ABSTRACT
Cryptosporidium parvum calcium-dependent protein kinase 1 (CpCDPK1) is a promising target for drug development against cryptosporidiosis. We report a series of low-nanomolar CpCDPK1 5-aminopyrazole-4-carboxamide (AC) scaffold inhibitors that also potently inhibit C. parvum growth in vitro. Correlation between anti-CpCDPK1 and C. parvum growth inhibition, as previously reported for pyrazolopyrimidines, was not apparent. Nonetheless, lead AC compounds exhibited a substantial reduction of parasite burden in the neonatal mouse cryptosporidiosis model when dosed at 25 mg/kg.
KEYWORDS: 5-aminopyrazole-4-carboxamide, bumped kinase inhibitors, Cryptosporidium parvum
TEXT
Cryptosporidiosis is an important global health problem that causes diarrheal diseases in humans (1, 2). Cryptosporidium parvum-associated diarrhea is self-limiting in healthy patients but can be life-threatening in immunocompromised patients and malnourished children (1, 3, 4). Furthermore, Cryptosporidium has the potential to be used for bioterrorism because oocysts can be readily obtained and dispersed in food and water supplies and are immediately infectious on passage in feces (5). Nitazoxanide, the only FDA-approved drug for cryptosporidiosis in humans (1), is not effective in immunocompromised people and only weakly active in malnourished children, thus new drugs are needed (6, 7).
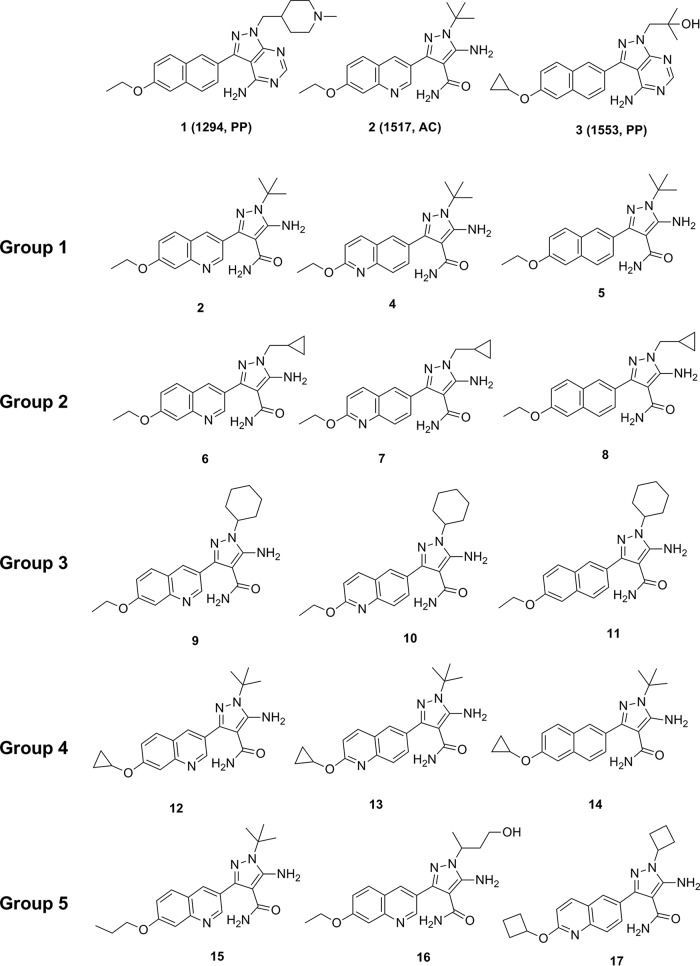
Previous studies suggested that chelation or depletion of intracellular calcium in C. parvum inhibited apical organelle discharge and decreased the gliding motility of sporozoites, which blocked parasite invasion of, but not attachment to, host cells (6). Several studies explored the activity of bumped kinase inhibitors (BKIs) on C. parvum calcium-dependent protein kinase 1 (CpCDPK1). Pyrazolopyrimidine (PP-BKIs) and 5-aminopyrazole-4-carboxamide (AC-BKIs) have been shown to block the activity of CpCDPK1 (7, 8), prevent the growth and proliferation of C. parvum in vitro (9), and reduce oocyst shedding in infected SCID beige mice (10, 11). Furthermore, BKIs 1 (compound 1294, PP), 2 (compound 1517, AC), and 3 (compound 1553, PP) were effective for treatment of clinical diarrhea and reduction of oocyst excretion in the neonatal calf C. parvum challenge model (Fig. 1) (9). Based on the cited studies, additional AC analogues were screened for activity against CpCDPK1 and inhibition of C. parvum growth. The anti-Cryptosporidium efficacies of select AC-BKIs (single 25-mg/kg dose) by gastric intubation were also evaluated in infected neonatal mice.
FIG 1.
Structures of BKIs 1, 2, and 3 and structures of select CpCDPK1 inhibitors (AC-BKIs).
New AC-BKIs (8, 11, 14, 17) were synthesized and characterized following previously reported procedures (8, 12, 13) (see Text S1 in the supplemental material). Methods for solubility testing, inhibition of CpCDPK1 and C. parvum cellular growth, drug pharmacokinetic and fecal concentration analysis, plasma protein binding, and determination of efficacy in neonatal mice were performed as previously described (9).
AC-BKI analogues with potent activity on CpCDPK1 (50% inhibitory concentration [IC50], <10 nM) can be structurally divided into 5 groups (group 1, 2/4/5; group 2, 6/7/8; group 3, 9/10/11; group 4, 12/13/14; and group 5, 15/16/17) (Table 1; Fig. 1). AC-BKIs 4 and 5 in group 1 are close derivatives of AC-BKI 2, with C3 substitution replaced by 2-ethoxyquinolin-6-yl and 6-ethoxynaphthyl-2-yl, respectively. Groups 2 and 3 maintained the quinoline/naphthyl variations of group 1, with changes on the N1 substitution (cyclopropylmethyl for group 2 and cyclohexyl for group 3). Group 4, compared with group 1, contains the cyclopropyloxy variation appending to the fused aromatic ring. Other potent BKIs in group 5 contain various substitutions on both ends: AC-BKI 15 contains a longer tail substitution at the quinoline ring, AC-BKI 16 contains a hydroxyl aliphatic chain at the N1 position, and AC-BKI 17 contains cyclobutyl group on both ends.
TABLE 1.
Data for CDPK1 inhibitors
| Compound no. | Compound | clogPa | CpCDPK1 IC50 (nM) | C. parvum EC50 (μM) | Src IC50 (μM) | CRL-8155 EC50 (μM) | hERG EC50 (μM) |
|---|---|---|---|---|---|---|---|
| 2 | 1517 | 2.61 | 1.4b | 0.65 | >30b | >30b | >30 |
| 4 | 1597 | 3.20 | 2.3 | 0.41 | >10b | >40b | >30 |
| 5 | 1473 | 3.44 | 3.6b | 1.00 | 7.3b | >30b | NDc |
| 6 | 1598 | 2.33 | 5.1 | 2.16 | >10b | >40b | ND |
| 7 | 1635 | 2.93 | 3.7 | 3.35 | 10b | >40b | ND |
| 8 | 1779 | 3.16 | 0.03 | 1.03 | >10 | >80 | ND |
| 9 | 1571 | 3.35 | 4.3 | 14.85 | >10b | >40b | 30.0 |
| 10 | 1633 | 3.95 | 2.5 | 6.66 | >10b | >40b | 14.1 |
| 11 | 1776 | 4.18 | 0.3 | 3.29 | >10 | 30 | ND |
| 12 | 1586 | 2.71 | 4.9 | 0.58 | 4.75b | >40b | 22.8 |
| 13 | 1643 | 3.31 | 2.5 | 0.41 | >10b | >40b | >30 |
| 14 | 1673 | 3.55 | 4.3 | 0.52 | >10 | >40 | 27.0 |
| 15 | 1566 | 3.13 | 3.1 | >20 | >10b | >40b | ND |
| 16 | 1608 | 1.34 | 5.5 | 0.71 | 3.5 | 40 | >30 |
| 17 | 1656 | 3.61 | 2.7 | 1.00 | >10 | >40 | 11.9 |
| 18 | Paromomycin | >2,000 | 96.6 | >10 | >40 | ND |
AC-BKIs were assayed for inhibition of C. parvum growth and proliferation in human HCT-8 cells, with 50% effective concentrations (EC50s) ranging from submicromolar to low micromolar (Table 1). We have shown that potencies of BKIs dramatically increased when added before host cell invasion versus after invasion (7). In this study, AC-BKIs were added before host cell invasion. Nine out of 15 compounds had EC50s of ≤1 μM, which were lower than those of PP-BKIs, which are uniformly of low concentration (14). Compounds within each group exhibited similar C. parvum EC50s. AC-BKIs in group 1 were all quite potent, with EC50s ranging from 0.41 to 1.0 μM, with a <3-fold inhibition difference. Group 2 AC-BKIs were less potent than compounds in group 1, exhibiting low micromolar activities. Group 3 AC-BKIs exhibited EC50s ranging from 3.3 to 14.9 μM, whereas group 4 showed comparable but less variable cellular inhibition than group 1, with EC50s ranging from 0.41 to 0.58 μM. AC-BKIs in group 5 contained various substituents, and their inhibition was significantly different, ranging from 0.71 to >20 μM. Overall, there was poor correlation between CpCDPK1 enzyme inhibition and direct phenotypic effect on C. parvum growth and proliferation (Fig. 2). This could be due to compound differences in lipophilicity, level of ionization, solubility, penetration to the intracellular Cryptosporidium parasite (15), or alternative non-CDPK1 AC-BKI C. parvum target(s) (11, 12). A previous study in T. gondii suggested that AC-BKIs act only partially on TgCDPK1 targets (13). Mitogen-activated protein kinase (UniProtKB A3FQ38_CRYPI) and cyclic GMP-dependent protein kinase (UniProtKB Q5CWD4_CRYPI) are being investigated as alternative AC-BKI targets in C. parvum.
FIG 2.
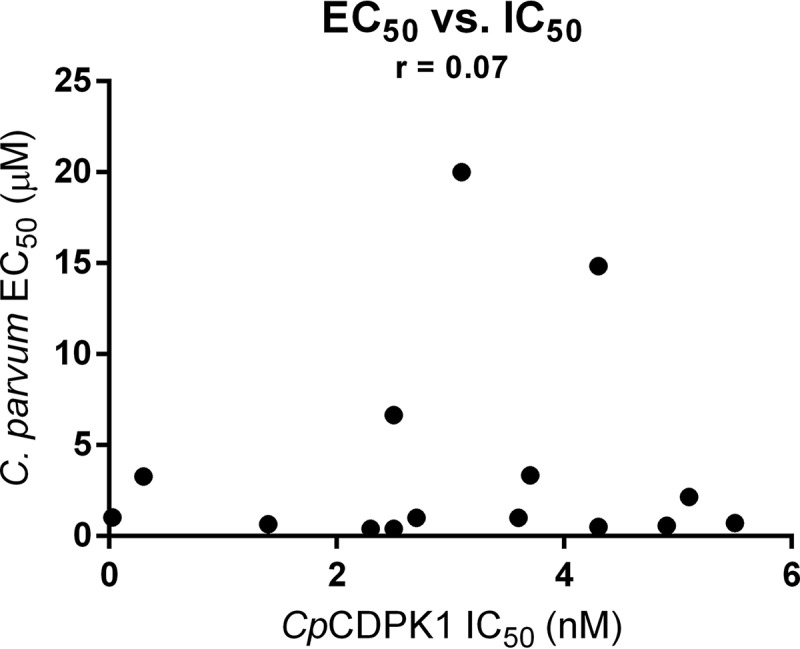
Cell inhibition EC50 versus CpCDPK1 IC50 plot to determine correlation. Plot and analysis showing the poor correlation between CpCDPK1 IC50 and C. parvum in vitro growth and proliferation EC50s. Pearson's correlation coefficient (r) was determined and added to the graph: r ranges were between 1 and −1, with 0 indicating no correlation at all, 0.1 to −0.1 also indicating no correlation, <0.5 indicating weak correlation, and 0.5 to 1 indicating strong correlation. Closer to 1 is stronger correlation and closer to 0 is weaker correlation, so r = 0.07 indicates extremely weak to no correlation.
Overall, these AC-BKIs had relatively safe profiles with high selectivity for CpCDPK1 over human tyrosine kinase (Src), no detectable growth inhibition of CRL-8155 human lymphocyte cells (12), and, most often, a lack of human Ether-à-go-go-related gene product (hERG) inhibitory activity (13). Of the nine AC-BKIs tested, five did not show 50% inhibition of hERG at ≥30 μM, two (12 and 14) had hERG EC50s between 20 and 30 μM, and two (7 and 10) had EC50s between 10 and 20 μM (Table 1).
All AC-BKIs that exhibited EC50s of <1 μM were selected to determine pharmacokinetic profiles in adult mice (Table 2). AC-BKIs 2 and 4 showed high maximum concentrations (Cmax) and areas under the curve (AUC). AC-BKIs 12, 13, and 14, all of which contain a cyclopropoxy-substituted aryl group at the C3 position, exhibited the highest Cmax and AUC values and fecal concentrations of >0.4 μM. AC-BKI 16 had low Cmax and AUC values but the highest fecal concentration, at 1.06 μM. The human plasma protein binding properties of these compounds were also measured, and the results ranged from 74% to 89%.
TABLE 2.
Pharmacokinetic, human plasma binding, stool level, and neonatal mouse C. parvum efficacy results
| Compound no. | Solubility pH 6.5 (μM) | Cmax (μM) | AUC (μM · min) | Human plasma binding (%) | Neonatal mice (25 mg/kg) (% reduction of infection)a | Fecal concentration (μM) |
|---|---|---|---|---|---|---|
| 2 | 26.0b | 9.9b | 2,428b | 74 | 39b | 0.45 |
| 4 | 83.5b | 5.3b | 1,694b | 89 | 36 | 0.05 |
| 7 | 10.9b | NDd | ND | ND | 8 | 0.83 |
| 12 | 83.7b | 23.4b | 18,450b | 84 | 37 | ND |
| 14 | 96.3b | 12.9 | 9,098 | 82 | 56 | 0.42 |
| 16 | >100c | 2.7 | 339 | 86 | 53 | 1.06 |
| 17 | 8.7 | ND | ND | 86 | 24 | ND |
| Paromomycin | ND | ND | ND | 18 | ND |
All AC-BKIs that showed potency in the C. parvum growth inhibition assay (EC50s, ≤1 μM), except AC-BKIs 5 and 13, were further tested for efficacy against C. parvum in the neonatal mouse model (Table 2). AC-BKI 7 (EC50, 3.35 μM) was included as a negative control and exhibited a low 8% reduction of infection. The positive control, paromomycin, exhibited 18% reduction of infection. Five of the seven AC-BKIs tested (2, 4, 12, 14, and 16) exhibited >30% reduction of infection compared with the untreated control. There was a statistically significant difference (t test, P < 0.05) between the controls and the AC-BKIs.
AC-BKIs 2, 4, 12, and 14 showed high Cmax and AUC levels. These four also reduced infection in the neonatal mouse model by >30%. However, AC-BKI 16, which exhibited low Cmax and AUC levels in adult mice, also showed a >50% reduction of infection in the neonatal mouse model. This indicates that systemic exposure in adult mice may not be the best predictor of efficacy in neonatal mice, as low exposure does not eliminate efficacy (r = −0.18). The poor correlation may be due to differences in drug exposure in adult and neonatal mice (14). AC-BKIs 4 and 16 were very active against C. parvum in vitro, but AC-BKI 4 demonstrated lower in vivo efficacy than AC-BKI 16. AC-BKI 4 had higher systemic levels and much lower stool levels than AC-BKI 16 (Table 2). The emerging consensus, based on a recent study (14), is that higher fecal levels of AC-BKI 16 may be a good proxy for elevated intraepithelial concentrations in the gastrointestinal tract, the site of drug action against C. parvum, whereas low fecal levels of AC-BKI 4 may indicate lower intraepithelial levels and low in vivo efficacy. Nevertheless, a number of AC-BKIs exhibited efficacy in neonatal mice, which is a good predictor of efficacy in the calf model, and remain promising for the treatment of cryptosporidiosis in animals and humans (16).
Supplementary Material
ACKNOWLEDGMENTS
Research described was supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development of the National Institutes of Health under award numbers R01AI089441, R01AI111341, and R01HD080670. The work was also supported by awards 2014-06183 and 2014-67015-22106 from the U.S. Department of Agriculture National Institute of Food and Agriculture and PATH Drug Solutions, South San Francisco, California (grant DFI 1850-02-405099).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00020-17.
REFERENCES
- 1.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White AC. 2015. Cryptosporidium species. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th ed Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 3.Shirley DA, Moonah SN, Kotloff KL. 2012. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis 25:555–563. doi: 10.1097/QCO.0b013e328357e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 5.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 6.Chen XM, O'Hara SP, Huang BQ, Nelson JB, Lin JJ, Zhu G, Ward HD, LaRusso NF. 2004. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton, and intracellular calcium dependent and required for host cell invasion. Infect Immun 72:6806–6816. doi: 10.1128/IAI.72.12.6806-6816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BG, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CL, White AC, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Ojo KK, Vidadala R, Huang W, Geiger JA, Scheele S, Choi R, Reid MC, Keyloun KR, Rivas K, Siddaramaiah LK, Comess KM, Robinson KP, Merta PJ, Kifle L, Hol WG, Parsons M, Merritt EA, Maly DJ, Verlinde CL, Van Voorhis WC, Fan E. 2014. Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 5:40–44. doi: 10.1021/ml400315s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SL, Rivas KL, Vidadala RS, Huang W, Barrett LK, Maly DJ, Fan E, Van Voorhis WC, Riggs MW. 2016. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J Infect Dis 214:1856–1864. doi: 10.1093/infdis/jiw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos-Gonzalez A, White AC, Ojo KK, Vidadala RS, Zhang Z, Reid MC, Fox AM, Keyloun KR, Rivas K, Irani A, Dann SM, Fan E, Maly DJ, Van Voorhis WC. 2013. A novel calcium-dependent protein kinase inhibitor as a lead compound for treating cryptosporidiosis. J Infect Dis 208:1342–1348. doi: 10.1093/infdis/jit327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellanos-Gonzalez A, Sparks H, Nava S, Huang W, Zhang Z, Rivas K, Hulverson MA, Barrett LK, Ojo KK, Fan E, Van Voorhis WC, White AC Jr. 2016. A novel calcium-dependent kinase inhibitor, bumped kinase inhibitor 1517, cures cryptosporidiosis in immunosuppressed mice. J Infect Dis 214:1850–1855. doi: 10.1093/infdis/jiw481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Ojo KK, Zhang Z, Rivas K, Vidadala RS, Scheele S, DeRocher AE, Choi R, Hulverson MA, Barrett LK, Bruzual I, Siddaramaiah LK, Kerchner KM, Kurnick MD, Freiberg GM, Kempf D, Hol WG, Merritt EA, Neckermann G, de Hostos EL, Isoherranen N, Maly DJ, Parsons M, Doggett JS, Van Voorhis WC, Fan E. 2015. SAR studies of 5-aminopyrazole-4-carboxamide analogues as potent and selective inhibitors of Toxoplasma gondii CDPK1. ACS Med Chem Lett 6:1184–1189. doi: 10.1021/acsmedchemlett.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Hulverson MA, Zhang Z, Choi R, Hart KJ, Kennedy M, Vidadala RS, Maly DJ, Van Voorhis WC, Lindner SE, Fan E, Ojo KK. 2016. 5-Aminopyrazole-4-carboxamide analogues are selective inhibitors of Plasmodium falciparum microgametocyte exflagellation and potential malaria transmission blocking agents. Bioorg Med Chem Lett 26:5487–5491. doi: 10.1016/j.bmcl.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulverson MA, Vinayak S, Choi R, Schaefer DA, Castellanos-Gonzalez A, Vidadala RS, Brooks CF, Herbert GT, Betzer DP, Whitman GR, Sparks HN, Arnold SL, Rivas KL, Barrett LK, White AC Jr, Dustin JM, Riggs MW, Striepen B, Van Voorhis WC, Ojo KK. 2017. Bumped-kinase inhibitors for therapy of cryptosporidiosis. J Infect Dis 215:1275–1284. doi: 10.1093/infdis/jix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster CRL. 2001. Pharmacology. Teton NewMedia, Jackson Hole, WY. [Google Scholar]
- 16.Imboden M, Riggs MW, Schaefer DA, Homan EJ, Bremel RD. 2010. Antibodies fused to innate immune molecules reduce initiation of Cryptosporidium parvum infection in mice. Antimicrob Agents Chemother 54:1385–1392. doi: 10.1128/AAC.00754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.