ABSTRACT
Coagulase-negative staphylococci (CoNS) are the major causative agents of foreign-body-related infections, including catheter-related bloodstream infections. Because of the involvement of biofilms, foreign-body-related infections are difficult to treat. P128, a chimeric recombinant phage-derived ectolysin, has been shown to possess bactericidal activity on strains of Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA). We tested the killing potential of P128 on three clinically significant species of CoNS, S. epidermidis, S. haemolyticus, and S. lugdunensis, under a variety of physiological conditions representing growing and nongrowing states. The MIC90 and minimum bactericidal concentration at which 90% of strains tested are killed (MBC90) of P128 on 62 clinical strains of CoNS were found to be 16 and 32 μg/ml (0.58 and 1.16 μM), respectively, demonstrating the bactericidal nature of P128 on CoNS strains. Serum showed a potentiating effect on P128 inhibition, as indicated by 4- to 32-fold lower MIC values observed in serum. P128 caused a rapid loss of viability in all CoNS strains tested. Persisters of CoNS that were enriched in the presence of vancomycin or daptomycin were killed by P128 at 1× the MIC in a rapid manner. Low concentrations of P128 caused a 2- to 5-log reduction in CFU in stationary-phase or poorly metabolizing CoNS cultures. P128 at low concentrations eliminated CoNS biofilms in microtiter plates and on the surface of catheters. Combinations of P128 and standard-of-care (SoC) antibiotics were highly synergistic in inhibiting growth in preformed biofilms. Potent activity on planktonic cells, persisters, and biofilms of CoNS suggests that P128 is a promising candidate for the clinical development of treatments for foreign-body-related and other CoNS infections.
KEYWORDS: P128, foreign-body-related infections, catheter-related infections, CRBSI, coagulase-negative staphylococci, CoNS, biofilms, persisters, stationary phase, synergy
INTRODUCTION
Insertion or implantation of medical devices is a common practice in the field of medicine. Foreign-body-related infections (FBRI), especially catheter-related bloodstream infections (CRBSI), are responsible for significant human morbidity, mortality, and economic loss throughout the world (1). Because of the involvement of biofilms, these infections are difficult to treat and are often chronic and recurrent in nature. Staphylococci, including Staphylococcus aureus and coagulase-negative staphylococci (CoNS), are involved in causing the maximum number of FBRI, including CRBSI (1, 2). Although CoNS are a part of the normal flora of human skin and mucosa, they can cause serious infections, especially in immunocompromised individuals and neonates. In cases of hospital-acquired bloodstream infections, CoNS account for the largest number of cases (3, 4). Out of the CoNS pathogens, Staphylococcus epidermidis is the most commonly isolated causative agent in CRBSI (5), and Staphylococcus haemolyticus is the second most common species isolated from human blood cultures (5, 6). Infections caused by Staphylococcus lugdunensis are rare, yet unlike other CoNS species, the clinical picture and pathogenesis of infectious endocarditis caused by S. lugdunensis are very similar to those of S. aureus; thus, S. lugdunensis is associated with high rates of mortality (7). In a survey involving data collected over a 13-year period (1999 to 2012), CoNS strains showed a trend toward increasing drug resistance (8). Currently, more than 90% of S. epidermidis strains are resistant to methicillin (8). The emergence of CoNS strains showing resistance to newer drugs, such as linezolid (9), has made the situation even more alarming. The prevalence of high rates of drug resistance in S. haemolyticus clinical isolates has been attributed to the highly plastic nature of its genome (6). In addition to the problem of widespread drug resistance among CoNS strains, the biofilm-forming ability of CoNS species poses an additional challenge, as this property is thought to be responsible for the chronic and recurrent nature of device-associated CoNS infections (5, 10). It has been amply proven that bacterial biofilms are highly tolerant to the action of antibiotics in vitro and in vivo (10). A number of factors, including the impermeable nature of biofilm matrix, the altered physiological state of bacteria in biofilms, and the presence of poorly metabolizing population of persister cells, have been implicated in conferring an antibiotic resistance phenotype on biofilms (11, 12). Out of these factors, the presence of a high percentage of persister population in biofilms is thought to be the major reason behind the recalcitrant nature of biofilms (13). A recent study has demonstrated that ATP depletion and expression of stationary-phase markers lead the bacterial cell into a physiological state in which it becomes tolerant to the action of antibiotics (14). It has been demonstrated that biofilms formed by S. epidermidis, S. haemolyticus, and S. lugdunensis in vitro are tolerant to the action of high concentrations of most of the antibiotics (15–18). Obviously, to control CoNS infections, there is a need to develop new therapeutics, especially those with good antibiofilm activity and the ability to kill persister cells. Engineered and native phage lysins are emerging as potential therapeutics for treating bacterial infections (19, 20) and have demonstrated promising efficacy in preclinical in vitro and in vivo studies involving drug-resistant human pathogens (20). Depending upon the site of action, the phage lysins can be broadly classified into two categories: endolysins, which help in releasing the intracellular virus particles by cleaving the peptidoglycan from inside (20), and ectolysins (also referred to as virion-associated peptidoglycan hydrolases [20, 21]), or tail-associated muralytic enzymes (TAME) (22), which help the phage inject DNA into the bacterial cell by cleaving the peptidoglycan on the external surface. P128 is a chimeric recombinant ectolysin derived from the tail of Staphylococcus phage K (22). It is a potent anti-Staphylococcus protein showing rapid bactericidal activity on planktonic cells and biofilms of S. aureus, including methicillin-resistant S. aureus (MRSA) (22, 23). In order to develop lysins as anti-Staphylococcus agents, it is desirable to have potent activity on sensitive and resistant strains of S. aureus and CoNS. A few lysins, such as Phi11 and LysK, have been reported to have inhibitory activities on CoNS isolates (24, 25). The anti-Staphylococcus lysins which have undergone phase 1 clinical trials for development as therapeutics for controlling systemic infections (26–28) either show negligible activity against S. epidermidis strains (27), or the anti-CoNS activity has not been demonstrated in a robust manner (26). Another well-known anti-Staphylococcus lysin of bacterial origin, lysostaphin, is also known to have poor activity on CoNS strains, especially on S. epidermidis (29). Thus, a lysin which is active both on S. aureus and CoNS will have a better clinical utility for treating FBRI because of its broader spectrum of activity covering all species of staphylococci causing infections. We report here an assessment of the bactericidal properties of P128 on planktonic cells and biofilms of three clinically significant species of CoNS, namely, S. epidermidis, S. haemolyticus and S. lugdunensis, and evaluate its ability to synergize with antibiotics in killing CoNS in biofilms. In addition, P128 was also tested for its ability to kill nongrowing population of bacteria under nutrient starvation conditions and antibiotic persisters which were refractory to the action of antibiotics. P128 showed potent bactericidal activity on growing and nongrowing cells of clinical strains of the three CoNS species and was found to be equally effective in disrupting biofilms and killing biofilm-embedded bacteria. Importantly, P128 showed a high degree of synergy in combination with standard-of-care (SoC) drugs in inhibiting CoNS growth, especially in preformed biofilms.
RESULTS
MIC and MBC of P128 on CoNS strains.
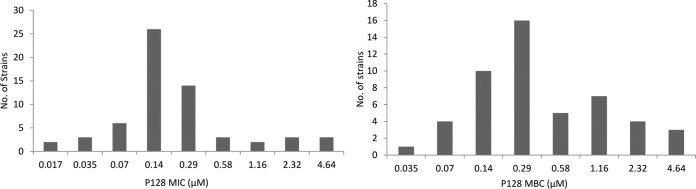
We tested P128 against 62 CoNS strains, including clinical isolates listed in Table S1 in the supplemental material (37 S. epidermidis, 16 S. haemolyticus, and 9 S. lugdunensis isolates). The strain set consisted of 49 strains with a known methicillin susceptibility profile (Table S3). Overall, 65% of the strains were resistant to methicillin; further breakup of data showed that 68%, 89%, and 17% of S. epidermidis, S. haemolyticus, and S. lugdunensis strains, respectively, were resistant to methicillin. The MIC of P128 on 62 strains of three CoNS species tested ranged from 0.5 to 128 μg/ml (0.017 to 4.64 μM) (Fig. 1). The MIC90 on CoNS strains was found to be 16 μg/ml (0.58 μM) (Table 1). P128 was equally effective on drug-sensitive and drug-resistant CoNS strains, as evidenced by similar MIC values on sensitive and resistant strains of CoNS. The MICs of P128 on a few strains of S. aureus and CoNS determined by a shorter assay format (6 h) were considerably lower than the 18-h-assay values (Table S4). Serum had a potentiating effect on P128 inhibition, as the serum MIC values on 2 strains each of S. epidermidis, S. haemolyticus, and S. lugdunensis were reduced 4- to 32-fold compared to the MIC values in cation-adjusted Mueller-Hinton broth (CAMHB) (Table S5). The range of minimum bactericidal concentration (MBC) values of P128 on CoNS isolates (1 to 128 μg/ml; Fig. 1) was comparable to that of the MICs on the same set of strains, suggesting a bactericidal effect. This was further supported by the MBC90 (32 μg/ml or 1.16 μM), which was only 2-fold higher than the corresponding MIC90 on CoNS strains. The three species of CoNS tested showed equal susceptibility to P128 in terms of the MIC90 and MBC90 values (Fig. 1, Table 1).
FIG 1.
MIC and MBC distributions of P128 on 62 CoNS strains belonging to S. epidermidis, S. haemolyticus, and S. lugdunensis. The assays were performed according to CLSI guidelines. The 0.017, 0.035, 0.07, 0.14, 0.29, 0.58, 1.16, 2.32, and 4.64 μM concentrations of P128 correspond to 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, and 128.0 μg/ml P128.
TABLE 1.
MIC and MBC of P128a on CoNS strains
| CoNS species (no.) | MIC (μg/ml [μM]) |
MBC (μg/ml [μM]) |
||||
|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | MBC range | MBC50 | MBC90 | |
| S. epidermidis (37) | 0.5–128 (0.017–4.64) | 4 (0.14) | 8 (0.29) | 0.5–128 (0.017–4.64) | 8 (0.29) | 32 (1.16) |
| S. haemolyticus (16) | 2–128 (0.07–4.64) | 8 (0.29) | 64 (2.32) | 4–128 (0.14–4.64) | 16 (0.58) | 64 (2.32) |
| S. lugdunensis (9) | 4–8 (0.14–0.29) | 4 (0.14) | 8 (0.29) | 8–16 (0.29–0.58) | 8 (0.29) | 16 (0.58) |
| Total (62) | 0.5–128 (0.017–4.64) | 4 (0.14) | 16 (0.58) | 1–128 (0.035–4.64) | 8 (0.29) | 32 (1.16) |
The molecular mass of P128 is 27 kDa.
Rapid killing of CoNS by P128.
We tested the bactericidal activity of P128 on 1 strain each of the three CoNS species, S. epidermidis, S. haemolyticus, and S. lugdunensis, using 1×, 4×, and 16× the MIC of P128 for 4 h in CAMHB and serum. P128 proved to be highly bactericidal in all the CoNS strains tested in broth, as P128 at 1× the MIC caused a >2- to 3-log reduction in CFU in 1 h (Fig. 2A). At the end of 4 h, a roughly 4-log CFU reduction was achieved using 1× the MIC of P128 in all three strains of CoNS tested. Compared to P128, the bactericidal effect of the SoC antibiotics vancomycin and daptomycin under these conditions was quite poor. Both of these drugs showed minimal killing of S. epidermidis cells at 1× the MIC after 6 h (Fig. 2B). On S. haemolyticus, the killing effect was observed only after 2 h, with vancomycin and daptomycin showing a 1- and 3-log CFU reduction, respectively, at 6 h. Contrary to the poor effect on S. epidermidis and S. haemolyticus, both vancomycin and daptomycin showed faster and greater killing of S. lugdunensis cells, albeit more slowly than seen with P128 (Fig. 2B). In order to find out if the lower MICs of P128 in serum would also be reflected in better killing in serum, time-kill kinetics (TKK) experiments were performed on the three CoNS strains using P128 at 1×, 4×, and 16× the serum MICs. The rate of killing of CoNS by P128 in serum was slightly lower than that in CAMHB, although at the end of 4 h in serum, 1× the MIC of P128 caused a >4-log CFU reduction (Fig. 2C). In both CAMHB and serum, the rate of killing was higher at 4× and 16× the MIC of P128 than at 1× the MIC, thus showing a dose-dependent effect. Overall, P128 at low concentrations showed rapid killing of CoNS strains in broth and serum far surpassing the bactericidal effects of SoC antibiotics. At 24 h, some bacterial cells were found to regrow in P128-treated samples in both MHB (data not shown) and serum (Fig. S1). Higher concentrations of P128 showed lesser regrowth. The regrown cells could again be killed by P128 in a rapid manner (data not shown), suggesting that these cells were still sensitive to P128.
FIG 2.
Time-kill kinetics (TKK) of P128 measured by CFU reduction assay using 1×, 4×, and 16× the MIC of P128 on S. epidermidis, S. haemolyticus, and S. lugdunensis under growing conditions. For broth TKK, the 6-h P128 MIC values on S. epidermidis, S. haemolyticus, and S. lugdunensis were 0.12 μg/ml, 8 μg/ml, and 2 μg/ml, respectively. For serum TKK, the 24-h P128 MIC values on S. epidermidis, S. haemolyticus, and S. lugdunensis were 0.25 μg/ml, 0.25 μg/ml, and 0.50 μg/ml, respectively. Each experiment was performed in triplicate and repeated twice. The error bars indicate the standard deviation in CFU seen across the replicates. (A) TKK in broth using increasing concentrations of P128. (B) Comparative TKK of P128, vancomycin, and daptomycin in broth using 1× the MIC of each drug. (C) TKK in serum using increasing concentrations of P128. Van, vancomycin; Dap, daptomycin.
P128 kills CoNS cells in stationary-phase or under nutrient starvation conditions.
Most of the antibiotics are known to be poorly effective on bacterial cells under slowly metabolizing conditions (e.g., in stationary phase or starvation) (30). In order to determine if the rapid killing ability of P128 seen on growing cells could be replicated on a slow-growing or poorly metabolizing population of cells, we performed a CFU reduction assay on CoNS cells grown to stationary phase and resuspended in spent medium or in lactated Ringer's solution (LRS). As shown in Fig. 3, vancomycin at 1× the MBC for 6 h did not show any significant CFU reduction either in LRS or spent medium. Daptomycin at 1× the MBC after 6 h showed a roughly 1-log CFU reduction in spent medium on two CoNS strains (S. epidermidis and S. haemolyticus), but no CFU reduction was observed in LRS on any of the CoNS strains tested. On the other hand, the killing ability of P128 on nongrowing cells remained unaltered, as cells treated with P128 at 1× the MIC resulted in a >4-log CFU reduction within 1 h in all three CoNS strains in LRS. In spent medium, P128 showed almost complete killing of S. epidermidis within 1 h, with 3-log killing of S. haemolyticus, reaching almost complete killing within 6 h, and 2-log killing of S. lugdunensis beginning at 1 h posttreatment (Fig. 3). The reason behind the poorer bactericidal effect of P128 on S. lugdunensis in spent medium is currently not understood.
FIG 3.
TKK of P128 measured by CFU reduction assay using 1× the MIC of P128 on stationary-phase-grown cells of S. epidermidis, S. haemolyticus, and S. lugdunensis under slow-growing or nongrowing conditions. Daptomycin and vancomycin at 1× the MIC each were also tested in parallel. Each experiment was performed in triplicate and repeated twice. The error bars indicate the standard deviation in CFU seen across the replicates. (A) TKK in lactated Ringer's solution. (B) TKK in spent broth. Van, vancomycin; Dap, daptomycin.
P128 kills antibiotic persisters of CoNS.
It has been shown that in vitro treatment of Staphylococcus or other bacteria by antibiotics does not lead to complete killing of bacteria (31). Instead, an initial killing curve is followed by a phase in which bacterial numbers do not undergo any change. The surviving cells, known as persisters, can tolerate the presence of high concentrations of antibiotics. In order to determine whether P128 could kill the CoNS persister population, we treated the persister cells of S. epidermidis, S. haemolyticus, and S. lugdunensis remaining after exposure to vancomycin or daptomycin at 50× or 100× the MIC with 1× the MIC of P128. As seen in Fig. 4, treatment of CoNS with daptomycin or vancomycin for 24 h resulted in survival of 103 to 105 CFU/ml out of a starting population of 108 CFU/ml. In S. epidermidis and S. haemolyticus, there was initially a linear drop in CFU in cultures treated with vancomycin or daptomycin up to 8 h; beyond this time point, there was hardly any killing with either of these drugs, suggesting the presence of persisters tolerant to the antibiotics used. As shown in Fig. 4, treatment of these persister cells with P128 at 1× the MIC for 1 h resulted in killing of almost the entire population of S. lugdunensis persisters, while very few cells of S. epidermidis and S. haemolyticus survived the action of P128. No persister cells were found to be viable in any of the CoNS cultures after treatment with 4× the MIC of P128 (Fig. S2). As expected, the cultures containing antibiotics only did not show any significant change in CFU up to 6 h. A subculture of persister cells in fresh medium followed by treatment with vancomycin or daptomycin showed that the culture was still sensitive to the action of antibiotics (data not shown), thus ruling out the possibility of genetic mutations in the persister population. These experiments proved that P128 can kill persister CoNS cells which are phenotypically refractory to the action of antibiotics.
FIG 4.
Killing of antibiotic persisters of S. epidermidis, S. haemolyticus, and S. lugdunensis by P128. The graphs show the time-kill kinetics (TKK) of 50× or 100× the MIC of vancomycin and daptomycin on CoNS strains showing initial killing of susceptible bacterial population by the antibiotics, followed by the presence of a constant number of persisters showing phenotypic resistance to antibiotics. The killing action of vancomycin and daptomycin persisters by P128 is shown by dotted lines. Each experiment was performed twice, and a similar killing trend was observed. The results obtained from one of the experiments are shown here. Van, vancomycin; Dap, daptomycin.
Combinations of P128 and antibiotics are synergistic.
In order to determine whether P128 and SoC antibiotics can act synergistically to inhibit CoNS, we tested combinations of various concentrations of P128 plus vancomycin, daptomycin, and linezolid on S. epidermidis, S. haemolyticus, and S. lugdunensis strains by checkerboard assays. Combinations of P128 and SoC drugs resulted in fractional inhibitory concentration (FIC) indices ranging from 0.18 to 1.0, suggesting a synergistic inhibition or an additive effect (Tables 2). The combination MIC values in majority of the drug combinations and strains tested were found to be lower than the individual MICs of P128 or the drugs. The MIC of P128 was lowered as much as 256-fold (in combination with daptomycin on S. haemolyticus B9478), while the MICs of the SoC antibiotics were reduced by 2- to 8-fold. No antagonism was observed with P128 and antibiotic combinations on any of the CoNS strains tested. The best synergy was observed with a combination of P128 and daptomycin on S. lugdunensis B9510, which showed an FIC index of 0.18 resulting from lower MIC values of P128 and daptomycin by 48- and 6-fold, respectively (Table 2).
TABLE 2.
Synergy of P128 and SoC antibiotics on planktonic cells of CoNS strains assayed by checkerboard microdilution methoda
| CoNS strain | Vancomycin |
Daptomycin |
Linezolid |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P128 MIC | Van MIC | P128 + Van |
FICI | P128 MIC | Dap MIC | P128 + Dap |
FICI | P128 MIC | Lin MIC | P128 + Lin |
FICI | ||||
| P128 MIC | Van MIC | P128 MIC | Dap MIC | P128 MIC | Lin MIC | ||||||||||
| S. epidermidis B9468 | 1.66 ± 0.57 | 1.66 ± 0.57 | 0.83 ± 0.28 | 0.33 ± 0.14 | 0.7 | 2 | 0.83 ± 0.28 | 0.16 ± 0.07 | 0.25 | 0.38 | 2 | 2 | 0.41 ± 0.14 | 0.5 | 0.45 |
| S. epidermidis B9470 | 2 | 2 | 1.41 ± 1.01 | 0.5 ± 0.43 | 0.95 | 2 | 1 | 0.20 ± 0.07 | 0.5 | 0.60 | 2.66 ± 1.15 | 2 | 0.41 ± 0.14 | 0.5 | 0.40 |
| S. epidermidis B9471 | 21.33 ± 9.23 | 2.66 ± 1.15 | 1.41 ± 1.01 | 2.66 ± 1.15 | 1.06 | 32 | 2 | 1.33 ± 0.57 | 0.5 | 0.29 | 26 ± 9.23 | 1 | 6.66 ± 2.30 | 0.25 | 0.50 |
| S. haemolyticus B9478 | 64 | 4 | 4 | 2 | 0.56 | 64 | 1 | 0.25 | 0.33 ± 0.14 | 0.33 | 64 | 1 | 3.33 ± 1.15 | 0.33 ± 0.14 | 0.38 |
| S. haemolyticus B9511 | 6.66 ± 2.30 | 1.33 ± 0.57 | 1.66 ± 0.57 | 0.18 ± 0.27 | 0.29 | 2.66 ± 1.15 | 0.83 ± 0.28 | 1.33 ± 0.57 | 0.18 ± 0.10 | 0.72 | 6.66 ± 2.30 | 1 | 0.83 ± 0.28 | 0.25 | 0.37 |
| S. lugdunensis B9476 | 8 | 1 | 0.5 ± 0.43 | 0.41 ± 0.14 | 0.47 | 16 | 1.33 ± 0.57 | 0.75 ± 0.43 | 0.33 ± 0.14 | 0.29 | 16 | 1 | 1.66 ± 0.57 | 0.25 | 0.35 |
| S. lugdunensis B9510 | 10.66 ± 4.61 | 2 | 0.41 ± 0.14 | 0.5 | 0.28 | 16 | 0.5 | 0.33 ± 0.14 | 0.08 ± 0.03 | 0.18 | 13.33 ± 4.61 | 1 | 2 | 0.25 | 0.40 |
MIC values (in micrograms per milliliter) are the average ± standard deviation (SD) of three values; where triplicate values were identical, no SD values are shown.
P128 rapidly eliminates CoNS biofilms on microtiter plates.
The antibiofilm activity of P128 on CoNS biofilms was assessed by its ability to eliminate preformed CoNS biofilms in a microtiter plate by the crystal violet staining assay (32). As seen in Fig. 5A, visual inspection of a crystal violet-stained well showed that P128 at 1× the MIC could destroy the biofilms of S. epidermidis, S. haemolyticus, and S. lugdunensis within 2 h. In contrast, none of the SoC antibiotics vancomycin, daptomycin, or linezolid tested at >100× the MIC showed any significant biomass removal, even after exposure up to 24 h. Quantitative analysis of the effect of P128 and antibiotics on biofilm biomass was also performed (Table S6). Under the assay conditions, S. haemolyticus B9511 formed the most luxuriant biofilms, with optical density at 540 nm (OD540) values of 18-h preformed biofilms ranging from 6.38 to 6.90, which did not show an appreciable increase in the next 24 h. The OD540 values of S. epidermidis B9470 and S. lugdunensis B9476 biofilms at time 0 ranged from 1.6 to 2.6, which showed a modest increase in OD during the next 24 h. The P128-treated biofilms of all three CoNS species showed a significant reduction in OD540 values after 2 h of treatment, whereas the antibiotic-treated biofilms did not show any significant change in OD values at this time point (Table S6). The inhibitory effect of P128 on CoNS biofilms was maintained for 24 h. The percent reduction in OD540 values (Table S6) shows that P128 consistently caused a reduction in OD at 24 h, ranging from 70 to 93%, in all three CoNS strains. A recently reported chimeric lysin, ClyF, at high concentration (100 μg/ml) was shown to remove biofilm biomass of S. aureus and CoNS species in a similar assay (33). In our study, linezolid did not reduce the biomass even after 24 h, as there was no significant reduction in OD540 values in any of the strains (Fig. 5B). Similarly, vancomycin and daptomycin at 24 h showed 34% and 53% reductions in OD540, respectively, but only in the case of S. lugdunensis B9476; no effect was observed on the other two strains (Fig. 5B and Table S6). It can be noticed that a relatively small percentage of biofilm removal caused by a high concentration of vancomycin or daptomycin observed in the quantitative assay was not apparent by visual examination of stained biofilm. Thus, P128 at low concentrations showed faster and more complete biofilm biomass reduction than did the high concentrations of SoC drugs.
FIG 5.
Effect of P128 on 24-h preformed CoNS biofilm biomass assayed by crystal violet staining. (A) Photograph showing stained biofilms after treatment with 1× the MIC (8 μg/ml) of P128 or >100× the MIC (250 μg/ml) of SoC antibiotics for 2 to 24 h. (B) Quantification of biofilm biomass in CoNS biofilms treated with P128 or SoC antibiotics for 24 h. The assay was performed by dissolving the crystal violet stain acquired by biofilms in acetic acid and measuring the OD at 540 nm. The assay was done in triplicate, and the standard deviation seen within the replicates is shown by error bars. Statistically significant removal of biofilm mass by P128 or SoC antibiotics is shown by asterisks. CC, cell control; lin, linezolid; van, vancomycin; dap, daptomycin.
SEM shows removal of CoNS biofilms from the surface of catheters.
The lytic and biomass removal potential of P128 was assessed on preformed CoNS biofilms on catheters by safranin staining and scanning electron microscopy (SEM). As seen in Fig. S3, S. epidermidis and S. lugdunensis formed luxuriant biofilms on the catheters which could be visualized by safranin staining. S. haemolyticus, on the other hand, formed thinner biofilms which could be seen only under SEM (Fig. 6C and S4). In S. epidermidis and S. lugdunensis, biofilm treatment with P128 resulted in a lack of safranin staining on catheters (Fig. S3). SEM images of all three species of CoNS showed the presence of a thick growth of staphylococci in biofilms (Fig. 6 and S4). In CoNS biofilms treated with 1× the MIC of P128, only cell debris could be seen, and no intact Staphylococcus cells were observed by SEM in any of the species. In biofilms treated with 30× the MIC of vancomycin, partial lysis of S. haemolyticus cells was observed, whereas no significant effect was seen on S. epidermidis and S. lugdunensis biofilms (Fig. 6). Thus, SEM studies clearly demonstrated that P128-mediated lysis of CoNS in biofilms leads to the elimination of biofilm biomass.
FIG 6.
Eradication of 48-h preformed CoNS biofilms on the surface of catheters (JMS infusion set) by P128 visualized by scanning electron microscopy. The biofilms were treated with the indicated concentrations of either P128 or vancomycin for 18 h and subjected to microscopy, as described in Materials and Methods. (A) S. epidermidis B9470. (B) S. lugdunensis B9510. (C) S. haemolyticus B9478.
Bactericidal activity of P128 on CoNS biofilms.
The ability of P128 to kill CoNS cells in preformed CoNS biofilms in microtiter plates and on the surface of catheters was further assessed by a CFU reduction assay. In microtiter plates, the preformed biofilms of S. epidermidis, S. haemolyticus, and S. lugdunensis harbored 107 to 108 CFU/well of viable bacteria. Treatment of preformed CoNS biofilms with increasing concentrations of P128 for 6 h showed dose-dependent killing of biofilm-embedded bacteria. In S. epidermidis and S. haemolyticus biofilms, >99% killing could be achieved with 15 to 31 μg/ml P128, while it required a slightly higher concentration of P128 (62.5 μg/ml) to achieve a similar killing of S. lugdunensis (Fig. 7A). As shown in Fig. 7B, the catheter-grown 48-h-old biofilm contained 107 CFU/ml of viable bacterial cells. Treatment of the S. epidermidis biofilm with 1× the MIC (8 μg/ml) of P128 led to a reduction of >2 log CFU, suggesting a strong bactericidal effect. Under similar conditions, daptomycin at 10× the MIC caused a roughly 1-log CFU reduction, while vancomycin at 10× the MIC failed to cause any loss of viability under these conditions (Fig. 7B).
FIG 7.
Killing of biofilm-embedded CoNS cells by P128 assayed by CFU reduction assay. (A) Reduction in cellular viability of S. epidermidis, S. haemolyticus, and S. lugdunensis in 24-h preformed biofilms in microtiter plates treated with increasing concentrations of P128 for 6 h. Each experiment was performed in triplicate and repeated twice. The error bars represent the standard deviation in CFU seen across the replicates. (B) Killing of S. epidermidis B9470 in 48-h preformed biofilms on the surface of catheters by 1× the MIC of P128 for 18 h. Vancomycin and daptomycin at their respective 10× the MIC were tested in parallel. The data were generated by performing the experiment on two sets of catheter pieces and plotting the average CFU obtained.
Combinations of P128 and antibiotics are synergistic on CoNS biofilms.
The efficacy of P128 and SoC antibiotics on CoNS biofilms, alone and in combination, was tested on three strains of S. epidermidis and one strain each of S. haemolyticus and S. lugdunensis by a minimum biofilm inhibitory concentration (MBIC) assay, as described in Materials and Methods. The MBIC values of P128 on 72-h preformed biofilms of 5 strains of CoNS ranged from 6.8 to 32 μg/ml (Table 3), suggesting that P128 can inhibit bacterial growth in CoNS biofilms in an efficient manner. Under these conditions, the SoC antibiotics showed much higher MBIC values, ranging from 7.8 μg/ml to >250 μg/ml. In general, the MBIC values of daptomycin on the strains tested were lower than the vancomycin and linezolid values (Table 3). The MBIC values of vancomycin and linezolid were found to be >200 μg/ml in the majority of the strains tested. In checkerboard assays, no antagonism was seen with any of the drugs used in combination with P128, as all the FIC index values were less than 0.5 (Table 3). Low FIC index values, ranging from 0.04 to 0.43, seen in combination with SoC drugs on various CoNS strains, meant that P128 and antibiotics inhibit CoNS growth in biofilms in a synergistic manner. P128 showed the best synergy in combination with linezolid on S. epidermidis B9472, as evidenced by a very low FIC index of 0.04 resulting from lowering of P128 and linezolid MBIC values by 26- and 328-fold, respectively. In a comparison of the data in Tables 2 and 3, it becomes obvious that in general, much lower FIC indices were obtained in combination studies on biofilms than those in planktonic cells. Overall, the lowering of P128 MBIC values in various combinations on CoNS strains ranged from 4- to 40-fold, and the antibiotic MBIC values were lowered in various combinations by 3- to 1,000-fold. The synergy data obtained with P128 and SoC antibiotics on planktonic cells and biofilms of S. epidermidis B9470 are presented in the form of isobolograms in Fig. 8. The greater synergy observed on biofilms than on planktonic cells can be clearly appreciated in the shapes of the curves in the isobolograms (Fig. 8).
TABLE 3.
Synergy of P128 and SoC antibiotics on 72-h preformed biofilms of CoNS strains assayed by checkerboard microdilution methoda
| CoNS strain | Vancomycin |
Daptomycin |
Linezolid |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P128 MBIC | Van MBIC | P128 + Van |
FICI | P128 MBIC | Dap MBIC | P128 + Dap |
FICI | P128 MBIC | Lin MBIC | P128 + Lin |
FICI | ||||
| P128 MBIC | Van MBIC | P128 MBIC | Dap MBIC | P128 MBIC | Lin MBIC | ||||||||||
| S. epidermidis B9470 | 26.66 ± 9.23 | 7.8 | 1.79 ± 1.84 | 2.56 ± 1.15 | 0.39 | 13.33 ± 4.61 | 20.8 ± 9.0 | 1 | 3.9 | 0.26 | 13.33 ± 4.61 | 7.8 | 1 | 0.45 | 0.13 |
| S. epidermidis B9471 | 26.66 ± 9.23 | 208.33 ± 72.16 | 0.76 ± 0.23 | 13 ± 4.50 | 0.09 | 26.66 ± 9.23 | 13.0 ± 4.5 | 1.33 ± 0.57 | 2.09 ± 1.71 | 0.21 | 10.66 ± 4.61 | 250 | 1.33 ± 0.57 | 1.23 ± 0.57 | 0.12 |
| S. epidermidis B9472 | 26.66 ± 9.23 | 250 | 6.53 ± 2.19 | 46.86 ± 27.07 | 0.43 | 32 | 7.8 | 1 | 0.68 ± 0.38 | 0.11 | 26.66 ± 9.23 | 250 | 1 | 0.76 ± 0.24 | 0.04 |
| S. haemolyticus B9511 | 8.19 ± 3.22 | 250 | 1.28 ± 0.88 | 16.9 ± 13.69 | 0.21 | 13.66 ± 2.02 | 250 | 1.37 ± 0.32 | 26.0 ± 9.0 | 0.20 | 6.83 ± 1.01 | 250 | 1.70 ± 0.25 | 0.25 ± 0.55 | 0.37 |
| S. lugdunensis B9510 | 32 | 250 | 0.83 ± 0.28 | 7.8 | 0.05 | 21.33 ± 9.23 | 15.6 | 2.66 ± 1.15 | 2.09 ± 1.70 | 0.25 | 21.33 ± 9.23 | 208.33 ± 72.16 | 1.66 ± 0.57 | 6.06 ± 8.54 | 0.10 |
MBIC values (in micrograms per milliliter) are the average ± standard deviation (SD); where triplicate values were identical, no SD values are shown. Van, vancomycin; Dap, daptomycin; Lin, linezolid.
FIG 8.
Synergy of P128 with SoC antibiotics, vancomycin, daptomycin, and linezolid, on planktonic cells and biofilms of S. epidermidis B9470 represented in the form of isobolograms. The isobolograms were generated by plotting fractional MIC or MBIC values of P128 against fractional MIC or MBIC values of the SoC drugs used in combination. The individual MIC or MBIC values of P128 and the drugs are joined by a solid line, while the MIC or MBIC values obtained in P128 and drug combinations are joined by a dotted line. (A) Isobolograms depicting effect of combination P128 and SoC antibiotics on planktonic cells of S. epidermidis B9470. (B) Isobolograms showing effect of combination P128 and SoC antibiotics on biofilms of S. epidermidis B9470.
DISCUSSION
The ability to adhere to and form biofilms on the surface of materials contributes towards the pathogenic potential of MRSA and CoNS in causing FBRI, such as CRBSI and other medical device-associated infections (2). Biofilms formed on the surface of medical devices, such as catheters, act as a reservoir of infection; thus, biofilm eradication is an important component of therapeutic intervention (5, 10). Although the removal of a catheter or administration of intravenous antibiotics constitutes the mainstay of treatment in these patients, the use of antibiotic-coated catheters and antibiotic lock solutions are also considered viable options (4). Due to the emergence of resistance to conventional antibiotics, efforts towards the development of non-small-molecule antibacterial therapeutics have gained increased impetus in recent years (28). Rapid killing, low rates of resistance, and profound antibiofilm activity (22, 23, 27, 34) are a few key properties of phage lysins, which can be exploited to develop clinically useful therapeutics. Currently, the lysins under clinical development (28) either show poor activity on CoNS strains (27) or have not been tested on CoNS strains in a comprehensive manner (26). Another anti-Staphylococcus lysin, lysostaphin, with potent efficacy on MRSA in vitro and in vivo, is also known to have poor activity on planktonic cells and biofilms of S. epidermidis (29, 35). PRF-119, a recombinant endolysin which shows potent activity on S. aureus or MRSA isolates, is ineffective on CoNS strains (36). The anti-CoNS activity of another potent anti-S. aureus endolysin, HY-133, has not been reported (37).
In this study, P128, an ectolysin which had shown potent bactericidal activity on methicillin-sensitive S. aureus (MSSA) and MRSA strains previously (22), was found to be equally effective on CoNS clinical strains. The MIC range, MIC50, and MBC50 values of P128 on CoNS were comparable to the corresponding values on MSSA and MRSA (38). The MIC90 of P128 on CoNS strains (0.58 μM, or 16 μg/ml) obtained in the present study is comparable to the MIC90 values of daptomycin and vancomycin on CoNS strains (0.31 μM and 1.35 μM, respectively) reported previously (39). The lower MICs of P128 observed in the 6-h CAMHB assay or in serum-based assays suggest that the actual potency of P128 is higher than what is reflected in a regular 18-h CAMHB assay. The property of P128 to inhibit both S. aureus/MRSA and CoNS equally well places it at an advantage in terms of coverage of the full spectrum of staphylococci of clinical significance compared to other anti-Staphylococcus lysins in preclinical or clinical development. The poor potency of lysostaphin on CoNS strains has been attributed to its inability to cleave serine- or alanine-containing peptide cross-bridges in the peptidoglycan (40). The potent activity of P128 on CoNS can be explained by the fact that this protein can cleave serine- or alanine-containing pentapeptides (41). The rate of killing of CoNS strains by P128 in broth or serum is much higher than that of the SoC antibiotics vancomycin and daptomycin. Moreover, the difference in the rates of killing between P128 and SoC antibiotics looks even more pronounced under nongrowing conditions (e.g., in spent medium and in LRS), where SoC antibiotics are poorly active, whereas P128 shows potent a bactericidal effect even at low concentrations. P128's ability to kill nongrowing stationary-phase cells of CoNS assumes significant importance in the light of recent findings linking stationary-phase markers or ATP depletion to the phenotype of antibiotic tolerance by persisters in S. aureus (14). The ability of P128 to kill a nongrowing bacterial population was again reflected in the remarkable CFU reduction it showed on persisters of CoNS enriched by the action of high concentrations of vancomycin or daptomycin. The antipersister activity of P128 is consistent with a similar effect shown by the LysH5 endolysin on S. aureus antibiotic persisters (35). The potent bactericidal activity of P128 on staphylococci in stationary phase, under conditions of nutrient starvation, or on cells which are phenotypically tolerant to the action of antibiotics makes P128 a promising antipersister therapeutic.
Treatment failures and late recovery in catheter-related CoNS bacteremia have often been attributed to the presence of biofilms found adhered to the surface of catheters (42). Bacteria residing in biofilms of even susceptible strains of bacteria exhibit phenotypic resistance to high concentrations of antibiotics (42). In addition to the physical factors associated with biofilms, the physiological state of bacteria in biofilms contributes to antibiotic tolerance (12). Thus, apart from the issues of the permeability barrier of a biofilm matrix, the presence of a high percentage of a persister cell population has been thought to be the reason behind the tolerance of biofilms to antibiotics (11, 18). Low MBIC values of P128 on CoNS biofilms comparable to its MIC values on planktonic cells result from the fact that it can inhibit growing and nongrowing cells equally well, and biofilm matrix probably does not act as a permeability barrier against P128. Given the variability of biofilm matrix compositions in CoNS (43, 44), it is likely that the strains used in this study will form biofilms with matrices containing carbohydrates, DNA, and proteins in various proportions. Since P128 inhibited biofilms of various CoNS isolates to a similar extent, it can be argued that the biofilm composition does not impact the efficacy of P128. P128 was able to eradicate the biofilm mass from the surface of microtiter plates and catheters with equal efficiency. The biofilm biomass elimination was accompanied by a significant loss of viability of biofilm-embedded bacteria, as detected by CFU reduction assays. Because P128 is not expected to act on constituents of the biofilm matrix, the disruption of biofilms, as confirmed by the absence of any structure observed under SEM, probably resulted from the lysis of biofilm-embedded CoNS bacteria. The tolerance of Staphylococcus biofilms to antibiotics has been attributed to the presence of a high percentage of persister cells in biofilms (13). The potent antibiofilm activity of P128 may therefore be attributed to its potent activity on nongrowing and persister cells.
The high degree of synergy between P128 and SoC antibiotics suggested by the low FIC indices observed in this study, particularly in biofilms, predicts that P128 may be effective in combination therapy with SoC antibiotics in serious CoNS infections involving biofilms. The substantial lowering of MICs of P128 and SoC antibiotics when used in combination (Table 2 and 3) suggests that the combination of P128 and antibiotics could be effective even on strains on which P128 has a higher MIC. The synergistic activity of P128 on planktonic cells and biofilms of CoNS isolates, irrespective of the antibiotic class, suggests that increased intracellular accumulation of antibiotics resulting from a loss of permeability in P128-treated CoNS cells could be the major mechanism contributing to synergy with antibiotics.
The potent bactericidal activity of P128 on planktonic cells and biofilms of MRSA shown earlier (22, 23, 34) and the equally potent efficacy on CoNS observed in this study make P128 a strong therapeutic candidate to be developed for treating catheter-related and other serious infections where a reservoir of bacteria in biofilms cause recurrent infections. The property of synergistic killing by P128 and SoC antibiotics should allow the development of effective combination therapy for treating chronic and recurrent Staphylococcus infections.
MATERIALS AND METHODS
The strains used in this study are listed in Table S1. CoNS cultures were routinely grown in Trypticase soy broth (TSB), Luria-Bertani (LB) broth, or LB agar at 37°C. The sources of the chemicals, reagents, and antibiotics used in this study are listed in Table S2. P128 was purified from an Escherichia coli overexpression strains, as described previously (22).
MIC, MBC, and synergy on planktonic cells.
The MICs of P128 and SoC drugs were determined using a modified Clinical and Laboratory Standards Institute (CLSI) broth microdilution procedure (45). Briefly, microtiter plate wells were precoated with 0.5% bovine serum albumin (BSA) to prevent nonspecific binding of P128 to the polystyrene plate. Two-fold dilutions of P128 were prepared in cation-adjusted Mueller-Hinton broth (CAMHB), and 50-μl aliquots of diluted P128 (0.125 to 256 μg/ml) were added to the wells. Bacterial suspensions (0.5 McFarland standard) were diluted in CAMHB to achieve 1 × 106 CFU/ml, and 50-μl aliquots of the cell suspension were added to the wells containing P128. The plates were incubated under static conditions at 35°C for 18 h. The MIC was defined as the lowest concentration of P128 in which no visible growth was observed at the end of the incubation period. The MBC was determined using the NCCLS procedure (46). Briefly, 100 μl from wells with the MIC, 2×, 4×, and 8× the MIC was plated on LB agar and incubated at 37°C overnight. The MBC was defined as the lowest concentration of P128 causing a >3-log CFU reduction under the assay conditions.
The synergistic potential of combinations of P128 with antibiotics on planktonic cells was assessed using the microdilution checkerboard technique described previously (23, 47). Briefly, 50 μl of a CoNS culture at a final density of 5 × 105 CFU/ml was added to wells of 96-well microtiter plates precoated with 0.5% BSA and containing 2-fold dilutions of P128 (25 μl) and/or an antibiotic (25 μl) in CAMHB. The CAMHB was supplemented with 50 μg/ml Ca2+ (as CaCl2) when testing daptomycin combinations. The microtiter plates were incubated at 35°C for 18 h, and the individual MICs and combination MICs were read. The fractional inhibitory concentration index (FICI) was determined using the following equation: FICI = (MIC of drug A in the combination/MIC of drug A alone) + (MIC of drug B in the combination/MIC of drug B alone). The combination was considered to be synergistic when the FICI was ≤0.5, additive when the FICI was 0.5 to 1.0, indifferent when the FICI was 1 to 4, and antagonistic when the FICI was ≥4. The experiments were performed in triplicate and repeated twice.
TKK.
To evaluate the concentration-dependent bactericidal activity of P128 on CoNS cultures, time-kill assays were performed in accordance with the CLSI guidelines. The strains were grown in CAMHB containing 0.1% BSA to a density of approximately 1 × 108 CFU/ml and diluted in CAMHB to obtain 20 ml of 5 × 105 CFU/ml culture, which was further grown for 1 h at 37°C with shaking at 200 rpm. A 300-μl aliquot of the cells was sampled for quantification (0-h reading). From the remaining suspension, 4 samples of 2.7 ml were dispensed in glass vials. One of the vials was left as a control, and 300 μl of P128 to achieve concentrations corresponding to the MIC, 4× the MIC, and 16× the MIC was placed in the remaining vials. The vials were incubated at 37°C with shaking at 200 rpm, and 300-μl samples were withdrawn at stipulated time points to assess the viability of the cultures. The CFU in each sample was determined by plating 100 μl of neat and diluted culture suspensions on LB agar plates, followed by incubation for 18 h at 37°C. For performing TKK in serum, the broth-grown cultures were pelleted and resuspended in fetal calf serum to obtain the required cell numbers. The rest of the procedure was the same as in the case of broth TKK. The detection limit in time-kill assays was 10 CFU/ml.
Killing activity of P128 on antibiotic persisters in planktonic cultures.
Antibiotic persisters were generated as per a previously described protocol (31, 35). Briefly, bacterial colonies were suspended in LB broth and allowed to grow at 37°C with shaking at 200 rpm for 2 h. The cultures were pelleted and resuspended in MHB, and the OD600 was adjusted to 0.5 to 1.0 to obtain 2 to 5 × 108 CFU/ml. Next, 2.7 ml of this culture was aliquoted into test tubes, and 300 μl of an antibiotic was added to achieve concentrations corresponding to 50× or 100× the MIC. The test tubes were incubated at 37°C with shaking at 200 rpm, and 250-μl aliquots were removed at 4-, 8-, and 24-h time points for CFU quantification. The aliquots were pelleted, washed, and resuspended in saline and then diluted and plated on LB agar plates. A rapid decrease in CFU, followed by stable CFU values up to 24 h, indicated the presence of antibiotic-tolerant persisters. Antibiotic-tolerant persisters were treated with P128 for 1 and 6 h by adding P128 at 1× the MIC to 450-μl aliquots of persisters. The control samples with antibiotics were further incubated for 6 h at 37°C with shaking at 200 rpm. The treated samples were subjected to CFU quantification on agar plates. In order to rule out the possibility of antibiotic resistance or tolerance resulting from genetic changes, the antibiotic-persister-containing sample was pelleted, washed in saline, and resuspended into fresh LB medium. The culture was allowed to grow at 37°C with shaking at 200 rpm until it reached an OD600 of 1.0. The culture was then subjected to persister generation, as described above. A typical biphasic growth curve in the presence of an antibiotic showing killing, followed by a constant number of CFU, indicated that the tolerance to the antibiotic was phenotypic and not caused by genetic mutations.
Bactericidal activity of P128 on slow-growing or nongrowing CoNS cells.
To simulate slow-growing or nongrowing conditions for cells, the bacterial cells were resuspended in spent medium or lactated Ringer‘s solution (LRS). Spent medium was prepared by inoculating S. epidermidis strain B9470 into 250 ml of MHB and incubating it at 37°C for 48 h. The cultures were centrifuged (48) for 10 min at 4°C to pellet bacterial cells. The supernatant was removed, passed through a 0.45-μm-pore filter, and supplemented with 0.1% BSA before using it for the assay. To obtain a population of stationary-phase cells, the cultures were grown overnight in LB. At the end of the incubation period, the culture was centrifuged, washed, and resuspended in spent medium or LRS. The OD600 of the suspension was adjusted to 0.2 (∼108 CFU/ml) and further diluted in spent medium or LRS to obtain 20 ml of an ∼1×105 CFU/ml culture suspension. A 300-μl aliquot of the cells was sampled for CFU quantification. From the remaining suspension, aliquots of 2.7 ml were dispensed in 4 glass vials. One of the vials was left as a control, and the rest received P128, daptomycin, or vancomycin at their respective MICs. The drug-treated cultures were incubated at 37°C with shaking at 200 rpm, and 300-μl samples were withdrawn at stipulated time points. The remaining CFU in each sample were determined by serially log diluting the suspension and plating 100 μl of the culture on LB agar plates, followed by incubation at 37°C overnight.
MBIC and synergy of P128 with antibiotics.
P128 was tested for its ability to inhibit biofilms of 3 strains of S. epidermidis and 2 strains each of S. haemolyticus and S. lugdunensis by the minimum biofilm inhibitory concentration (MBIC) assay described previously (23). For this purpose, overnight-grown cultures of CoNS strains were diluted 1:40 in LB broth, and 200 μl of the diluted culture was aliquoted into microtiter plate wells. The microtiter plates were placed in a shaker-incubator at 37°C and 100 rpm for 24 h, followed by a 48-h incubation under static conditions at 37°C to allow biofilm formation. The supernatants were aspirated out and discarded. The wells were washed twice with 1× PBS, and the presence of biofilm in the wells at the end of 72-h period was quantified by the metabolic dye reduction assay method using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. In this assay, the color formation upon reduction of the dye by living cells can be read at 570 nm, and the intensity of color can be correlated to the number of live cells. After washing the biofilm, 100 μl of PBS was added to the wells, along with 10 μl of MTT solution. The plate was incubated for 2 h in the dark. After this, 110 μl of solvent solution (solubilizing agent) was added to the wells, and the plate was further incubated for 15 minutes at ambient temperature with gentle agitation. The absorbance at 570 nm was read in a microplate reader. For biofilm inhibition studies, the biofilm-containing wells were washed twice with 1× PBS, challenged with various concentrations of P128 or other antibiotic drugs in LB, and incubated for 6 h at 37°C. LB was supplemented with 50 μg/ml CaCl2 during daptomycin treatment. After treatment, the supernatant was aspirated out and discarded. The biofilm adhered to the wells was quantified by an MTT assay, as described above. The MBIC was defined as the minimum concentration of P128 or the drug showing no color development. To determine whether P128 showed synergy with other drugs, combinations of P128 and antibiotics were tested by the microdilution checkerboard method described previously (23). For comparison, the MBIC of each drug was also determined individually in each experiment. The fractional MBICs were determined by an MTT dye assay, as described above. The FICI and synergy were also calculated using the formula described above for synergy in planktonic cells. To determine the bactericidal effect of P128 on CoNS biofilms, a set of four wells was processed for harvesting the biofilm and number of CFU determined by plating on solid medium.
Effect of P128 on biomass of CoNS biofilms.
A slight modification of the protocol described previously by Christensen et al. (32) was followed. Briefly, S. epidermidis B9470, S. haemolyticus B9511, or S. lugdunensis B9510 was inoculated into TSB with 2% glucose, and the cultures were grown overnight. The cultures were further diluted 1:50 in TSB to achieve an OD600 of 0.1. Two hundred microliters of the diluted culture was added to 1.8 ml of TSB medium aliquoted into each well of a 24-well microtiter plate to achieve approximately 5 × 105 CFU per well. The plates were incubated at 37°C under static conditions for 18 h, the supernatants were aspirated out, and the wells were washed twice with 2 ml of 1× PBS. To treat the biofilms with P128 or antibiotics, 1 ml of TSB plus 1 ml of the drug were added to each well and incubated at 37°C for 0, 2, 4, and 24 h. TSB supplemented with 50 μg/ml Ca2+ was used for the daptomycin treatment wells. The supernatants were aspirated out at the stipulated time points, and the wells were washed with 2 ml of 1× PBS. The wells were allowed to dry at 37°C for 15 min and stained with 1 ml of 1% crystal violet (CV) for 5 min for visual observations or 0.1% CV for quantitative reading of the wells. For visual observation, the wells were washed with 1 ml of 1× PBS, air-dried, and observed for intensity of blue color. For quantification of biomass, 1 ml of 30% acetic acid was added to each well and the plate incubated in for 5 to 10 min at ambient temperature. The contents in the well were mixed thoroughly, and the OD at 570 nm was read. The assay was performed in triplicate and repeated twice.
Formation of CoNS biofilms on catheters and treatment with P128 or antibiotics.
Cultures of S. epidermidis B9470, S. haemolyticus B9511, and S. lugdunensis B9510 were tested for their ability to form biofilms on the surface of catheters. An overnight-grown culture was diluted 1:40 in TSB containing 4% NaCl for S. epidermidis and S. lugdunensis and 1% NaCl plus 3% glucose for S. haemolyticus. Catheter (JMS infusion set or Romolene enteral feeding catheter) pieces of 1 to 2 cm in size were cut, slit into two halves, and submerged in the culture. The cultures with catheter pieces were incubated at 37°C with shaking at 100 rpm for 24 h. Postincubation, the catheters were removed and rinsed twice in PBS to remove the adhering planktonic cells. The biofilms on catheters were challenged with 8 μg/ml P128 or 30 μg/ml vancomycin by transferring the catheter pieces into tubes containing the drugs. The tubes were further incubated at 37°C under static conditions for 18 h. The antibiofilm activity was assessed by safranin staining, scanning electron microscopy (SEM), and a CFU reduction assay. For staining, the catheters were removed from the tubes, rinsed once in PBS, and immersed in 0.1% safranin for 5 min. The stained catheters were washed once in PBS and allowed to dry. For microscopy, the dried samples were fixed on aluminum stubs with double-sided carbon adhesive tape, coated with 5- to 7-nm-thick gold using a sputter-coating system (Q150T; Quorum Technologies), and examined by SEM (Neon 40; Carl Zeiss) for the presence of biofilm structures. In order to assess the bactericidal effect of P128 or antibiotics on CoNS biofilms by CFU drop assay, the catheters were removed from the tubes, rinsed twice in PBS, and placed in Eppendorf tubes containing 1 ml of PBS. To release the adhered biofilm into PBS, the catheters were scraped using an inoculation loop. The samples were vortexed thoroughly, and appropriate dilutions were plated on LB agar plates.
Statistical analysis.
To determine the statistical significance of the difference between two values, one-way analysis of variance (ANOVA) was applied, and P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Ramachandran, M. Jayasheela, and T. S. Balganesh for constant encouragement and providing suggestions during the study. Thanks are due to Vivek Paul, Candida Braithwaite, Bharathi Sriram, and Amy Percy for reviewing the manuscript. We thank the following institutions for providing bacterial strains: BEI Resources, USA, and Kanva Diagnostics, Bangalore, India. We also thank Ambica R., Bangalore Medical College, Bangalore; Girish N. Swami, Vydehi Institiute of Medical Sciences and Research Centre, Bangalore; R. Ravikumar, National Institute of Mental Health and Neurosciences, Bangalore; Anuradha K., Mysore Medical College, Mysore; and Gokul B. N., Mallige Medical Centre, Bangalore, for the kind gift of bacterial strains used in this study. We appreciate the support provided by Shashimohan Keelara in arranging catheters for the biofilm studies and Jagadeesh Bhat for procuring bacterial strains from various hospitals. We are thankful to Aloysius Daniel, Central Manufacturing Technology Institute, Bangalore, for providing the technical expertise for scanning electron microscopy of the biofilm samples. We thank the anonymous reviewers for providing suggestions for improving the manuscript.
N.P., S.N., S.D., D.T., D.H., and T.M. planned and executed the experiments and did the troubleshooting. A.V. designed the experiments and analyzed the data. U.S. analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00457-17.
REFERENCES
- 1.Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. 2014. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci. 4:162–167. doi: 10.4103/2229-5151.134184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Eiff C, Jansen B, Kohnen W, Becker K. 2005. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs 65:179–214. doi: 10.2165/00003495-200565020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Zakhour R, Chaftari AM, Raad II. 2016. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis 16:e241–e250. doi: 10.1016/S1473-3099(16)30213-4. [DOI] [PubMed] [Google Scholar]
- 5.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czekaj T, Ciszewski M, Szewczyk EM. 2015. Staphylococcus haemolyticus—an emerging threat in the twilight of the antibiotics age. Microbiology 161:2061–2068. doi: 10.1099/mic.0.000178. [DOI] [PubMed] [Google Scholar]
- 7.Frank KL, Del Pozo JL, Patel R. 2008. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21:111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May L, Klein EY, Rothman RE, Laxminarayan R. 2014. Trends in antibiotic resistance in coagulase-negative staphylococci in the United States, 1999 to 2012. Antimicrob Agents Chemother 58:1404–1409. doi: 10.1128/AAC.01908-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. 2013. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 68:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. [DOI] [PubMed] [Google Scholar]
- 12.Stewart PS. 2015. Antimicrobial tolerance in biofilms. Microbiol Spectr 3:. doi: 10.1128/microbiolspec.MB-0010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Ray P, Das A, Sharma M. 2009. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol 58:1067–1073. doi: 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 14.Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. 2016. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 18:16051. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 15.Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, Azeredo J. 2005. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother 56:331–336. doi: 10.1093/jac/dki217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank KL, Reichert EJ, Piper KE, Patel R. 2007. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51:888–895. doi: 10.1128/AAC.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredheim EG, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flaegstad T, Sollid JE. 2009. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol 47:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Hay ID, Cameron DR, Speir M, Cui B, Su F, Peleg AY, Lithgow T, Deighton MA, Qu Y. 2015. Antibiotic regimen based on population analysis of residing persister cells eradicates Staphylococcus epidermidis biofilms. Sci Rep 5:18578. doi: 10.1038/srep18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Yu J, Wei H. 2014. Engineered bacteriophage lysins as novel anti-infectives. Front Microbiol 5:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roach DR, Donovan DM. 2015. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Rubio L, Martínez B, Donovan DM, Rodríguez A, García P. 2013. Bacteriophage virion-associated peptidoglycan hydrolases: potential new enzybiotics. Crit Rev Microbiol 39:427–434. doi: 10.3109/1040841X.2012.723675. [DOI] [PubMed] [Google Scholar]
- 22.Paul VD, Rajagopalan SS, Sundarrajan S, George SE, Asrani JY, Pillai R, Chikkamadaiah R, Durgaiah M, Sriram B, Padmanabhan S. 2011. A novel bacteriophage tail-associated muralytic enzyme (TAME) from phage K and its development into a potent antistaphylococcal protein. BMC Microbiol 11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair S, Desai S, Poonacha N, Vipra A, Sharma U. 2016. Antibiofilm activity and synergistic inhibition of S. aureus biofilms by bactericidal protein P128 in combination with antibiotics. Antimicrob Agents Chemother 60:7280–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol 187:7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan DM, Lardeo M, Foster-Frey J. 2006. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett 265:133–139. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 26.Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH. 2011. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 55:1764–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, Henderson IR, Hilpert K, Jones BV, Kadioglu A, Knowles D, Ólafsdóttir S, Payne D, Projan S, Shaunak S, Silverman J, Thomas CM, Trust TJ, Warn P, Rex JH. 2016. Alternatives to antibiotics–a pipeline portfolio review. Lancet Infect Dis 16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 29.Zygmunt WA, Browder HP, Tavormina PA. 1968. Susceptibility of coagulase-negative staphylococci to lysostaphin and other antibiotics. Appl Microbiol 16:1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35:1824–1828. doi: 10.1128/AAC.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechner S, Lewis K, Bertram R. 2012. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. J Mol Microbiol Biotechnol 22:235–244. doi: 10.1159/000342449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Zhang H, Wang J, Yu J, Wei H. 2017. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci Rep 7:40182. doi: 10.1038/srep40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drilling AJ, Cooksley C, Chan C, Wormald PJ, Vreugde S. 2016. Fighting sinus-derived Staphylococcus aureus biofilms in vitro with a bacteriophage-derived muralytic enzyme. Int Forum Allergy Rhinol 6:349–355. doi: 10.1002/alr.21680. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. 2014. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idelevich EA, von Eiff C, Friedrich AW, Iannelli D, Xia G, Peters G, Peschel A, Wanninger I, Becker K. 2011. In vitro activity against Staphylococcus aureus of a novel antimicrobial agent, PRF-119, a recombinant chimeric bacteriophage endolysin. Antimicrob Agents Chemother 55:4416–4419. doi: 10.1128/AAC.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idelevich EA, Schaumburg F, Knaack D, Scherzinger AS, Mutter W, Peters G, Peschel A, Becker K. 2016. The recombinant bacteriophage endolysin HY-133 exhibits in vitro activity against different African clonal lineages of the Staphylococcus aureus complex, including Staphylococcus schweitzeri. Antimicrob Agents Chemother 60:2551–2553. doi: 10.1128/AAC.02859-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vipra AA, Desai SN, Roy P, Patil R, Raj JM, Narasimhaswamy N, Paul VD, Chikkamadaiah R, Sriram B. 2012. Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol 12:41. doi: 10.1186/1471-2180-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biedenbach DJ, Arhin FF, Moeck G, Lynch TF, Sahm DF. 2015. In vitro activity of oritavancin and comparator agents against staphylococci, streptococci and enterococci from clinical infections in Europe and North America, 2011–2014. Int J Antimicrob Agents 46:674–681. doi: 10.1016/j.ijantimicag.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Sugai M, Fujiwara T, Ohta K, Komatsuzawa H, Ohara M, Suginaka H. 1997. epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus. J Bacteriol 179:4311–4318. doi: 10.1128/jb.179.13.4311-4318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundarrajan S, Raghupatil J, Vipra A, Narasimhaswamy N, Saravanan S, Appaiah C, Poonacha N, Desai S, Nair S, Bhatt RN, Roy P, Chikkamadaiah R, Durgaiah M, Sriram B, Padmanabhan S, Sharma U. 2014. Bacteriophage-derived CHAP domain protein, P128, kills Staphylococcus cells by cleaving interpeptide cross-bridge of peptidoglycan. Microbiology 160:2157–2169. doi: 10.1099/mic.0.079111-0. [DOI] [PubMed] [Google Scholar]
- 42.Uçkay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann Med 41:109–119. doi: 10.1080/07853890802337045. [DOI] [PubMed] [Google Scholar]
- 43.Frank KL, Patel R. 2007. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun 75:4728–4742. doi: 10.1128/IAI.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira F, Lima CA, Brás S, França Â, Cerca N. 2015. Evidence for inter- and intraspecies biofilm formation variability among a small group of coagulase-negative staphylococci. FEMS Microbiol Lett 362:. [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 46.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. NCCLS document M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 47.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 48.Mascio CT, Alder JD, Silverman JA. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother 51:4255–4260. doi: 10.1128/AAC.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.