptomatic and symptomatic infections), 41 |
| 1/γabx | Average duration of infection (treated) | 2.26 days | 3, 41 |
| vabx | Risk ratio of DS infection given antibiotic chemoprophylaxis | 0.34 | 3, 42; see supplemental material for sensitivity analyses |
| βa/β | Relative infectiousness of asymptomatic infections | 10% | 3, 43, 44; see supplemental material for additional details and results based on 1% and 50% |
| 1/δ | Incubation period | 1.55 days | 3, 45 |
| σvaxm | Multiplier: relative probability of symptoms among vaccinated individuals (vs unvaccinated) | 0.35 | Protective efficacy in older children and adults is 0.66 (0.57, 0.73) (46) |
| βvaxm | Multiplier: relative infectiousness of vaccinated asymptomatic individuals (vs unvaccinated asymptomatic) | 0.37 | Assuming a log-linear relationship between infectiousness and vibrio density (6); see supplemental material for details and sensitivity analyses |
Unif, numbers are drawn according to a uniform distribution with the provided max and min; 10^Unif, the exponent of 10 is drawn according to a uniform distribution with the provided max and min.
Modeling infections among arriving peacekeepers.
For a given parameter set, we first estimated the probability of cholera importation to the host country by the 500 peacekeepers. Similar to the authors of reference 3, we assumed that the probability of an individual peacekeeper being infected at the time of departure was proportional to cholera prevalence in the departure country, estimated as the average duration of infection (1/δ + 1/λ) multiplied by the combined incidence of symptomatic and asymptomatic infections (j/σ). Because existing protocols include screening for diarrheal symptoms (5), we assumed that only asymptomatically infected peacekeepers (probability 1 − σvaxmσ) would be missed in predeparture screening. We allowed the infecting strains to be either DS or DR (with probability rR representing prevalence of drug resistance in the country of origin) and did not differentiate between exposed and infectious individuals. In the absence of chemoprophylaxis, the probability of a peacekeeper being asymptomatically infected by a DS or DR strain, respectively, was thus calculated as
We assumed that chemoprophylaxis reduced both risk and duration of DS infections and could lead to resistance acquisition (probability aprop) but had no effect on DR infections. In the presence of chemoprophylaxis, the probability of a peacekeeper being asymptomatically infected by a DS or DR strain, respectively, was thus calculated as
The numbers of peacekeepers that were uninfected, infected with DS V. cholerae, and infected with DR V. cholerae at departure were drawn according to a multinomial distribution based on these infection probabilities. To account for possible transmission events among peacekeepers while in transit and prior to drug clearance, we assumed that the second generation of cholera infections occurred solely among peacekeepers still experiencing the effects of chemoprophylaxis. The number of secondary infections per index case was drawn from a Poisson distribution with rate parameter equal to the basic reproductive number for asymptomatic vaccinated cases: βaβvaxmN/γ for the no-chemoprophylaxis scenarios and vabxβaβvaxmN/γabx for the chemoprophylaxis scenarios. The number of these secondary cases that were symptomatic was then drawn according to a binomial distribution with symptom probability σvaxmσ, and the number of DS cases that acquired resistance from residual antibiotic concentration in the chemoprophylaxis scenarios was drawn according to a binomial distribution with probability of acquired resistance aprop.
From the third generation onwards, we assumed random mixing with the general population of the host country and ran the model forwards for 1,000 days as described below. Instances in which at least one member of the general population of the host country was infected were counted as importation events. For each parameter set, the model runs were repeated and results of each recorded until 2,000 such importation events had occurred.
Modeling transmission within the host population.
To determine the course of an epidemic in the general population following importation, we created a stochastic transmission model (Fig. 4). We assumed at baseline a susceptible host population with a size of 1 million, treating anyone not susceptible as removed from the population. Because cholera infection is rapidly transmitted and infrequently fatal, we did not incorporate births, deaths, or latency into our model. Instead, we assumed that all infected people immediately became infectious and eventually recovered with stable immunity over the course of the epidemic. These assumptions resulted in a discrete SIR-type epidemic. We ran the model for 1,000 days, substantially later than the last case of a random sample of epidemics, to capture all cases occurring within the epidemic in a closed population.
FIG 4.

Structure of the stochastic transmission model within the host population. See Table 1 for parameter details and values. Model states are as follows: S, susceptible; Ira, infectious, DR, asymptomatic; Ir, infectious, DR, symptomatic; TS, on treatment, DS; IS, infectious, DS, symptomatic; Isa, infectious, DS, asymptomatic. Not shown are asymptomatic DS and DR peacekeepers Isap and Irap, who are considered only for their role in seeding a potential epidemic.
Individuals within our model could experience symptomatic or asymptomatic infection with either DS or DR phenotypes, depending on the resistance phenotype of the infecting individual. All transmission was assumed to occur in direct proportion to the number of prevalent symptomatic and asymptomatic infections, i.e.,
and
We assumed exponentially distributed recovery times, which allowed us to account for the right-tailed duration for which individuals may transmit infection, for instance, due to persistence of V. cholerae in the environment. We allowed some fraction of symptomatic individuals to receive antibiotic treatment, which could accelerate their recovery or, alternatively, lead to acquired resistance either through mutation or horizontal transfer from other bacterial species. We exclusively considered resistance acquisition in the presence of antibiotic treatment for symptomatic cholera and did not account for horizontal transfer of resistance elements between infected individuals. These simplifying assumptions may have underestimated the risks of resistance emergence after DS cholera importation, and our estimated benefits of prophylaxis may therefore be too low.
Parameter values.
As shown in Table 1, we explored wide ranges for the six parameters that we judged most uncertain or likely to vary between settings. These ranges were informed by empirical studies. A more detailed description of how these values and ranges relate to the cited studies is provided in the supplemental material. The proportions of individuals starting treatment or acquiring resistance were converted to rates by comparing the proportion of individuals leaving these compartments through each route, and the transmission parameters β and βa were back-calculated from the values of R0 (in the absence of treatment or vaccination), based on the relationship
We calculated partial rank correlation coefficients between the six uncertain parameters and our outcomes of interest to determine the impact of these parameters on our results. Sensitivity analyses based on the impact of vaccination, relative infectiousness of asymptomatic cases, efficacy of prophylaxis, and population size are summarized in Results, with additional details provided in the supplemental material.
Computation.
For our baseline analysis, we ran both chemoprophylaxis and no-chemoprophylaxis scenarios for 10,000 parameter sets each chosen by Latin hypercube sampling. We ran the model repeatedly for each parameter set and chemoprophylaxis scenario until 2,000 importation events (i.e., runs with at least one case in the host population) had accrued, recording the results for each run. All computing was conducted in R, with epidemics simulated using an adaptive tau-leap algorithm (package “adaptivetau”) that switches between exact and approximate methods based on the state of the system (29). Our code is included in the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
A.K., J.A.L., V.E.P., and T.C. conceived the study; A.K. developed the model with assistance from J.A.L., V.E.P., and T.C.; A.K., J.A.L., V.E.P., and T.C. interpreted model findings; A.K. wrote the first draft of the paper; and A.K., J.A.L., V.E.P., and T.C. contributed to the writing of the final version of the paper.
Funding was received from the National Institute of General Medical Sciences of the National Institutes of Health under award number U54GM088558 (T.C.), the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI112970 (V.E.P.), and the Wellcome Trust under award number 106158/Z/14/Z (V.E.P.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00026-17.
REFERENCES
- 1.Luquero FJ, Rondy M, Boncy J, Munger A, Mekaoui H, Rymshaw E, Page AL, Toure B, Degail MA, Nicolas S, Grandesso F, Ginsbourger M, Polonsky J, Alberti KP, Terzian M, Olson D, Porten K, Ciglenecki I. 2016. Mortality rates during cholera epidemic, Haiti, 2010-2011. Emerg Infect Dis 22:410–416. doi: 10.3201/eid2203.141970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lantagne D, Balakrish Nair G, Lanata CF, Cravioto A. 2014. The cholera outbreak in Haiti: where and how did it begin? Curr Top Microbiol Immunol 379:145–164. [DOI] [PubMed] [Google Scholar]
- 3.Lewnard JA, Antillon M, Gonsalves G, Miller AM, Ko AI, Pitzer VE. 2016. Strategies to prevent cholera introduction during international personnel deployments: a computational modeling analysis based on the 2010 Haiti outbreak. PLoS Med 13:e1001947. doi: 10.1371/journal.pmed.1001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piarroux R, Frerichs RR. 2015. Cholera and blame in Haiti. Lancet Infect Dis 15:1380–1381. doi: 10.1016/S1473-3099(15)00411-9. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Department of Peacekeeping Operations, Department of Field Support. 2015. Medical support manual for United Nations field missions, 3rd ed United Nations Department of Field Support, New York, NY. [Google Scholar]
- 6.Black RE, Levine MM, Clements ML, Young CR, Svennerholm AM, Holmgren J. 1987. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun 55:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, Cabezas C, Watts DM, Svennerholm AM, Sadoff JC, Taylor DN. 1994. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet 344:1273–1276. doi: 10.1016/S0140-6736(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 8.United Nations. 2014. United Nations follow-up to the recommendations of the Independent Panel of Experts on the Cholera Outbreak in Haiti. http://www.un.org/News/dh/infocus/haiti/Follow-up-to-Recommendations-of-IPE_no2.pdf. Accessed 5 April 2016.
- 9.Medrano Rojas P. 2014. Assistant Secretary General's response. https://spdb.ohchr.org/hrdb/28th/Haiti_ASG_25.11.14_(3.2014).pdf. Accessed 5 April 2016.
- 10.Knox R. 5 February 2016. A $1 pill that could save thousands of lives: research suggests cheap way to avoid U.N.-caused cholera. WBUR CommonHealth. http://www.wbur.org/commonhealth/2016/02/05/antiobiotic-pill-cholera-united-nations.
- 11.Kunkel A, Colijn C, Lipsitch M, Cohen T. 2015. How could preventive therapy affect the prevalence of drug resistance? Causes and consequences. Philos Trans R Soc Lond B Biol Sci 370:20140306. doi: 10.1098/rstb.2014.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruque AS, Alam K, Malek MA, Khan MG, Ahmed S, Saha D, Khan WA, Nair GB, Salam MA, Luby SP, Sack DA. 2007. Emergence of multidrug-resistant strain of Vibrio cholerae O1 in Bangladesh and reversal of their susceptibility to tetracycline after two years. J Health Popul Nutr 25:241–243. [PMC free article] [PubMed] [Google Scholar]
- 13.Marin MA, Thompson CC, Freitas FS, Fonseca EL, Aboderin AO, Zailani SB, Quartey NK, Okeke IN, Vicente AC. 2013. Cholera outbreaks in Nigeria are associated with multidrug resistant atypical El Tor and non-O1/non-O139 Vibrio cholerae. PLoS Negl Trop Dis 7:e2049. doi: 10.1371/journal.pntd.0002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixit SM, Johura FT, Manandhar S, Sadique A, Rajbhandari RM, Mannan SB, Rashid MU, Islam S, Karmacharya D, Watanabe H, Sack RB, Cravioto A, Alam M. 2014. Cholera outbreaks (2012) in three districts of Nepal reveal clonal transmission of multi-drug resistant Vibrio cholerae O1. BMC Infect Dis 14:392. doi: 10.1186/1471-2334-14-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan WA, Saha D, Rahman A, Salam MA, Bogaerts J, Bennish ML. 2002. Comparison of single-dose azithromycin and 12-dose, 3-day erythromycin for childhood cholera: a randomised, double-blind trial. Lancet 360:1722–1727. doi: 10.1016/S0140-6736(02)11680-1. [DOI] [PubMed] [Google Scholar]
- 16.Saha D, Karim MM, Khan WA, Ahmed S, Salam MA, Bennish ML. 2006. Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med 354:2452–2462. doi: 10.1056/NEJMoa054493. [DOI] [PubMed] [Google Scholar]
- 17.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2014. Use of antibiotics for cholera. http://who.int/cholera/prevention_control/recommendations/en/index4.html. Accessed 16 April 2016.
- 19.Towner KJ, Pearson NJ, Mhalu FS, O'Grady F. 1980. Resistance to antimicrobial agents of Vibrio cholerae E1 Tor strains isolated during the fourth cholera epidemic in the United Republic of Tanzania. Bull World Health Organ 58:747–751. [PMC free article] [PubMed] [Google Scholar]
- 20.Weber JT, Mintz ED, Canizares R, Semiglia A, Gomez I, Sempertegui R, Davila A, Greene KD, Puhr ND, Cameron DN, Tenover FC, Barrett TJ, Bean NH, Ivey C, Tauxe RV, Blake PA. 1994. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 112:1–11. doi: 10.1017/S0950268800057368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mhalu FS, Mmari PW, Ijumba J. 1979. Rapid emergence of El Tor Vibrio cholerae resistant to antimicrobial agents during first six months of fourth cholera epidemic in Tanzania. Lancet i:345–347. doi: 10.1016/S0140-6736(79)92889-7. [DOI] [PubMed] [Google Scholar]
- 22.de Zoysa I, Feachem RG. 1985. Interventions for the control of diarrhoeal diseases among young children: chemoprophylaxis. Bull World Health Organ 63:295–315. [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas UL, Glaubius R, Mubayi A, Hood G, Mellors JW. 2013. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis 208:224–234. doi: 10.1093/infdis/jit150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel A, Crawford FW, Shepherd J, Cohen T. 2016. Benefits of continuous isoniazid preventive therapy may outweigh resistance risks in a declining tuberculosis/HIV coepidemic. AIDS 30:2715–2723. doi: 10.1097/QAD.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teboh-Ewungkem MI, Mohammed-Awel J, Baliraine FN, Duke-Sylvester SM. 2014. The effect of intermittent preventive treatment on anti-malarial drug resistance spread in areas with population movement. Malar J 13:428. doi: 10.1186/1475-2875-13-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper BS, Medley GF, Scott GM. 1999. Preliminary analysis of the transmission dynamics of nosocomial infections: stochastic and management effects. J Hosp Infect 43:131–147. doi: 10.1053/jhin.1998.0647. [DOI] [PubMed] [Google Scholar]
- 27.Gomes MF, Pastore YPA, Rossi L, Chao D, Longini I, Halloran ME, Vespignani A. 2014. Assessing the international spreading risk associated with the 2014 West African Ebola outbreak. PLoS Curr 6:ecurrents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin JT, Bhatt S, Sinka ME, Gething PW, Lynch M, Patouillard E, Shutes E, Newman RD, Alonso P, Cibulskis RE, Ghani AC. 2016. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis 16:465–472. doi: 10.1016/S1473-3099(15)00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Gillespie DT, Petzold LR. 2007. Adaptive explicit-implicit tau-leaping method with automatic tau selection. J Chem Phys 126:224101. doi: 10.1063/1.2745299. [DOI] [PubMed] [Google Scholar]
- 30.Ali M, Nelson AR, Lopez AL, Sack DA. 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longini IM Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. 2007. Controlling endemic cholera with oral vaccines. PLoS Med 4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG Jr. 2011. Estimating the reproductive numbers for the 2008-2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci U S A 108:8767–8772. doi: 10.1073/pnas.1019712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson BR, Talkington DF, Pruckler JM, Fouche MD, Lafosse E, Nygren B, Gomez GA, Dahourou GA, Archer WR, Payne AB, Hooper WC, Tappero JW, Derado G, Magloire R, Gerner-Smidt P, Freeman N, Boncy J, Mintz ED. 2013. Seroepidemiologic survey of epidemic cholera in Haiti to assess spectrum of illness and risk factors for severe disease. Am J Trop Med Hyg 89:654–664. doi: 10.4269/ajtmh.13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward WE, Mosley WH. 1972. The spectrum of cholera in rural Bangladesh. II. Comparison of El Tor Ogawa and classical Inaba infection. Am J Epidemiol 96:342–351. [DOI] [PubMed] [Google Scholar]
- 35.Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque AS, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2009. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis 49:1473–1479. doi: 10.1086/644779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamayo JF, Mosley WH, Alvero MG, Joseph PR, Gomez CZ, Montague T, Dizon JJ, Henderson DA. 1965. Studies of cholera El Tor in the Philippines. 3. Transmission of infection among household contacts of cholera patients. Bull World Health Organ 33:645–649. [PMC free article] [PubMed] [Google Scholar]
- 37.Glass RI, Holmgren J, Haley CE, Khan MR, Svennerholm AM, Stoll BJ, Belayet Hossain KM, Black RE, Yunus M, Barua D. 1985. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol 121:791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- 38.Lindenbaum J, Greenough WB, Islam MR. 1967. Antibiotic therapy of cholera. Bull World Health Organ 36:871–883. [PMC free article] [PubMed] [Google Scholar]
- 39.Kobari K, Takakura I, Nakatomi M, Sogame S, Uylangco C. 1970. Antibiotic-resistant strains of E1 Tor vibrio in the Philippines and the use of furalazine for chemotherapy. Bull World Health Organ 43:365–371. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Lou J, Liu J, Zhang L, Li J, Kan B. 2012. Antibiotic resistance of Vibrio cholerae O1 El Tor strains from the seventh pandemic in China, 1961–2010. Int J Antimicrob Agents 40:361–364. doi: 10.1016/j.ijantimicag.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, Paul M. 2014. Antimicrobial drugs for treating cholera. Cochrane Database Syst Rev 6:Cd008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reveiz L, Chapman E, Ramon-Pardo P, Koehlmoos TP, Cuervo LG, Aldighieri S, Chambliss A. 2011. Chemoprophylaxis in contacts of patients with cholera: systematic review and meta-analysis. PLoS One 6:e27060. doi: 10.1371/journal.pone.0027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cash RA, Music SI, Libonati JP, Snyder MJ, Wenzel RP, Hornick RB. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis 129:45–52. [DOI] [PubMed] [Google Scholar]
- 44.Sack DA, Tacket CO, Cohen MB, Sack RB, Losonsky GA, Shimko J, Nataro JP, Edelman R, Levine MM, Giannella RA, Schiff G, Lang D. 1998. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect Immun 66:1968–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azman AS, Rudolph KE, Cummings DA, Lessler J. 2013. The incubation period of cholera: a systematic review. J Infect 66:432–438. doi: 10.1016/j.jinf.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. 2011. Oral vaccines for preventing cholera. Cochrane Database Syst Rev doi: 10.1002/14651858.CD008603.pub2:Cd008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.