ABSTRACT
Candida species were tested for susceptibility to caspofungin, anidulafungin, and micafungin in order to evaluate the roles of Etest and Sensititre YeastOne in antifungal susceptibility testing for daily routines and to survey resistance. A total of 104 Candida species isolates detected from blood cultures were investigated. With EUCAST broth microdilution as the reference method, essential agreement (EA), categorical agreement (CA), very major errors (VME), major errors (ME), and minor (MIN) errors were assessed by reading MICs at 18, 24, and 48 h. By use of EUCAST broth microdilution and species-specific clinical breakpoints (CBPs), echinocandin resistance was not detected during the study period. Using EUCAST CBPs, MIC readings at 24 h for the Etest and Sensititre YeastOne resulted in CA levels of 99% and 93% for anidulafungin and 99% and 97% for micafungin. Using revised CLSI CBPs for caspofungin, CA levels were 92% and 99% for Etest and Sensititre YeastOne. The Etest proved an excellent, easy-to-handle alternative method for testing susceptibility to anidulafungin and micafungin. Due to misclassifications, the Etest is less suitable for testing susceptibility to caspofungin (8% of isolates falsely tested resistant). The CA levels of Sensititre YeastOne were 93% and 97% for anidulafungin and micafungin (24 h) by use of EUCAST CBPs and increased to 100% for both antifungals if CLSI CBPs were applied and to 100% and 99% if Sensititre YeastOne epidemiological cutoff values (ECOFFs) were applied. No one echinocandin could be demonstrated to be superior to another in vitro. Since resistance was lacking among our Candida isolates, we cannot derive any recommendation from accurate resistance detection by the Etest and Sensititre YeastOne.
KEYWORDS: antifungal susceptibility testing, echinocandins, Candida species, Etest, Sensititre YeastOne
INTRODUCTION
Candida species are leading pathogens causing health care-associated bloodstream infections with septic shock and high mortality (1, 2). Anidulafungin, caspofungin, and micafungin are recommended as first-line drugs for the treatment of invasive candidiasis (3, 4). Echinocandin resistance among Candida species is rising, and Candida glabrata shows cross-resistance to azoles, limiting the therapeutic options available (5–8). The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) have developed standardized microdilution methods for antifungal susceptibility testing (9, 10). These two organizations have determined different species-specific clinical breakpoints (CBPs) for the interpretation of MICs defining susceptible, intermediately susceptible, and resistant isolates. Since these reference methods are time-consuming and too elaborate for the daily routine, easier-to-handle methods, such as the Etest and Sensititre YeastOne methods, have become widely accepted. However, data on their suitability compared to the EUCAST method are missing. Hence, the aims of this study were to survey the echinocandin resistance of Candida bloodstream isolates and to evaluate whether the Etest and Sensititre YeastOne may serve as smart replacements of the EUCAST reference methodology for routine use. Specifically, we were interested in examining whether in vitro differences among the three echinocandins exist and whether the method of testing, the time of MIC reading, and the underlying fungal species influence any in vitro results.
RESULTS
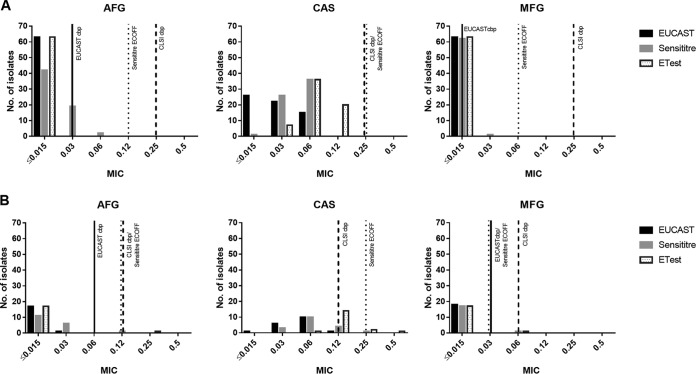
Overall, 104 Candida sp. isolates were tested, including Candida albicans (n = 63), C. glabrata (n = 18), C. tropicalis (n = 8), C. krusei (n = 5), C. parapsilosis (n = 4), C. dubliniensis (n = 2), C. kefyr (n = 1), C. lusitaniae (n = 1), C. orthopsilosis (n = 1), and C. pararugosa (n = 1). Using EUCAST broth microdilution and EUCAST and CLSI species-specific CBPs (Table 1), none of the isolates tested was classified as resistant. Table 2 gives an overview of Candida susceptibility and shows species-related MIC distributions. Figure 1 shows echinocandin MIC values for C. albicans and C. glabrata by the different test methods, indicating EUCAST and CLSI CBPs and Sensititre YeastOne epidemiological cutoff values (ECOFFs). The EUCAST method resulted in comparable MIC90 values (0.016 μg/ml) for anidulafungin and micafungin; caspofungin MIC90s were 2 dilutions higher (0.063 μg/ml). Interestingly, such differences were not observed for C. parapsilosis and C. orthopsilosis; caspofungin values were lower than anidulafungin values. The Etest showed the lowest MIC90 values for anidulafungin (0.016 μg/ml), followed by micafungin (0.064 μg/ml) and caspofungin (0.354 μg/ml). These MIC differences were not observed for C. parapsilosis and C. orthopsilosis; on the contrary, caspofungin exhibited 2-fold lower values than anidulafungin. Sensititre YeastOne resulted in lower MIC90 values for micafungin (0.064 μg/ml) than for anidulafungin and caspofungin (0.108 μg/ml and 0.120 μg/ml). Caspofungin demonstrated 4-fold-lower MIC90s for C. parapsilosis than anidulafungin and micafungin.
TABLE 1.
Breakpoint table for interpretation of EUCAST and CLSI MICs of echinocandins and Sensititre ECOFFs
| Antifungal agent | Species | MIC breakpoint (mg/liter) |
Sensititre ECOFF (μg/ml) | ||||
|---|---|---|---|---|---|---|---|
| EUCAST |
CLSI |
||||||
| S | R | S | I | R | |||
| Anidulafungin | C. albicans | ≤0.03 | >0.03 | ≤0.25 | 0.5 | ≥1 | 0.12 |
| C. glabrata | ≤0.06 | >0.06 | ≤0.12 | 0.25 | ≥0.5 | 0.12 | |
| C. krusei | ≤0.06 | >0.06 | ≤0.25 | 0.5 | ≥1 | 0.25 | |
| C. parapsilosis | ≤0.002 | >4 | ≤2 | 4 | ≥8 | 4 | |
| C. tropicalis | ≤0.06 | >0.06 | ≤0.25 | 0.5 | ≥1 | 0.5 | |
| Caspofungin | C. albicans | IEa | ≤0.25 | 0.5 | ≥1 | 0.25 | |
| C glabrata | IE | ≤0.12 | 0.25 | ≥0.5 | 0.25 | ||
| C. krusei | IE | ≤0.25 | 0.5 | ≥1 | 1 | ||
| C. parapsilosis | IE | ≤2 | 4 | ≥8 | 2 | ||
| C. tropicalis | IE | ≤0.25 | 0.5 | ≥1 | 0.25 | ||
| Micafungin | C. albicans | ≤0.016 | >0.016 | ≤0.25 | 0.5 | ≥1 | 0.06 |
| C. glabrata | ≤0.03 | >0.03 | ≤0.06 | 0.12 | ≥0.25 | 0.03 | |
| C. krusei | IE | ≤0.25 | 0.5 | ≥1 | 0.25 | ||
| C. parapsilosis | ≤0.002 | >2 | ≤2 | 4 | ≥8 | 4 | |
| C. tropicalis | IE | ≤0.25 | 0.5 | ≥1 | 0.06 | ||
IE, insufficient evidence.
TABLE 2.
In vitro susceptibilities of Candida species to anidulafungin, caspofungin, and micafungin as determined by EUCAST, Etest, and Sensititre after 24 h, including EA and CA
| Drug and species | No. of isolates | EUCAST MIC |
Etest MICa |
Sensititre MIC |
EA (%) |
CA with EUCAST (%) |
CA with CLSI (%) |
CA by use of Sensititre ECOFF (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Etest | Sensititre | Etest | Sensititre | Etest | Sensititre | |||
| Anidulafungin | |||||||||||||||||
| Total | 104 | 0.002–0.5 | 0.009 | 0.016 | 0.002–1.5 | 0.003 | 0.016 | 0.015–1 | 0.015 | 0.108 | 97 | 92 | 99 | 93 | 99 | 100 | 100 |
| C. albicans | 63 | 0.002–0.016 | 0.002 | 0.016 | 0.002–0.006 | 0.003 | 0.004 | 0.015–0.06 | 0.015 | 0.030 | 100 | 100 | 100 | 97 | 100 | 100 | 100 |
| C. dubliniensis | 2 | 0.002–0.002 | 0.002 | 0.002 | 0.002–0.006 | 0.004 | 0.006 | 0.015–0.12 | 0.068 | 0.110 | 100 | 50 | 100 | 50 | 100 | 100 | |
| C. glabrata | 18 | 0.002–0.031 | 0.016 | 0.016 | 0.002–0.25 | 0.007 | 0.008 | 0.015–0.12 | 0.015 | 0.030 | 94 | 100 | 94 | 94 | 94 | 100 | 100 |
| C. kefyr | 1 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.015 | 0.015 | 0.015 | 100 | 100 | NAb | NA | NA | NA | |
| C. krusei | 5 | 0.016–0.031 | 0.016 | 0.025 | 0.012–0.023 | 0.016 | 0.020 | 0.03–0.06 | 0.030 | 0.048 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| C. lusitaniae | 1 | 0.016 | 0.016 | 0.016 | 0.032 | 0.032 | 0.032 | 0.12 | 0.120 | 0.120 | 100 | 0 | NA | NA | NA | NA | |
| C. orthopsilosis | 1 | 0.031 | 0.031 | 0.031 | 0.38 | 0.380 | 0.380 | 0.25 | 0.250 | 0.250 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
| C. parapsilosis | 4 | 0.125–0.5 | 0.375 | 0.500 | 0.38–1.5 | 1.000 | 1.350 | 0.5–1 | 0.750 | 1.000 | 75 | 75 | 100 | 100 | 100 | 100 | 100 |
| C. pararugosa | 1 | 0.002 | 0.002 | 0.002 | 0.032* | 0.032* | 0.032* | 0.06* | 0.06* | 0.06* | 100 | 0 | NA | NA | NA | NA | |
| C. tropicalis | 8 | 0.002–0.016 | 0.016 | 0.016 | 0.003—0.008 | 0.006 | 0.007 | 0.015–0.12 | 0.060 | 0.120 | 100 | 63 | 100 | 63 | 100 | 100 | 100 |
| Caspofungin | |||||||||||||||||
| Total | 104 | 0.002–0.25 | 0.031 | 0.063 | 0.023–0.5 | 0.094 | 0.354 | 0.015–0.5 | 0.060 | 0.120 | 74 | 98 | NA | NA | 92 | 99 | 100 |
| C. albicans | 63 | 0.002–0.062 | 0.031 | 0.062 | 0.023–0.125 | 0.064 | 0.094 | 0.015–0.06 | 0.060 | 0.060 | 71 | 100 | NA | NA | 100 | 100 | 100 |
| C. dubliniensis | 2 | 0.031–0.031 | 0.031 | 0.031 | 0.047–0.094 | 0.071 | 0.089 | 0.03–0.12 | 0.075 | 0.111 | 100 | 100 | NA | NA | 100 | 100 | |
| C. glabrata | 18 | 0.016–0.125 | 0.062 | 0.063 | 0.064–0.38 | 0.125 | 0.190 | 0.03–0.25 | 0.060 | 0.120 | 89 | 100 | NA | NA | 83 | 94 | 100 |
| C. kefyr | 1 | 0.031 | 0.031 | 0.031 | 0.023 | 0.023 | 0.023 | NA | 0.015 | 0.015 | 100 | 100 | NA | NA | NA | NA | |
| C. krusei | 5 | 0.063–0.125 | 0.125 | 0.125 | 0.38–0.5 | 0.380 | 0.452 | 0.12–0.25 | 0.250 | 0.250 | 60 | 100 | NA | NA | 0 | 100 | 100 |
| C. lusitaniae | 1 | 0.063 | 0.063 | 0.063 | 0.25 | 0.250 | 0.250 | NA | 0.060 | 0.060 | 100 | 100 | NA | NA | NA | NA | |
| C. orthopsilosis | 1 | 0.031 | 0.031 | 0.031 | 0.38 | 0.380 | 0.380 | NA | 0.250 | 0.250 | 0 | 0 | NA | NA | 100 | 100 | 100 |
| C. parapsilosis | 4 | 0.062–0.25 | 0.125 | 0.213 | 0.38–0.38 | 0.380 | 0.380 | 0.12–0.5 | 0.250 | 0.425 | 75 | 100 | NA | NA | 100 | 100 | 100 |
| C. pararugosa | 1 | 0.002 | 0.002 | 0.002 | 0.19* | 0.19* | 0.19* | 0.12* | 0.12* | 0.12* | 0 | 0 | NA | NA | NA | NA | |
| C. tropicalis | 8 | 0.016–0.063 | 0.031 | 0.063 | 0.032–0.094 | 0.079 | 0.094 | 0.03–0.06 | 0.060 | 0.060 | 75 | 100 | NA | NA | 100 | 100 | 100 |
| Micafungin | |||||||||||||||||
| Total | 104 | 0.002–0.25 | 0.002 | 0.016 | 0.003–0.5 | 0.006 | 0.064 | 0.008–1 | 0.015 | 0.060 | 92 | 92 | 99 | 97 | 99 | 100 | 99 |
| C. albicans | 63 | 0.002–0.016 | 0.002 | 0.016 | 0.003–0.012 | 0.006 | 0.008 | 0.008–0.03 | 0.008 | 0.015 | 100 | 100 | 100 | 98 | 100 | 100 | 100 |
| C. dubliniensis | 2 | 0.002–0.002 | 0.002 | 0.002 | 0.004–0.008 | 0.006 | 0.008 | 0.015–0.06 | 0.038 | 0.056 | 100 | 100 | 100 | 50 | 100 | 100 | |
| C. glabrata | 18 | 0.002–0.016 | 0.002 | 0.016 | 0.004–0.064 | 0.006 | 0.008 | 0.008–0.06 | 0.015 | 0.015 | 94 | 100 | 94 | 94 | 94 | 100 | 94 |
| C. kefyr | 1 | 0.016 | 0.016 | 0.016 | 0.032 | 0.032 | 0.032 | 0.03 | 0.030 | 0.030 | 100 | 100 | NA | NA | NA | NA | |
| C. krusei | 5 | 0.016–0.062 | 0.016 | 0.043 | 0.064–0.094 | 0.064 | 0.094 | 0.12–0.12 | 0.120 | 0.120 | 20 | 20 | NA | NA | 100 | 100 | 100 |
| C. lusitaniae | 1 | 0.016 | 0.016 | 0.016 | 0.032 | 0.032 | 0.032 | 0.03 | 0.030 | 0.030 | 100 | 100 | NA | NA | NA | NA | |
| C. orthopsilosis | 1 | 0.016 | 0.016 | 0.016 | 0.19 | 0.190 | 0.190 | 0.5 | 0.500 | 0.500 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
| C. parapsilosis | 4 | 0.062–0.25 | 0.188 | 0.250 | 0.25–0.5 | 0.315 | 0.464 | 0.25–1 | 0.750 | 1.000 | 75 | 50 | 100 | 100 | 100 | 100 | 100 |
| C. pararugosa | 1 | 0.002 | 0.002 | 0.002 | 0.032* | 0.032* | 0.032* | 0.12* | 0.12* | 0.12* | 100 | 0 | NA | NA | NA | NA | |
| C. tropicalis | 8 | 0.002–0.016 | 0.016 | 0.016 | 0.008–0.12 | 0.012 | 0.047 | 0.015–0.03 | 0.030 | 0.030 | 88 | 100 | NA | NA | 100 | 100 | 100 |
*, read after 48 h.
NA, not applicable, since no breakpoints were defined.
FIG 1.
MIC values of anidulafungin (AFG), caspofungin (CAS), and micafungin (MFG) for C. albicans (A) and C. glabrata (B) by different test methods. EUCAST and CLSI CBPs and Sensititre YeastOne ECOFFs are indicated.
Etest (24-h) essential agreement (EA) levels were 97%, 74%, and 92% for anidulafungin, caspofungin, and micafungin, respectively. Categorical agreement (CA) levels were 99% for both anidulafungin and micafungin (Table 2). Minor (MIN) errors occurred for caspofungin when CLSI CBPs for C. glabrata (3/18 isolates) and C. krusei (5/5 isolates) were applied (Table 3). Sensititre YeastOne (24-h) EA levels were 92%, 98%, and 92% for anidulafungin, caspofungin, and micafungin, respectively. CA levels were 93% and 97% for anidulafungin and micafungin, respectively. Major errors (ME) were detected mainly for anidulafungin (3/8 C. tropicalis, 2/63 C. albicans, 1/2 C. dubliniensis, and 1/18 C. glabrata isolates), followed by micafungin (1/63 C. albicans, 1/2 C. dubliniensis, and 1/18 C. glabrata isolates) (Tables 2 and 3). In addition, MICs were read at 18, 24, and 48 h, at which times Etest EA levels were 97%, 97%, and 95%, and Sensititre YeastOne EA levels were 97%, 92%, and 85%, for anidulafungin; Etest EA levels were 73%, 74%, and 66%, while Sensititre YeastOne EA levels were 99%, 98%, and 80%, for caspofungin; and Etest EA levels were 93%, 92%, and 90%, while Sensititre YeastOne EA levels were 95%, 92%, and 89%, for micafungin, respectively. Etest CA levels were 99%, 99%, and 99%, while Sensititre YeastOne CA levels were 96%, 93%, and 69%, for anidulafungin; Etest CA levels were 99%, 99%, and 97%, while Sensititre YeastOne CA levels were 97%, 97%, and 88%, for micafungin; and Etest CA levels were 82%, 92%, and 82%, while Sensititre YeastOne CA levels were 99%, 99%, and 92%, for caspofungin, respectively. For several species, we lack CBPs; by use of EUCAST microdilution, a supposed micafungin cutoff of 0.03 μg/ml (which is the CBP for C. glabrata) for C. krusei and C. tropicalis categorizes only one isolate of C. krusei (MIC, 0.06 μg/ml) as resistant. While only 1 of 8 C. tropicalis isolates was classified as resistant by the Etest, and none were so classified by Sensititre YeastOne, all of the C. krusei isolates (5/5) exhibited MICs of >0.03 μg/ml. The MICs of anidulafungin and micafungin for C. kefyr, C. lusitaniae, and C. pararugosa as determined by the EUCAST method and Etest are low (≤0.03 μg/ml), in contrast to the Sensititre MICs (both the anidulafungin MIC for C. lusitaniae and the micafungin MIC for C. pararugosa were 0.12 μg/ml).
TABLE 3.
VME, ME, and MIN according to EUCAST CBPs, CLSI CBPs, and Sensititre ECOFFsa
| Drug and species | No. of isolates | Etest compared to CBPs |
Sensititre compared to CBPs |
Sensititre ECOFF |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST |
CLSI |
EUCAST |
CLSI |
|||||||||||||
| VME | ME | MIN | VME | ME | MIN | VME | ME | MIN | VME | ME | MIN | VME | ME | MIN | ||
| Anidulafungin | ||||||||||||||||
| Total | 104 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. albicans | 63 | 2 | ||||||||||||||
| C. dubliniensis | 2 | 1 | ||||||||||||||
| C. glabrata | 18 | 1 | 1 | 1 | ||||||||||||
| C. kefyr | 1 | NAb | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C. krusei | 5 | |||||||||||||||
| C. lusitaniae | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| C. orthopsilosis | 1 | |||||||||||||||
| C. parapsilosis | 4 | |||||||||||||||
| C. pararugosa | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C. tropicalis | 8 | 3 | ||||||||||||||
| Caspofungin | ||||||||||||||||
| Total | 104 | NA | NA | NA | 0 | 0 | 8 | NA | NA | NA | 0 | 0 | 1 | 0 | 0 | 0 |
| C. albicans | 63 | NA | NA | NA | NA | NA | NA | |||||||||
| C. dubliniensis | 2 | NA | NA | NA | NA | NA | NA | |||||||||
| C. glabrata | 18 | NA | NA | NA | 3 | NA | NA | NA | 1 | |||||||
| C. kefyr | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| C. krusei | 5 | NA | NA | NA | 5 | NA | NA | NA | ||||||||
| C. lusitaniae | 1 | NA | NA | NA | NA | NA | NA | |||||||||
| C. orthopsilosis | 1 | NA | NA | NA | NA | NA | NA | |||||||||
| C. parapsilosis | 4 | NA | NA | NA | NA | NA | NA | |||||||||
| C. pararugosa | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||
| C. tropicalis | 8 | NA | NA | NA | NA | NA | NA | |||||||||
| Micafungin | ||||||||||||||||
| Total | 104 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | ||
| C. albicans | 63 | 1 | ||||||||||||||
| C. dubliniensis | 2 | 1 | ||||||||||||||
| C. glabrata | 18 | 1 | 1 | 1 | 1 | |||||||||||
| C. kefyr | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C. krusei | 5 | NA | NA | NA | NA | NA | NA | |||||||||
| C. lusitaniae | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| C. orthopsilosis | 1 | |||||||||||||||
| C. parapsilosis | 4 | |||||||||||||||
| C. pararugosa | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| C. tropicalis | 8 | NA | NA | NA | NA | NA | NA | |||||||||
VME, very major error; ME, major error; MIN, minor error; ECOFF, epidemiological cutoff value.
NA, not applicable, since no breakpoints were defined.
DISCUSSION
This 1-year survey showed none of the Candida spp. tested against the echinocandins to be resistant in vitro. This is in contrast to recent reports showing an increase of as much as 13% in echinocandin resistance among Candida spp. (5–8). The Etest, for which the levels of EA with EUCAST microdilution are 97% and 92% for anidulafungin and micafungin and the CA is 99%, might be an excellent alternative method for testing susceptibility to echinocandins in routine laboratories. Only one C. glabrata isolate was classified as resistant by Etest, irrespective of the drug tested (anidulafungin and micafungin). Furthermore, the Etest provides very robust results with stable MICs over time, giving routine laboratories a timely way of reading the MICs. However, Arendrup and Pfaller (11), showed that the Etest misclassified 8% of caspofungin-susceptible isolates as intermediately susceptible or resistant. Similar misclassifications were observed among 17% of C. glabrata and 100% of C. krusei isolates in our study. Given these numbers of errors, the generally low Etest EA for caspofungin (74%), and the absence of defined CBPs by EUCAST due to significant interlaboratory variability in MIC ranges, the Etest cannot be recommended for testing susceptibility to caspofungin (12).
In contrast to the EUCAST method, where the same MIC90 values for anidulafungin and micafungin were observed, the Etest generates MIC90 values 2 steps higher for micafungin than for anidulafungin in our study. In contrast, with a large collection of >1,000 Candida isolates tested against micafungin, EUCAST MICs were shown to be 1 to 2 dilutions higher than those observed by Etest (13). However, as in our study, these MIC differences did not effectively influence CA and the susceptibility classification of isolates.
For several species, EUCAST lacks CBPs; hence, data interpretation is challenging in the clinic. Results might be based either on CBPs established for other species, on breakpoints defined for a different method of susceptibility testing (i.e., the CLSI method), or on epidemiological cutoff values (ECOFFs). Here we offer an example with C. krusei, where CBPs for micafungin are lacking: while the EA of the Etest and Sensititre YeastOne with the EUCAST method is excellent (100%) for anidulafungin, it is low for micafungin (20%). Applying CBPs for C. glabrata and micafungin (0.03 μg/ml) classifies all C. krusei MICs obtained by the Etest and Sensititre YeastOne as resistant (>0.03 μg/ml). Applying ECOFFs for C. krusei and micafungin (0.25 μg/ml) classifies all the isolates as wild type, not indicating acquired or mutational resistance mechanisms (14). However, although MICs for C. krusei are known to be three 2-fold dilution steps higher than those for C. albicans, there is insufficient evidence as to whether strains within the wild-type distribution predict susceptibility. According to CLSI CBPs, these strains are categorized as susceptible. At present, no procedure for dealing with such issues in the daily routine has been clearly defined; we advise against the use of species-specific CBPs for other species, since this could result in false data interpretation. Currently, it is recommended that anidulafungin-susceptible isolates be classified as susceptible to caspofungin (15). Hence, testing of anidulafungin against C. krusei may be the best way to predict susceptibility to micafungin.
With CA levels of 93% and 97% for anidulafungin and micafungin, Sensititre YeastOne provides acceptable results but seems to be inferior to the Etest. The errors detected are not related only to some specific species but are distributed among all the species. In contrast to those obtained by Etest, MICs obtained by Sensititre YeastOne might be read after 18 to 24 h to avoid misclassification.
However, while equivalent MIC90 values were observed for anidulafungin and micafungin by the EUCAST microdilution method, anidulafungin demonstrates the lowest MIC90 values by Etest, and micafungin has the lowest values by Sensititre YeastOne. No echinocandin can be reliably demonstrated in vitro to be superior to another. Interestingly, caspofungin exhibits the lowest MIC values for C. parapsilosis among the species tested irrespective of the method used. However, very different results, with 4-fold- to 8-fold-lower MIC90 values of anidulafungin than of caspofungin and micafungin against C. parapsilosis, have been demonstrated with the CLSI method (16). Differences in MICs obtained by different laboratories have been described as being >2 2-fold dilutions (13). Minor MIC differences without a major impact on the final susceptibility result may be due to interlaboratory variability rather than to real differences in action among the echinocandins. While the EUCAST and the CLSI have developed their standardized test methods, including their particular CBPs, specific CBPs for the Etest and Sensititre YeastOne are lacking. Therefore, MICs obtained by Etest and Sensititre YeastOne can be interpreted only by using EUCAST or CLSI CBPs. Alternatively, species-specific ECOFFs, which were determined for Sensititre YeastOne by Espinel-Ingroff et al., might be used (17). The CA of the Etest remains high (99% each for anidulafungin and micafungin) irrespective of the CBPs applied (EUCAST versus CLSI). The CA levels of Sensititre YeastOne increase from 93% and 97% for anidulafungin and micafungin by use of EUCAST CBPs to 100% for each if CLSI CBPs are used and to 100% and 99% if Sensititre YeastOne ECOFFs are used. Consequently, while the Etest might be used irrespective of the CBPs applied, Sensititre YeastOne might be far better if based on CLSI CBPs or Sensititre YeastOne ECOFFs.
The major limitation of our study is the lack of resistant isolates in our collection. Antifungal susceptibility testing aims to reliably identify isolates with in vitro (microbiological) resistance predicting in vivo (clinical) resistance associated with treatment failure. Echinocandin resistance relies largely on mutations in the two hot spot regions of the fks1 and fks2 genes, encoding a subunit of the 1,3-β-d glucan synthase protein, the target of the echinocandins (18–21). Irrespective of the method and CBPs used, isolates harboring these mutations, which result in elevated MICs, should be safely distinguished from wild-type, echinocandin-susceptible isolates (22, 23). The EUCAST EDef 7.1 method was found to be suitable for detecting fks hot spot mutants; the Etest, resulting in very major errors (VME) and ME of 8% and 3%, respectively, was less suitable (24). Using Sensititre YeastOne and the species-specific ECOFFs determined for anidulafungin, caspofungin, and micafungin, 89%, 91%, and 94% of Candida isolates with fks mutations were correctly classified, respectively (17). Since no resistance was detected among our Candida isolates, we cannot derive any recommendation from accurate resistance detection by Etest and Sensititre YeastOne.
MATERIALS AND METHODS
Isolates.
A total of 104 Candida spp. were isolated from blood cultures during 2014 and 2015 at the Division of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck, Austria. The isolates were stored at −20°C and were recultivated on a chromogenic medium (chromID Candida; reference no. 43631; bioMérieux) for confirmation of purity and further susceptibility testing. Species were identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry.
Antifungal susceptibility testing.
The EUCAST microdilution method, the Etest (AB Biodisk, Solna, Sweden), and the Sensititre YeastOne colorimetric microdilution method (TREK Diagnostic Systems, Oxoid, Germany) were performed according to EUCAST definitive document EDef 7.3 (9) and the manufacturer's instructions, respectively. EUCAST microdilution served as the reference method. MICs were read after 18, 24, and 48 h. C. parapsilosis ATCC 22019 was used as the quality control strain throughout the experiments. For comparisons, MICs determined by the Etest and Sensititre YeastOne were rounded up to the next matching EUCAST MIC cutoff values. MICs within 2 dilutions of the reference values were categorized as being in essential agreement (EA). For the calculation of categorical agreement (CA), we applied EUCAST and CLSI species-specific CBPs as well as Sensititre YeastOne species-specific ECOFFs (Table 1). CA was reached when test and reference MICs fell within the same interpretive category. Very major errors (VME) described the proportion of isolates resistant by the EUCAST microdilution method but susceptible by the test method (false-susceptible fungi). Major errors (ME) described the proportion of isolates susceptible by the EUCAST microdilution method but resistant by the test method (false-resistant fungi). Minor (MIN) errors described MICs categorized as susceptible or resistant by the EUCAST microdilution method but intermediate by the test method, or vice versa.
ACKNOWLEDGMENT
This study was financially supported by Astellas Pharma.
REFERENCES
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paiva J-A, Pereira JM, Tabah A, Mikstacki A, de Carvalho FB, Koulenti D, Ruckly S, Cakar N, Misset B, Dimopoulos G, Antonelli M, Rello J, Ma X, Tamowicz B, Timsit J-F. 2016. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: data from the EUROBACT study. Crit Care 20:53. doi: 10.1186/s13054-016-1229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical Practice Guideline for the Management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts. Fourth informational supplement. CLSI M27-S4 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Arendrup MC, Pfaller MA. 2012. Caspofungin Etest susceptibility testing of Candida species: risk of misclassification of susceptible isolates of C. glabrata and C. krusei when adopting the revised CLSI caspofungin breakpoints. Antimicrob Agents Chemother 56:3965–3968. doi: 10.1128/AAC.00355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletiadis J, Geertsen E, Curfs-Breuker I, Meis JF, Mouton JW. 2016. Intra- and interlaboratory agreement in assessing the in vitro activity of micafungin against common and rare Candida species with the EUCAST, CLSI, and Etest methods. Antimicrob Agents Chemother 60:6173–6178. doi: 10.1128/AAC.01027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. 2013. Micafungin and Candida spp.: rationale for the clinical breakpoints, version 1.0. http://www.eucast.org.
- 15.European Committee on Antimicrobial Susceptibility Testing. 2013. Anidulafungin: rationale for the clinical breakpoints, version 2.0. http://www.eucast.org.
- 16.Ghannoum MA, Chen A, Buhari M, Chandra J, Mukherjee PK, Baxa D, Golembieski A, Vazquez JA. 2009. Differential in vitro activity of anidulafungin, caspofungin and micafungin against Candida parapsilosis isolates recovered from a burn unit. Clin Microbiol Infect 15:274–279. doi: 10.1111/j.1469-0691.2008.02660.x. [DOI] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff A, Alvarez-Fernandez M, Cantón E, Carver PL, Chen SC, Eschenauer G, Getsinger DL, Gonzalez GM, Govender NP, Grancini A, Hanson KE, Kidd SE, Klinker K, Kubin CJ, Kus JV, Lockhart SR, Meletiadis J, Morris AJ, Pelaez T, Quindos G, Rodriguez-Iglesias M, Sanchez-Reus F, Shoham S, Wengenack NL, Borrell Solé N, Echeverria J, Esperalba J, Gómez-G de la Pedrosa E, García García I, Linares MJ, Marco F, Merino P, Pemán J, Pérez Del Molino L, Roselló Mayans E, Rubio Calvo C, Ruiz Pérez de Pipaon M, Yagüe G, Garcia-Effron G, Guinea J, Perlin DS, Sanguinetti M, Shields R, Turnidge J. 2015. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59:6725–6732. doi: 10.1128/AAC.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother 52:2263–2265. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Kelly R, Kahn JN, Robles J, Hsu M-J, Register E, Li W, Vyas V, Fan H, Abruzzo G, Flattery A, Gill C, Chrebet G, Parent SA, Kurtz M, Teppler H, Douglas CM, Perlin DS. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother 49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat 10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, Ghannoum M, Bonilla H. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother 54:2225–2227. doi: 10.1128/AAC.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela J-L, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and IsoSensitest media. Antimicrob Agents Chemother 54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]