ABSTRACT
Bisphosphonates are widely used for the treatment of bone disorders. These drugs also inhibit the growth of a variety of protozoan parasites, such as Toxoplasma gondii, the etiologic agent of toxoplasmosis. The target of the most potent bisphosphonates is the isoprenoid biosynthesis pathway enzyme farnesyl diphosphate synthase (FPPS). Based on our previous work on the inhibitory effect of sulfur-containing linear bisphosphonates against T. gondii, we investigated the potential synergistic interaction between one of these derivatives, 1-[(n-heptylthio)ethyl]-1,1-bisphosphonate (C7S), and statins, which are potent inhibitors of the host 3-hydroxy-3-methyl glutaryl-coenzyme A reductase (3-HMG-CoA reductase). C7S showed high activity against the T. gondii bifunctional farnesyl diphosphate (FPP)/geranylgeranyl diphosphate (GGPP) synthase (TgFPPS), which catalyzes the formation of FPP and GGPP (50% inhibitory concentration [IC50] = 31 ± 0.01 nM [mean ± standard deviation]), and modest effect against the human FPPS (IC50 = 1.3 ± 0.5 μM). We tested combinations of C7S with statins against the in vitro replication of T. gondii. We also treated mice infected with a lethal dose of T. gondii with similar combinations. We found strong synergistic activities when using low doses of C7S, which were stronger in vivo than when tested in vitro. We also investigated the synergism of several commercially available bisphosphonates with statins both in vitro and in vivo. Our results provide evidence that it is possible to develop drug combinations that act synergistically by inhibiting host and parasite enzymes in vitro and in vivo.
KEYWORDS: bisphosphonate, statins, synergy, Toxoplasma gondii, isoprenoids
INTRODUCTION
Toxoplasma gondii is a ubiquitous intracellular apicomplexan parasite that can infect humans and a number of animal species. Human infections are usually asymptomatic, but the parasite can persist in the form of tissue cysts controlled by the immune system, which can be reactivated when there is immunosuppression due to organ transplant or cancer chemotherapy (1) or in people infected with HIV (2). Infection of the fetus during pregnancy causes congenital toxoplasmosis (3). Some strains of T. gondii also cause severe ocular disease in immunocompetent patients (4). Current chemotherapy does not prevent the disease progression that leads to blindness in ocular toxoplasmosis patients (4). Toxoplasmosis represents a serious public health problem, and no prophylactic or therapeutic vaccines are available for humans. The available chemotherapy constitutes the only possibility for control of the disease worldwide. The drugs presently used against toxoplasmosis do not eradicate the chronic infection, and as many as 50% of the patients treated do not respond to the therapy (5). Most of the drugs currently used are poorly distributed to the central nervous system, and they trigger allergic reactions in a large number of patients (5). In addition to these disadvantages, the first line of treatment has recently become very expensive (6). In summary, there is a compelling need for safe and effective treatments for toxoplasmosis (7, 8). A comprehensive analysis of the present stage of toxoplasmosis treatments has recently been published (5, 9).
The isoprenoid pathway has been particularly useful for the identification of new targets against intracellular parasites. Isoprenoids are lipid compounds with many important functions. The enzymes that synthesize and use isoprenoids are among the most important drug targets for the treatment of cardiovascular disease, osteoporosis, and bone metastases and have shown promise as antimicrobials in a number of systems (10). T. gondii lacks the mevalonate pathway for the synthesis of isoprenoid precursors that is used by mammals but harbors a prokaryotic-type 1-deoxy-d-xylulose-5-phosphate (DOXP) pathway in the apicoplast (11). This pathway generates isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). We previously demonstrated that the DOXP pathway is essential in T. gondii (11). Knockout of either LytB, which catalyzes the generation of IPP and DMAPP in the final step of the DOXP pathway, or DOXP reductoisomerase (DOXPRI), which catalyzes the second step of the DOXP pathway, was lethal (11). We also characterized the key downstream enzyme for isoprenoid synthesis in T. gondii, farnesyl diphosphate synthase (TgFPPS) (12). Interestingly, this enzyme is bifunctional and can catalyze the condensation of IPP with three allylic substrates: DMAPP, geranyl diphosphate (GPP), and farnesyl disphosphate (FPP). The enzyme thus generates not only 15-carbon FPP but also 20-carbon geranylgeranyl diphosphate (GGPP) (12).
We recently demonstrated that T. gondii TgFPPS knockout mutants have only a mild growth phenotype due to the ability of the parasite to salvage FPP and/or GGPP from the host, where they are produced through the mevalonate pathway (13). We observed that genetic deletion of TgFPPS renders the parasites more susceptible to inhibition of the host isoprenoid pathway with atorvastatin, and we proposed a double-hit strategy combining inhibitors of host and parasite pathways as a novel approach against toxoplasmosis (13). We tested and demonstrated in vitro synergism by inhibiting the parasite enzyme TgFPPS with zoledronic acid, a bisphosphonate, and the host enzyme 3-hydroxymethyl-3-glutaryl-coenzyme A (3-HMG-CoA) reductase with atorvastatin (13).
Bisphosphonates are metabolically stable pyrophosphate analogues in which a methylene group replaces the oxygen atom bridge between the two phosphorus atoms of the pyrophosphate (Fig. 1). These compounds target the enzyme farnesyl diphosphate synthase (14) and are used in medicine for the treatment and prevention of osteoporosis, Paget's disease, hypercalcemia, tumor bone metastases, and other bone diseases (15, 16). The drugs are selective because they can bind to the bone mineral (17). By replacing the carbon atom with different side chains, it is possible to generate a large variety of bisphosphonate derivatives. Interestingly, bisphosphonates also have antibacterial (17) and anticancer activities (18) and are able to stimulate γδ T cells (19). Several of these compounds have antiparasitic action (20–23). It was shown that the enzyme FPPS from these parasites is targeted by bisphosphonates (14).
FIG 1.
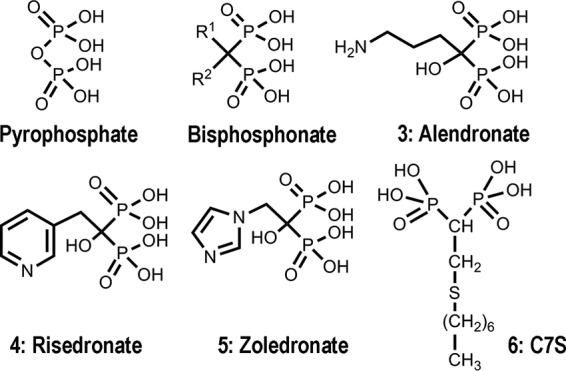
Structures of the compounds discussed in this work.
In the present work, we report synergistic combinations of the most potent sulfur-containing bisphosphonate, 1-[(n-heptylthio)ethyl]-1,1-bisphosphonate, hereinafter called C7S or compound 6 (Fig. 1), and other commercially available bisphosphonates with several statins both in vitro and in vivo against acute T. gondii infection. We tested these synergistic treatments in vivo using mice infected with a lethal dose of the hypervirulent RH strain of T. gondii.
RESULTS
Inhibition of T. gondii growth by bisphosphonates and statins.
Bisphosphonates are known to inhibit the isoprenoid pathway, and they have been shown to be effective against T. gondii growth (12, 13). We tested several commercially available bisphosphonates (Table 1 and Fig. 1) and compared their activities with C7S against tachyzoite forms. We also tested the inhibition of TgFPPS, as well as of the human enzyme (Homo sapiens FPPS [HsFPPS]). C7S has good activity against intracellular tachyzoites (50% effective concentration [EC50] of 1.49 ± 0.38 μM [mean ± standard deviation]) and excellent activity against TgFPPS (50% inhibitory concentration [IC50] = 0.031 ± 0.01 μM), with low toxicity against fibroblasts. C7S was tested at levels as high as 7 times the EC50 for Toxoplasma growth inhibition, and no detectable inhibition of host cell growth was observed (see Table S1 in the supplemental material). Atorvastatin has low cytotoxicity when used at up to 4 times its EC50 growth inhibition value. Significant toxicity to host cells was observed when using a concentration of atorvastatin of 7 times its EC50. At this concentration, ∼40% of host cells showed growth inhibition (Table S1). When looking at the cytotoxicities of the combinations, the effects were similar to the cytotoxicity of atorvastatin alone, confirming that there is no detectable cytotoxicity of C7S at the concentrations tested in vitro. We also tested several statins against parasite growth in vitro and detected only modest inhibitory effects (Table 2).
TABLE 1.
Biological activities of C7S and other bisphosphonates against T. gondii tachyzoites, TgFPPS, and HsFPPSa
| Compound | Mean T. gondii EC50 ± SD (μM) | Mean IC50 ± SD (μM) for: |
|
|---|---|---|---|
| TgFPPS | HsFPPS | ||
| C7S | 1.49 ± 0.38 | 0.03 ± 0.000 | 1.31 ± 0.53 |
| Risedronate | 2.40 ± 0.7 | 0.44 ± 0.01 | 0.40 ± 0.01 |
| Zoledronate | 0.84 ± 0.04 | 0.07 ± 0.02 | 0.43 ± 0.04 |
| Alendronate | 32.88 | 0.14 ± 0.05 | 5.13 ± 1.77 |
| Atovaquone | 0.032 ± 0.019 | ND | ND |
Results are expressed as mean values ± SD from three independent experiments except for growth with alendronate, which has very low in vitro activity. ND, not determined.
TABLE 2.
In vitro growth inhibition of Toxoplasma gondii by several statins
| Compound | Mean EC50 ± SD (μM)a |
|---|---|
| Atorvastatin | 34.86 ± 5.00 |
| Lovastatin | 22.73 ± 4.90 |
| Mevastatin | 27.63 ± 9.84 |
| Simvastatin | 6.67 ± 1.13 |
| Cerivastatin | 10.64 ± 3.73 |
| Pitavastatin | 20.66 ± 3.82 |
Results are expressed as mean values ± SD from three independent experiments.
In vivo effect of C7S.
Several schemes of infection and treatment were first tested to evaluate the percentages of survival among treated mice. Since RH is a hypervirulent T. gondii strain, infection with more than 50 parasites or initiating the treatment several hours after the infection led to only a marginal difference in survival between control and treated groups. This was the case when atorvastatin (10 and 20 mg/kg of body weight/day) was administered 24 h after infection with 20 parasites (see Fig. S1A), contrasting with the results obtained with risedronate (1 mg/kg) starting at 6 or 54 h postinfection (p.i.) (Fig. S1B). Risedronate administration at 6 h p.i. led to the survival of all 5 treated mice; at 54 h p.i., the treated mice survived for a longer time but succumbed due to complications (paralysis of rear legs due to brain damage). These results indicate that early treatment is key to the cure of infection with a type I strain of T. gondii. We also tested infection with the less virulent ΔTgFPPS strain, which we characterized previously (13). Animals were infected with 20 or 100 parasites of the ΔTgFPPS strain. Atorvastatin treatment (20 mg/kg/day) protected mice by 80% after infection with 20 parasites, but the same dose was ineffective against a lethal infection with 100 parasites of the ΔTgFPPS strain. In both cases, treatment was started 6 h p.i. (Fig. S1C). Based on these results, we established our animal protocol by inoculating 20 parasites per mouse and starting treatment 6 h p.i.
We next tested the efficacy of C7S (0.1 to 2.0 mg/kg/day) and atovaquone (0.5 to 5 mg/kg/day) against T. gondii infection using the hypervirulent RH strain (Fig. 2). Figure 2A shows a summary of the results of four experiments using groups of 5 mice treated with different doses of C7S. All control mice (negative control) died between 9 and 12 days postinfection, while 60 to 80% of mice treated with doses of 1 or 2 mg/kg per day survived more than 30 days. For C7S, the in vivo 50% effective dose (ED50; amount of drug that cures 50% of mice) was determined to be 0.87 mg/kg/day (Table 3). Fig. S2 shows the results from the individual experiments that were performed with a variety of doses of C7S. These results were combined to generate Fig. 2A.
FIG 2.
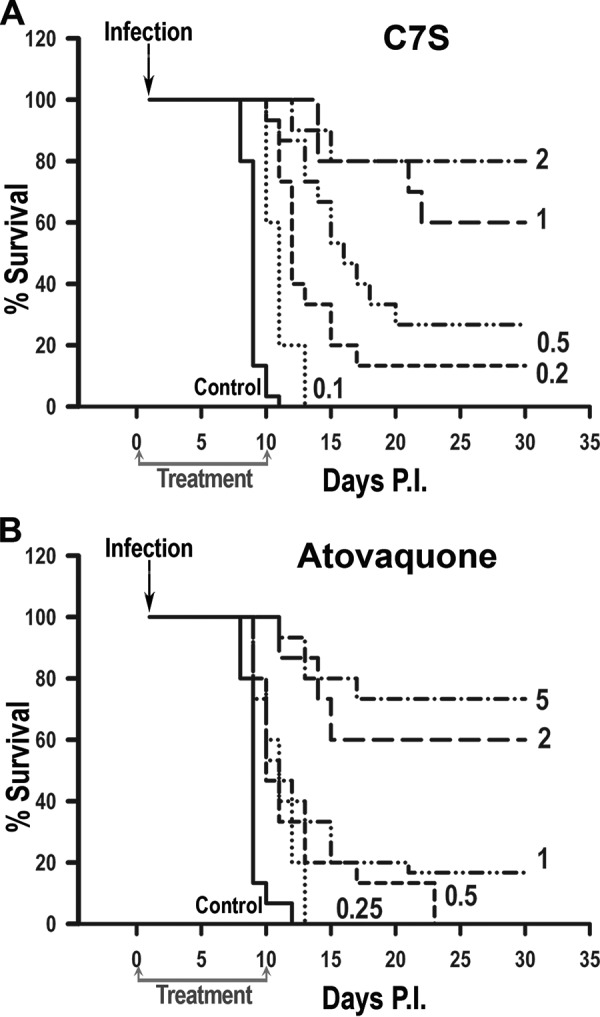
Effects of various doses of C7S (A) or atovaquone (B) on survival of mice treated with a lethal dose of RH tachyzoites (infection). Treatment was once a day for 10 days starting 6 h after infection. The ED50s calculated were 0.87 mg/kg/day for C7S and 2.53 mg/kg/day for atovaquone. The graphs show the combination of the results of four individual experiments, shown in Fig. S2 (C7S) and Fig. S3 (atovaquone) in the supplemental material. For each experiment, the controls received only the drug solvent (10% Kolliphor HS 15) instead of the treatment. As expected, all control animals succumbed to the infection at 9 to 11 days. Statistical analysis using the Kaplan-Meier log rank test (GraphPad Prism 7 software) indicated significant differences (P < 0.0001) between the results for the control animals (untreated) and those that received various doses of C7S or atovaquone. The number next to each line indicates the dose in mg/kg/day.
TABLE 3.
ED50s of different anti-Toxoplasma drugs in vivo
| Compound | ED50 (range) in mice (mg/kg/day)a |
|---|---|
| C7S | 0.87 (0.77–0.89) |
| Risedronate | 0.22 (0.12–0.68) |
| Atorvastatin | 32.4 (14.1–70.4) |
| Zoledronate | 0.25 (0.17–0.51) |
| Atovaquone | 2.53 (1.82–4.17) |
The drugs were administered i.p. to groups of 5 mice during 10 days, and the results are expressed as the ED50 values calculated from the results of all experiments combined; the ranges of ED50s are calculated from the results of each of the three to four independent experiments using each drug.
We then used the same protocol to test atovaquone (0.5 to 5.0 mg/kg/day), our positive control. Figure 2B shows a summary of the results of four experiments in which infected mice were treated with various doses of atovaquone. The results of the individual experiments are shown in Fig. S3. Atovaquone can cure mice infected with RH in a dose-dependent manner, and the ED50 calculated from these experiments was 2.53 mg/kg (Table 3), which was comparable to the ED50 previously obtained in a type II model (24).
Uninfected mice receiving 2 mg C7S/kg/day for 10 days showed no signs of toxicity, while at 5 mg/kg/day, they began to die after 4 days of treatment. Similar toxicity results were obtained with zoledronic acid, which is an FDA-approved drug. Atorvastatin alone was tested at 40 mg/kg/day, and the mice were healthy during the 10 days of the treatment. Previous work showed that atorvastatin could be used at 200 mg/kg/day for 2 years orally without toxic effects (25).
Combination of bisphosphonates with statins against T. gondii in vitro and in vivo.
We had previously reported synergy between statins and zoledronic acid against T. gondii growth in vitro (13). However, other commercial bisphosphonates, like risedronate, did not show similar synergistic interaction with atorvastatin against tachyzoite growth. C7S showed synergistic effects with a number of statins in vitro (Table 4), suggesting that such combinations might have similar effects in vivo.
TABLE 4.
Combinations of statins and C7S against T. gondii in vitro
| Combination tested | Mean sum FICa | Synergyb |
|---|---|---|
| Cerivastatin + C7S | 0.18 ± 0.09 | ++ |
| Pitavastatin + C7S | 0.25 ± 0.07 | ++ |
| Atorvastatin + C7S | 0.32 ± 0.11 | ++ |
| Lovastatin + C7S | 0.43 ± 0.12 | + |
| Simvastatin + C7S | 0.51 ± 0.26 | +− |
| Mevastatin + C7S | 0.54 ± 0.19 | +− |
| Atorvastatin + atovaquone | 0.55 ± 0.13 | +− |
Results are expressed as mean values ± SD from three independent experiments.
++, strong synergistic interaction (sum FIC < 40); +, synergy (sum FIC < 50); +−, very mild synergy, not a very strong interaction (sum FIC < 60).
Figure 3A shows the results of experiments combining 16.2 mg/kg atorvastatin and 0.44 mg of C7S for 10 consecutive days. This combination cured the majority of mice previously infected with RH parasites. Surviving mice were challenged with 5,000 RH tachyzoites (Fig. 3A, arrow), and follow-up showed that the majority of them survived (Fig. 3A), confirming the initial infection. Statistical analysis using the Kaplan-Meier log rank test revealed that, compared with ∼20% protection by treatment with single drugs, mouse survival was significantly increased with the combined use of both drugs (P < 0.0001). The results shown in Fig. 3B show protection by treatment with both drugs combined at various doses. We tested combinations of doses (with a constant ratio between the two drugs) representing different fractions of their ED50s. For example, considering that the ED50 for atorvastatin is 32.4 mg/kg and the ED50 for C7S is 0.87 mg/kg (Table 3), a 0.5× ratio would be a combination of 16.2 mg/kg atorvastatin and 0.44 mg/kg C7S. We found a correlation between the levels of protection and the doses used for the combinations (Fig. 3B). We tested 6 different combinations and analyzed the results with CompuSyn, which calculated the combination index (CI) for each concentration. The combination of 16.2 mg/kg atorvastatin and 0.44 mg/kg C7S from 3 independent experiments resulted in a CI of ∼0.37, indicating strong synergy in vivo. This CI value was consistent with the sum of the fractional inhibitory concentrations (sum FIC) (0.32) obtained from the in vitro synergy test (Table 4). We looked at the relationship of the CI and the doses used of both drugs (Fig. 3C). Synergy was found for all 5 concentrations (0.5×, 0.25×, 0.15×, 0.075×, and 0.04×) in the data shown in Fig. 3B and also for the 0.02× concentrations (data not shown). The average CI for these 6 doses (85 mice total) was 0.295. Interestingly, we found a strong synergistic interaction (CI < 0.06) when using very low doses of atorvastatin and bisphosphonate. The CI values obtained when combining low doses of drugs are remarkably low (Fig. 3C).
FIG 3.
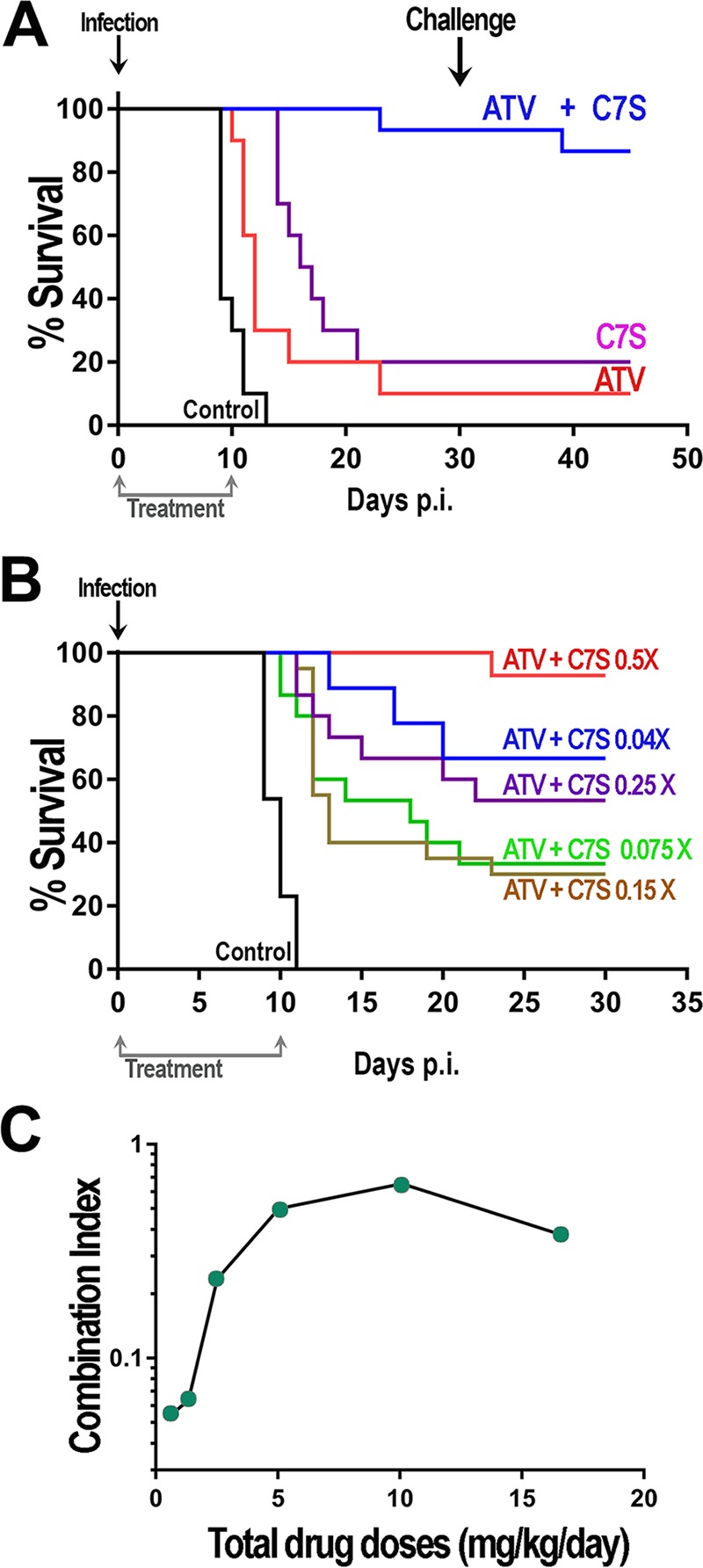
Effects of various combinations of C7S and atorvastatin on survival of mice infected with tachyzoites. (A) Mouse survival after treatment with atorvastatin (ATV) (16.2 mg/kg/day) and C7S (0.44 mg/kg/day) as single drugs or in combination (ATV + C7S) for 10 days using 5 mice/experimental group. Results are from 2 or 3 experiments. (B) Survival curves after treatment with various doses of C7S and atorvastatin (0.075, 0.15, 0.25, and 0.5× ED50); the ED50s were 0.87 mg/kg/day and 32.4 mg/kg/day, respectively. Results are from 2 to 4 experiments using 5 mice per combination. Mice in the control groups received only 10% Kolliphor HS 15 and, as expected, succumbed to the infection at 9 to 10 days. (C) Data from panel B and 0.02× ED50s were used to calculate the combination index (CI) values, and the results graphed against the total drug doses (atorvastatin + C7S). Statistical analysis using the Kaplan-Meier log rank test indicated significant differences (P < 0.0001) between the results for the control (untreated) animals and those that received C7S or atorvastatin together and each drug separately or controls (A) and the different drug combinations compared to controls (B).
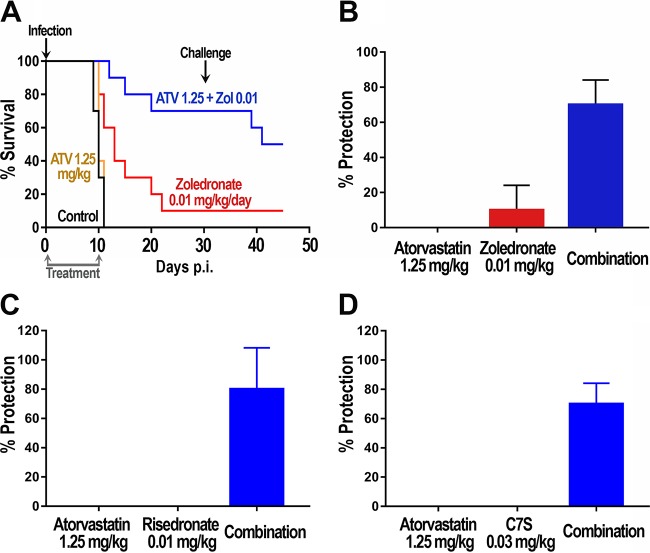
We next investigated whether this strong synergistic activity using low doses of C7S in combination with atorvastatin could be reproduced with other bisphosphonates. We tested combinations of atorvastatin with two FDA-approved bisphosphonates, zoledronate and risedronate. In previous work, we found no synergy between atorvastatin and risedronate in in vitro growth assays (data not shown). Single drug treatments (0.04 × ED50 of both drugs) did not result in significant protection against T. gondii lethality at 30 days postinfection (Fig. 4A to C). Interestingly, the combination of these compounds at those low doses showed 60 to 80% protection against the same lethal infection with tachyzoites. Figure 4 shows the results for combinations of atorvastatin (1.25 mg/kg) with zoledronate (0.01 mg/kg) (Fig. 4B), risedronate (0.01 mg/kg) (Fig. 4C), and C7S (0.03 mg/kg) (Fig. 4D).
FIG 4.
Effects of combinations of atorvastatin (ATV) with several bisphosphonates on survival of mice infected with tachyzoites. (A) Groups of mice were either untreated or treated with zoledronate alone (0.01 mg/kg/day) or the combination of zoledronate (0.01 mg/kg/day) and atorvastatin (1.25 mg/kg/day). (B) Survival at 30 days of mice treated with atorvastatin (1.25 mg/kg/day) or zoledronate (0.01 mg/kg/day) alone or both in combination. (C) Survival at 30 days of mice treated with atorvastatin (1.25 mg/kg/day) or risedronate (0.01 mg/kg/day) alone or both in combination. (D) Survival at 30 days of mice treated with atorvastatin (1.25 mg/kg/day) or C7S (0.03 mg/kg/day) alone or both in combination. In all cases, treatment was started 6 h after initial infection and administered daily for 10 days, and 5 mice per group were used. The results for each combination are from two independent experiments. Statistical analysis using the Kaplan-Meier log rank test indicated significant differences (P < 0.0001) between the results for control animals (untreated) and animals treated with the different drug combinations (A to D). Error bars show standard deviations.
DISCUSSION
Isoprenoids are essential for all cells and most apicomplexans. Their 5-carbon precursors, IPP and DMAPP, are produced by the apicoplast (26, 27). The synthesis of these precursors is the most important function of this organelle (11). Interestingly, TgFPPS, the enzyme that synthesizes FPP and GGPP from IPP and DMAPP, is dispensable for T. gondii growth in vitro, which is surprising when considering that FPPS-catalyzed reactions are essential in most organisms. The reason is that T. gondii is not only able to make its own isoprenoids but can also import specific intermediates from the host cell (13). This ability of the parasite to not only use its own metabolites but also manipulate the host cell metabolism and salvage its products makes drug therapy a challenge. However, in the case of isoprenoid metabolism, this split reliance may also prove to be an opportunity, as it makes the parasite vulnerable to inhibition of the host mevalonate pathway. In our previous work, we showed that inhibition of the host mevalonate pathway with statins resulted in modest IC50s. However, when we genetically or pharmacologically inhibited the parasite's synthesis of isoprenoids, the IC50 for atorvastatin decreased almost 20 times (13). Combinations of bisphosphonates with statins had synergistic effects against T. gondii growth in vitro (13).
In the present work, we describe the effects of the sulfur-containing bisphosphonate derivative C7S and its synergistic interaction with several statins in vitro. C7S was highly effective alone when used to treat a lethal infection of mice with T. gondii. We compared the efficacy of C7S with that of atovaquone under similar conditions of infection and treatment, and we found it to be almost three times more effective. Atovaquone can be used for the treatment of toxoplasmosis in immunocompetent patients or in patients with a history of allergy to pyrimethamine. We chose atovaquone for comparison with bisphosphonates because it is known that the targets for atovaquone are the mitochondria (5), which are probably the targets that are affected by inhibiting isoprenoid synthesis, as we have shown previously (13).
We showed that the strategy of combining bisphosphonates with statins works in vivo using mice infected with a lethal dose of T. gondii. We validated our proposed strategy that blocking both host and parasite isoprenoid synthesis produces stronger effects and affords considerable protection in vivo. Our results show that it would be possible to develop combination therapies targeting both parasite and host. This strategy would be of general interest, because it will be harder for the parasite to develop resistance, resulting in more effective therapies. The basic observation is therefore significant in the context of developing combination therapy approaches against toxoplasmosis.
Interestingly, we found that using very low doses of bisphosphonates and statins results in a stronger synergistic interaction. This could be the result of a strong host participation in the supply of isoprenoids that becomes especially evident upon infection. It has been shown that several enzymes of the isoprenoid pathway become upregulated upon infection with intracellular pathogens like T. gondii (28) and Trypanosoma cruzi (29). Isoprenoid metabolites are essential molecules for cells, and during their replicative stage, parasites need a constant and reliable supply of them. This means that the supply of isoprenoid metabolites is a viable drug target. We predict that the strategy of combining drugs that inhibit both host and parasite pathways will work for other intracellular parasites, as well as against T. gondii.
Conclusions.
T. gondii is an obligate intracellular parasite and is not able to replicate outside the host cell. The parasite lives in a specialized parasitophorous vacuole and is in contact with the host cytoplasm through the parasitophorous vacuole membrane. We previously showed an active exchange of isoprenoid metabolites between T. gondii and its host cell. Parasites salvage farnesyl diphosphate (FPP) and/or geranylgeranyl diphosphate (GGPP) from the host, and they are able to grow even when its endogenous production is shut down. This metabolic interaction between the parasite and its host cell ensures a steady supply of important metabolites for growth of the parasite. The pathway for the synthesis of isoprenoids in the host cell differs in several steps from the parasite pathway, and we showed that T. gondii growth is sensitive to inhibitors of both pathways. Mammalian cells use the mevalonate pathway, which is susceptible to statins. These drugs showed a modest inhibitory effect against T. gondii when tested alone. However, when statins were combined with inhibitors of the parasite isoprenoid pathway (bisphosphonates), a strong synergistic effect was observed. This double-hit strategy combining inhibitors of host and parasite pathways represents a novel therapeutic approach against apicomplexan parasites. We found that very low doses of both drugs could be used for treatment, which significantly decreases the chances for potential toxicity. We used the hypervirulent strain RH and tested this strategy in the acute model of virulence. Future work will test this strategy in an established chronic infection model.
MATERIALS AND METHODS
Ethics statement.
All animal care and therapy studies were carried out in strict accordance with the NIH guidelines. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia.
Synthesis of the inhibitor C7S.
The compound C7S (Fig. 1, compound 6) was prepared in multigram scale as described previously (30). The purity of this compound was found to be >97% according to homogeneity of the corresponding hydrogen-1 nuclear magnetic resonance (1H-NMR), 13C-NMR, and 31P-NMR spectra. High-performance liquid chromatography (HPLC) analysis of this compound, employing a 5 μM, 250- by 10-mm Beckman Ultrasphere ODS-2 column and eluting with water-acetonitrile (9:1) at 3.00 ml/min with a refractive index detector, also showed a purity of >97%.
Enzymatic determinations.
Recombinant TgFPPS (31) and HsFPPS (32) were purified and their activities determined as described in the references, with some modifications. Briefly, DMAPP (100 μM) and [14C]IPP (4.7 μM) were mixed with various concentrations of the inhibitors, and the reaction was initiated by the addition of TgFPPS. The reaction was allowed to proceed at 37°C for 30 min and stopped by the addition of 6 N HCl. Labeled isoprenoids were extracted with hexane after neutralization with NaOH. The activity of the enzyme and IC50s of inhibitors were calculated as described previously (12).
In vitro drug screening.
Experiments on T. gondii tachyzoites were carried out using parasites expressing red fluorescent protein (RFP) (33) with the modification described by Recher et al. (30). Tachyzoites were maintained in human fibroblasts (hTert cells). For drug testing, parasites were purified by passing them through a 27-gauge needle, followed by filtration through a 3-μm filter. Human fibroblasts were cultured in 96-well plates for 24 h prior to the addition of 4,000 fluorescent tachyzoites/well. Fluorescence values were measured for 3 to 4 days, and both excitation (544 nm) and emission (590 nm) were read from the bottom of the plates in a Molecular Devices plate reader. For studies of in vitro synergism, a checkerboard technique was used as described before (13, 34). The results were expressed as the sums of the fractional inhibitory concentrations (sum FIC) as described by Berenbaum (35), as follows: sum FIC = (IC50 of drug A in mixture/IC50 of drug A alone) + (IC50 of drug B in mixture/IC50 of drug B alone) (35). Sum FIC values show the types of interactions between drugs as follows: <0.5, synergistic; 1, additive; >2, antagonistic.
Cytotoxicity to hTert cells.
The cytotoxicity was tested using the alamarBlue assay (30), and the results are shown in Table S1 in the supplemental material.
In vivo drug screening.
In vivo drug screening experiments were carried out as described previously (13), using 20 T. gondii tachyzoites of the RH strain to infect Webster mice, unless indicated under Results. Drugs were dissolved in 10% Kolliphor HS 15 and were inoculated intraperitoneally (i.p.). Treatment was initiated 6 h after infection and administered daily for 10 days.
A combination index (CI) for each combination of drugs was calculated using CompuSym (version 1.0) (36), and the values are expressed as follows: <1, synergism; 1, additive effect; >1, antagonism (37, 38). Negative controls received the vehicle alone.
In vivo toxicity.
Mice received various doses of drugs simulating treatment after infection, and signs of toxicity, including moribund state, body weight loss, mortality, or any other sign of toxicity, were recorded.
Statistics.
Experimental data are expressed as the mean values ± standard deviations (SD) from at least three independent experiments unless indicated otherwise. The results of in vivo studies were analyzed using the Kaplan-Meier log rank test (GraphPad Prism 7 software). A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
Melissa Storey and Omar Salas helped with the host toxicity assays. Beejan Asady assisted with the maintenance of parasites and host cells.
This work was supported by grants from the U.S. National Institutes of Health, to S.N.J.M. (AI-102254), and the National Research Council of Argentina (grant number PIP 0797), ANPCyT (grant number PICT 2012 #0457), and the Universidad de Buenos Aires (grant number 20020130100223BA) to J.B.R.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02628-16.
REFERENCES
- 1.Israelski DM, Remington JS. 1993. Toxoplasmosis in patients with cancer. Clin Infect Dis 17(Suppl 2):S423–S435. doi: 10.1093/clinids/17.Supplement_2.S423. [DOI] [PubMed] [Google Scholar]
- 2.Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM, Bourland DD III, Uttamchandani R, Fuhrer J, Jacobson J, Morlat P, Vilde J-L, Remington JS, ACTG 077p/ANRS 009 Study Team. 1993. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med 329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 3.Wong SY, Remington JS. 1994. Toxoplasmosis in pregnancy. Clin Infect Dis 18:853–861. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN. 2004. Ocular toxoplasmosis: a global reassessment. Part II. Disease manifestations and management. Am J Ophthalmol 137:1–17. doi: 10.1016/j.ajo.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Neville AJ, Zach SJ, Wang X, Larson JJ, Judge AK, Davis LA, Vennerstrom JL, Davis PH. 2015. Clinically available medicines demonstrating anti-Toxoplasma activity. Antimicrob Agents Chemother 59:7161–7169. doi: 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer O. 2015. Company reneges on promise to cut price of toxoplasmosis drug. BMJ 351:h6472. doi: 10.1136/bmj.h6472. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez JB, Szajnman SH. 2012. New antibacterials for the treatment of toxoplasmosis; a patent review. Expert Opin Ther Pat 22:311–333. doi: 10.1517/13543776.2012.668886. [DOI] [PubMed] [Google Scholar]
- 8.Derouin F. 2001. Anti-toxoplasmosis drugs. Curr Opin Investig Drugs 2:1368–1374. [PubMed] [Google Scholar]
- 9.Wei HX, Wei SS, Lindsay DS, Peng HJ. 2015. A Systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One 10:e0138204. doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldfield E. 2010. Targeting isoprenoid biosynthesis for drug discovery: bench to bedside. Acc Chem Res 43:1216–1226. doi: 10.1021/ar100026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair SC, Brooks CF, Goodman CD, Sturm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SN, Striepen B. 2011. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J Exp Med 208:1547–1559. doi: 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling Y, Li ZH, Miranda K, Oldfield E, Moreno SN. 2007. The farnesyl-diphosphate/geranylgeranyl-diphosphate synthase of Toxoplasma gondii is a bifunctional enzyme and a molecular target of bisphosphonates. J Biol Chem 282:30804–30816. doi: 10.1074/jbc.M703178200. [DOI] [PubMed] [Google Scholar]
- 13.Li ZH, Ramakrishnan S, Striepen B, Moreno SN. 2013. Toxoplasma gondii relies on both host and parasite isoprenoids and can be rendered sensitive to atorvastatin. PLoS Pathog 9:e1003665. doi: 10.1371/journal.ppat.1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin MB, Arnold W, Heath HT III, Urbina JA, Oldfield E. 1999. Nitrogen-containing bisphosphonates as carbocation transition state analogs for isoprenoid biosynthesis. Biochem Biophys Res Commun 263:754–758. doi: 10.1006/bbrc.1999.1404. [DOI] [PubMed] [Google Scholar]
- 15.Rodan GA. 1998. Mechanisms of action of bisphosphonates. Annu Rev Pharmacol Toxicol 38:375–388. doi: 10.1146/annurev.pharmtox.38.1.375. [DOI] [PubMed] [Google Scholar]
- 16.Russell RG. 2011. Bisphosphonates: the first 40 years. Bone 49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Reddy R, Dietrich E, Lafontaine Y, Houghton TJ, Belanger O, Dubois A, Arhin FF, Sarmiento I, Fadhil I, Laquerre K, Ostiguy V, Lehoux D, Moeck G, Parr TR Jr, Rafai Far A. 2008. Bisphosphonated benzoxazinorifamycin prodrugs for the prevention and treatment of osteomyelitis. ChemMedChem 3:1863–1868. doi: 10.1002/cmdc.200800255. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. 2009. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew Chem Int Ed Engl 48:2949–2954. doi: 10.1002/anie.200805133. [DOI] [PubMed] [Google Scholar]
- 19.Sanders JM, Ghosh S, Chan JM, Meints G, Wang H, Raker AM, Song Y, Colantino A, Burzynska A, Kafarski P, Morita CT, Oldfield E. 2004. Quantitative structure-activity relationships for gammadelta T cell activation by bisphosphonates. J Med Chem 47:375–384. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- 20.Linares GE, Ravaschino EL, Rodriguez JB. 2006. Progresses in the field of drug design to combat tropical protozoan parasitic diseases. Curr Med Chem 13:335–360. doi: 10.2174/092986706775476043. [DOI] [PubMed] [Google Scholar]
- 21.Docampo R, Moreno SN. 2001. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Curr Drug Targets Infect Disord 1:51–61. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- 22.Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, Docampo R. 1999. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J Biol Chem 274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- 23.Martin MB, Grimley JS, Lewis JC, Heath HT III, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SN, Docampo R, Croft SL, Oldfield E. 2001. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J Med Chem 44:909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 24.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordon C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Physicians' Desk Reference. 2005. Physicians' desk reference, 59th ed, p 2585 Medical Economics Company, Montvale, NJ. [Google Scholar]
- 26.Seeber F, Soldati-Favre D. 2010. Metabolic pathways in the apicoplast of apicomplexa. Int Rev Cell Mol Biol 281:161–228. doi: 10.1016/S1937-6448(10)81005-6. [DOI] [PubMed] [Google Scholar]
- 27.Wiesner J, Reichenberg A, Heinrich S, Schlitzer M, Jomaa H. 2008. The plastid-like organelle of apicomplexan parasites as drug target. Curr Pharm Des 14:855–871. doi: 10.2174/138161208784041105. [DOI] [PubMed] [Google Scholar]
- 28.Blader IJ, Manger ID, Boothroyd JC. 2001. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, El-Sayed NM, Burleigh BA. 2016. Transcriptome remodeling in Trypanosoma cruzi and human cells during intracellular infection. PLoS Pathog 12:e1005511. doi: 10.1371/journal.ppat.1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Recher M, Barboza AP, Li ZH, Galizzi M, Ferrer-Casal M, Szajnman SH, Docampo R, Moreno SN, Rodriguez JB. 2013. Design, synthesis and biological evaluation of sulfur-containing 1,1-bisphosphonic acids as antiparasitic agents. Eur J Med Chem 60:431–440. doi: 10.1016/j.ejmech.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ZH, Cintron R, Koon NA, Moreno SN. 2012. The N-terminus and the chain-length determination domain play a role in the length of the isoprenoid product of the bifunctional Toxoplasma gondii farnesyl diphosphate synthase. Biochemistry 51:7533–7540. doi: 10.1021/bi3005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Cao R, Yin F, Hudock MP, Guo RT, Krysiak K, Mukherjee S, Gao YG, Robinson H, Song Y, No JH, Bergan K, Leon A, Cass L, Goddard A, Chang TK, Lin FY, Van Beek E, Papapoulos S, Wang AH, Kubo T, Ochi M, Mukkamala D, Oldfield E. 2009. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J Am Chem Soc 131:5153–5162. doi: 10.1021/ja808285e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. 2008. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc Natl Acad Sci U S A 105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai SK, Moellering RC, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–440. In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams & Wilkins Co., Philadelphia, PA. [Google Scholar]
- 35.Berenbaum MC. 1978. A method for testing for synergy with any number of agents. J Infect Dis 137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 36.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 37.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Chou TC, Martin N. 2005. CompuSyn for drug combinations: PC software and user's guide. A computer program for quantitation of synergism and antagonism in drug combinations and the determination of IC50 and Ed50 and LD50 values. ComboSyn, Inc., Paramus, NJ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.