ABSTRACT
Moxidectin is under consideration for development as a treatment for human scabies. As some arthropods show decreased sensitivity to moxidectin relative to ivermectin, it was important to assess this for Sarcoptes scabiei. In vitro assays showed that the concentration of moxidectin required to kill 50% of mites was lower than that of ivermectin (0.5 μM versus 1.8 μM at 24 h; P < 0.0001). This finding provides further support for moxidectin as a candidate for the treatment of human scabies.
KEYWORDS: ivermectin, moxidectin, Sarcoptes scabiei, scabies, treatment
TEXT
The macrocyclic lactones ivermectin and moxidectin have been widely utilized in veterinary practice for the treatment of Sarcoptes scabiei infestation. For human scabies, ivermectin is the only licensed oral acaricide, with moxidectin under recent consideration for development as an alternative to ivermectin (1). As moxidectin has a prolonged plasma half-life (t1/2) in humans (29 to 47 days versus 14 h) (2–4), it is anticipated that it may be more suitable than ivermectin as a single oral dose, providing coverage over the entire mite life cycle.
Ivermectin is administered at a concentration of approximately 200 μg/kg of body weight for scabies, with rationale for this based on its activity against nematodes. There have been no empirical dose-finding studies for scabies. It is acknowledged that doses of ≤150 μg/kg have reduced efficacy (5–7), and it has been suggested that higher doses may be advantageous (8). Suboptimal responses to ivermectin in some groups (9–11) indicate that a more detailed investigation of optimal therapeutic doses in scabies is warranted. This is particularly relevant with the utilization of ivermectin for scabies mass treatment, where a single-dose regimen is desirable (12, 13). Moxidectin has demonstrated activity against sarcoptic mange, although several studies suggest that a single 200-μg/kg dose is still insufficient to achieve cure (14, 15), with long-acting formulations or higher doses required (16). Other reports show 100% efficacy following a single 200- to 300-μg/kg dose (17, 18).
Differences between ivermectin's and moxidectin's activities are apparent, especially against arthropods (19–22). From this, questions emerge regarding the threshold for acaricidal activity of both drugs over the mite life cycle and how this relates to therapeutically relevant concentrations, bioavailability, and safety margins. In vitro studies are a useful preclinical measure of relative toxicity and are routinely used to measure acaricide's activity against S. scabiei. In this study, we compared the in vitro toxicities of ivermectin and moxidectin in S. scabiei var. suis.
Mites were harvested from pigs maintained at the Queensland Agricultural Science Precinct (QASP), University of Queensland, Australia, with ethical approval from the Queensland Department of Agriculture, Forestry, and Fisheries (approval SA 2015-03-504). Scabies mites in this colony were originally obtained from naturally infested pigs and have had no known acaricide exposure. Establishment of this model and protocols for mite collection have been described in detail previously (23). Briefly, skin scrapings were obtained from the ears of infested pigs and transported to the laboratory. To isolate mites, skin crusts were placed on glass petri dishes and incubated at 28°C, which encourages mites to move out of the crusts toward the heat source.
In vitro assays were commenced within 4 h of collecting skin crusts. For all assays, mortality was defined as the absence of any movement when mites were gently touched with a probe. We used injectable solutions of ivermectin (Ivomec; Merial) and moxidectin (Cydectin; Virbac). An initial analysis was performed to compare the effects of diluents on mite survival, using 10 adult female mites exposed to 50 μM and 100 μM acaricides diluted in phosphate-buffered saline (PBS), mineral oil, or polyethylene glycol (PEG). Negative controls (n = 10) consisted of diluent in the absence of acaricide. Mortality was measured at hourly intervals for up to 5 h and then again at 24 h. Kaplan-Meier survival curves were generated, and the curves were compared statistically using the log rank (Mantel-Cox) test (GraphPad Prism 7). Results from these assays were used to confirm the most appropriate diluent for subsequent assays.
Next, assays to determine the concentration required to kill 50% of mites (50% lethal concentration [LC50]) were conducted using female mites as previously described (24). Stock solutions (200 μM) and 2-fold serial dilutions (1.6 to 100 μM) were prepared in PBS immediately prior to use. Mortality was recorded at 1, 3, and 24 h postexposure. Each compound was assayed in duplicate, with 10 mites per concentration, and assays were repeated on three separate occasions (60 mites were tested at each concentration). LC50s were determined by normalized dose-response analysis and best-fit curves generated by nonlinear regression. The two regression models and LC50s were compared statistically using the extra sum-of-squares F test (GraphPad Prism 7).
The LC50 assays were restricted to adult female mites to limit variation in susceptibility due to differences in activities associated with developmental stages. To assess this, we then compared the survival of adult females to that of juvenile and adult male mites when exposed to a fixed concentration of acaricide (6.5 μM moxidectin or 12.5 μM ivermectin) diluted in PBS. Fifteen mites per life stage were assessed in duplicate assays (n = 30).
These assays revealed that moxidectin was significantly more active than ivermectin at all time points tested (Fig. 1; Table 1). At 1 and 3 h postexposure, the reductions in moxidectin's micromolar LC50 relative to that of ivermectin were 6.2-fold and 7.5-fold, respectively. LC50s decreased for both acaricides over time of exposure, and the magnitude of difference in the LC50s of the drugs at 24 h was smaller (3.6-fold) but still significant (Table 1).
FIG 1.
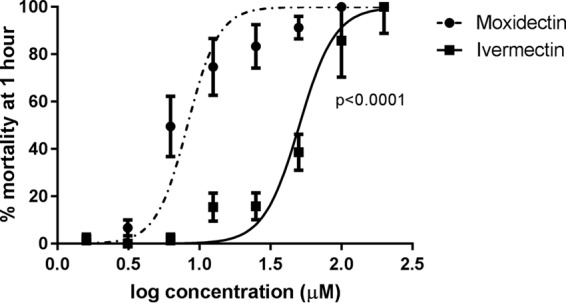
Dose-response curves of S. scabiei mortality after 1 h of in vitro exposure to serial dilutions of 0 to 200 μM ivermectin and moxidectin. Points show median mortality; bars show standard errors. Sixty mites per concentration were tested.
TABLE 1.
Concentrations of ivermectin and moxidectin required to kill 50% of S. scabiei mites at 1, 3, and 24 h postexposurea
| Drug | 1 h |
3 h |
24 h |
|||
|---|---|---|---|---|---|---|
| LC50 (μM) | 95% CI | LC50 (μM) | 95% CI | LC50 (μM) | 95% CI | |
| Ivermectin | 50.5 | 45.4–56.4 | 10.5 | 8.2–13.5 | 1.8 | 1.18–2.68 |
| Moxidectin | 8.2b | 7.6–8.9 | 1.4b | 1.1–1.9 | 0.5b | 0.34–0.77 |
Sixty mites per concentration were tested.
P < 0.0001.
Survival analysis revealed significant differences associated with developmental stages. For ivermectin (12.5 μM), median survival was 30 min for larvae, 1 h for nymphs and adult males, and 2 h for adult females (Fig. 2A). For moxidectin (6.25 μM), the median survival time was 30 min for juvenile and male mites, compared to the 1 h for females (Fig. 2B).
FIG 2.
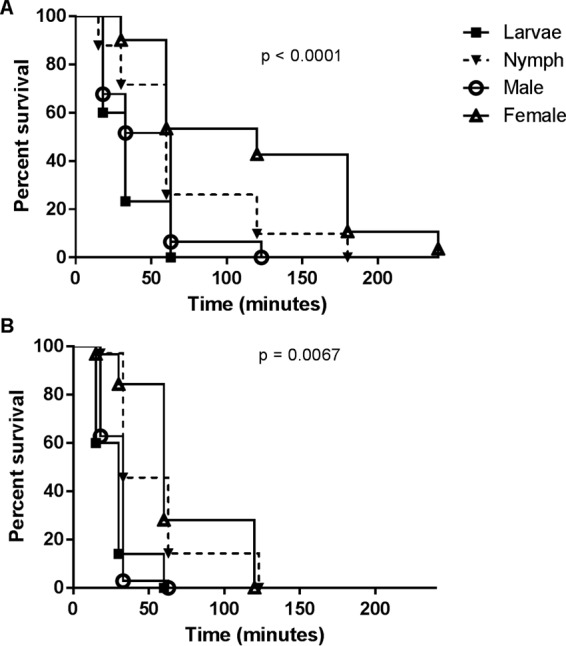
In vitro survival of S. scabiei life stages upon exposure to 12.5 μM ivermectin (A) or 6.25 μM moxidectin (B). Thirty mites at each life stage were tested.
We also found that survival times of adult female mites exposed to 50 μM ivermectin were significantly different depending on the diluent (1 to 2 h in PBS or PEG versus 4 h in mineral oil; P = 0.0003). For 50 μM moxidectin, no significant differences between diluents were apparent. Notably in all diluents, survival times with moxidectin were significantly reduced compared to those with ivermectin (P < 0.0001). The median survival time of negative controls for all assays exceeded 20 h, with no statistical differences between diluents. These findings suggest that differences in diluent should be considered when comparing data from other in vitro studies, at least for ivermectin. Considering this, the median survival time for ivermectin in mineral oil (1 h at 100 μM) was consistent with previously reported in vitro data from S. scabiei var. hominis mites prior to ivermectin exposure (for these S. scabiei var. hominis assays, ointment containing 100 μM ivermectin in paraffin oil was used). After 10 years of ivermectin use, the median in vitro survival times with ivermectin doubled for these mites (11). Precise LC50 estimates have not yet been undertaken in S. scabiei var. hominis, nor has moxidectin been assessed in this population.
The in vitro LC50s reported here are in the range documented for other parasitic arthropods and nematodes. The LC50 for ivermectin at 24 h (1.8 μM) is similar to that for a Cooperia sp. (1.7 μM), Haemonchus contortus, and Strongyloides ratti (∼1.14 μM) (25–27), although in the last two nematodes, a high-throughput motility assay demonstrated lower IC50s (<0.34 μM) (28). Where the filarial nematodes Brugia malayi and Dirofilaria immitis are concerned, the ivermectin IC50s ranged from 4.6 to 28.2 μM, depending on life stage (27, 29). These concentrations are generally higher than have been documented for arthropods for which ivermectin LC50s range from 23 nM (Cimex lectularius, Musca domestica, Anopheles gambiae) to 0.69 μM (Aedes aegypti) (19, 20, 22). There have been few studies on mites, although one recent report shows ivermectin's extremely high activity against spider mites (Tetranychus cinnabarinus; 10.3 nM) (30). Variation in reported values are likely due to different assay methodologies, particularly in arthropod assays which involve assessment of mortality after direct feeding (from a spiked blood meal, for example), whereas our S. scabiei in vitro assays are primarily contact based.
The finding that moxidectin was more toxic than ivermectin to S. scabiei is in contrast to findings reported for other arthropods. The LC50 of moxidectin at 24 h for A. gambiae (4.2 μM) is over 100-fold higher than that of ivermectin (20). In spider mites, ivermectin was 2-fold more active than milbemycin (30), although newly developed synthetic milbemycins had higher activity (0.03 to 0.17 μM) (31). Equivalent concentrations of moxidectin were less active than ivermectin against bed bugs (C. lectularius) (22). Ivermectin was approximately 10 times more potent than moxidectin in a variety of fly species (19). Differences in toxicity between the macrocyclic lactones are less apparent in nematodes (27); however, studies of B. malayi and Caenorhabditis elegans show differences between phenotypic effects of the drugs, suggesting that different target sites exist (32, 33). While moxidectin and ivermectin have similar mechanisms of action, the existence of different binding targets is further reflected by differences in ABC transporter-mediated efflux, for example (21). It is important to note that in other parasites, considerable variation exists between in vitro susceptibility and plasma drug concentrations, with the latter invariably lower (29). This is likely due to the aforementioned differences in modes of drug delivery (direct ingestion of skin and sera versus absorption of the drug through the mite cuticle). Thus, while these in vitro studies are a useful measure of relative toxicity which may aid clinical decision-making, the LC50s cannot be directly transferred to the in vivo setting.
Notwithstanding the above, when considering the clinical relevance of these results, how do these in vitro concentrations compare to bioavailability in skin? A recent study of pig skin measured the maximum concentration (Cmax) of moxidectin in skin as 0.94 μM and the t1/2 as 8.6 days, compared to only 0.069 μM and 1 day for ivermectin (17). While the moxidectin levels are within our in vitro susceptibility range, it is possible that these levels of ivermectin might be insufficient to kill all mites. In the above-described study, ivermectin could not be detected in pig skin beyond 9 to 12 days posttreatment, suggesting little activity against newly hatched eggs, although it is seen in our data that juvenile mites are susceptible to lower drug concentrations. Our results explain in part why single-dose moxidectin achieved 100% efficacy in pigs at day 14, compared to ivermectin's 62% efficacy (17). While moxidectin has excellent retention in the skin of pigs and cattle (34), different pharmacokinetics may relate to observed differences in treatment efficacy in other species. There have been few reports regarding the skin concentrations of ivermectin and moxidectin in humans. One study of five participants measured an ivermectin Cmax of 0.023 μM on the skin surface, with levels declining after 24 h (35). Determination of the concentrations of moxidectin in human skin would be of great benefit, complementing the work herein and informing future dose-finding studies.
This work contributes important preclinical data toward recently funded human phase II efficacy and dose-finding studies for moxidectin use in cases of scabies (36). Our findings confirm that S. scabiei is highly susceptible to moxidectin and that moxidectin is superior to ivermectin in vitro. This increased susceptibility, combined with enhanced bioavailability (4), provide strong support for the development of moxidectin for human scabies.
ACKNOWLEDGMENTS
We thank the staff of the Queensland Animal Science Precinct, Meredith Johnson and Mallory King, for assistance with mite collections and in vitro assays.
This work was supported by a University of the Sunshine Coast faculty grant.
REFERENCES
- 1.Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. 2016. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis 10:e0004389. doi: 10.1371/journal.pntd.0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, Rock D, Chen CY, Schwertschlag US. 2003. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol 43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez Canga A, Sahagun Prieto AM, Jose Diez Liebana M, Martinez NF, Vega MS, Vieitez JJ. 2009. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet J 179:25–37. doi: 10.1016/j.tvjl.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Korth-Bradley JM, Parks V, Patat A, Matschke K, Mayer P, Fleckenstein L. 2012. Relative bioavailability of liquid and tablet formulations of the antiparasitic moxidectin. Clin Pharmacol Drug Dev 1:32–37. doi: 10.1177/2160763X11432508. [DOI] [PubMed] [Google Scholar]
- 5.Chouela EN, Abeldano AM, Pellerano G, La Forgia M, Papale RM, Garsd A, del Carmen Balian M, Battista V, Poggio N. 1999. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the treatment of human scabies. Arch Dermatol 135:651–655. doi: 10.1001/archderm.135.6.651. [DOI] [PubMed] [Google Scholar]
- 6.Glaziou P, Cartel JL, Alzieu P, Briot C, Moulia-Pelat JP, Martin PM. 1993. Comparison of ivermectin and benzyl benzoate for treatment of scabies. Trop Med Parasitol 44:331–332. [PubMed] [Google Scholar]
- 7.Ly F, Caumes E, Ndaw CAT, Ndiaye B, Mahe A. 2009. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: a randomized controlled trial. Bull World Health Org 87:424–430. doi: 10.2471/BLT.08.052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence G, Leafasia J, Sheridan J, Hills S, Wate J, Wate C, Montgomery J, Pandeya N, Purdie D. 2005. Control of scabies, skin sores and haematuria in children in the Solomon Islands: another role for ivermectin. Bull World Health Org 83:34–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Currie BJ, Harumal P, McKinnon M, Walton SF. 2004. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 39:e8–e12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto K, Kawasaki Y, Morimoto K, Kikuchi I, Kawana S. 2014. Treatment for crusted scabies: limitations and side effects of treatment with ivermectin. J Nippon Med Sch 81:157–163. doi: 10.1272/jnms.81.157. [DOI] [PubMed] [Google Scholar]
- 11.Mounsey K, Holt D, McCarthy J, Currie B, Walton S. 2009. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol 145:840. doi: 10.1001/archdermatol.2009.125. [DOI] [PubMed] [Google Scholar]
- 12.Kearns TM, Speare R, Cheng AC, McCarthy J, Carapetis JR, Holt DC, Currie BJ, Page W, Shield J, Gundjirryirr R, Bundhala L, Mulholland E, Chatfield M, Andrews RM. 2015. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis 9:e0004151. doi: 10.1371/journal.pntd.0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, Tuicakau M, Koroi A, Andrews R, Kaldor JM, Steer AC. 2015. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med 373:2305–2313. doi: 10.1056/NEJMoa1500987. [DOI] [PubMed] [Google Scholar]
- 14.Fthenakis GC, Papadopolous E, Himonas C, Leontides L, Kritas S, Papatsas J. 2000. Efficacy of moxidectin against sarcoptic mange and effects on milk yield of ewes and growth of lambs. Vet Parasitol 87:207–216. doi: 10.1016/S0304-4017(99)00182-X. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo Arguello MR, Diez-Banos N, Martinez-Gonzalez B, Rojo-Vazquez FA. 2001. Efficacy of moxidectin 1% injectable against natural infection of Sarcoptes scabiei in sheep. Vet Parasitol 102:143–150. doi: 10.1016/S0304-4017(01)00517-9. [DOI] [PubMed] [Google Scholar]
- 16.Astiz S, Legaz-Huidobro E, Mottier L. 2011. Efficacy of long-acting moxidectin against sarcoptic mange in naturally infested sheep. Veterinary Record 169:637a. doi: 10.1136/vr.100188. [DOI] [PubMed] [Google Scholar]
- 17.Bernigaud C, Fang F, Fischer K, Lespine A, Aho LS, Dreau D, Kelly A, Sutra JF, Moreau F, Lilin T, Botterel F, Guillot J, Chosidow O. 2016. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl Trop Dis 10:e0005030. doi: 10.1371/journal.pntd.0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Losson B, Lonneux JF. 1993. Field efficacy of injectable moxidectin in cattle naturally infested with Chorioptes bovis and Sarcoptes scabiei. Vet Parasitol 51:113–121. doi: 10.1016/0304-4017(93)90202-X. [DOI] [PubMed] [Google Scholar]
- 19.Blanckenhorn WU, Puniamoorthy N, Scheffczyk A, Rombke J. 2013. Evaluation of eco-toxicological effects of the parasiticide moxidectin in comparison to ivermectin in 11 species of dung flies. Ecotoxicol Environ Saf 89:15–20. doi: 10.1016/j.ecoenv.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Butters MP, Kobylinski KC, Deus KM, da Silva IM, Gray M, Sylla M, Foy BD. 2012. Comparative evaluation of systemic drugs for their effects against Anopheles gambiae. Acta Trop 121:34–43. doi: 10.1016/j.actatropica.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prichard R, Menez C, Lespine A. 2012. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist 2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheele JM, Ridge GE. 2016. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug, Cimex lectularius L. Parasitol Res 115:3071–3081. doi: 10.1007/s00436-016-5062-x. [DOI] [PubMed] [Google Scholar]
- 23.Mounsey K, Ho MF, Kelly A, Willis C, Pasay C, Kemp DJ, McCarthy JS, Fischer K. 2010. A tractable experimental model for study of human and animal scabies. PLoS Negl Trop Dis 4:e756. doi: 10.1371/journal.pntd.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasay C, Mounsey K, Stevenson G, Davis R, Arlian L, Morgan M, Vyszenski-Moher D, Andrews K, McCarthy J. 2010. Acaricidal activity of eugenol based compounds against scabies mites. PLoS One 5:e12079. doi: 10.1371/journal.pone.0012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotze AC, Clifford S, O'Grady J, Behnke JM, McCarthy JS. 2004. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am J Trop Med Hyg 71:608–616. [PubMed] [Google Scholar]
- 26.Kotze AC, Le Jambre LF, O'Grady J. 2006. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet Parasitol 137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, Howell S, Sakanari J, Wolstenholme A, Kaplan R. 2014. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “The Worminator.” Int J Parasitol Drugs Drug Resist 4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smout MJ, Kotze AC, McCarthy JS, Loukas A. 2010. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis 4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolstenholme AJ, Maclean MJ, Coates R, McCoy CJ, Reaves BJ. 2016. How do the macrocyclic lactones kill filarial nematode larvae? Invert Neurosci 16:7. doi: 10.1007/s10158-016-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Chen AL, Zhang H, Yu Z, Li MH, Li N, Lin JT, Bai H, Wang JD, Zheng YG. 2015. Gene replacement for the generation of designed novel avermectin derivatives with enhanced acaricidal and nematicidal activities. Appl Environ Microbiol 81:5326–5334. doi: 10.1128/AEM.01025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan JJ, Wan X, Zhang H, Chen Z, Huang J, Yang B, Chen AL, Wang JD. 2016. Three new milbemycins from a genetically engineered strain S. avermitilis MHJ1011. J Antibiot (Tokyo) 69:104–107. doi: 10.1038/ja.2015.90. [DOI] [PubMed] [Google Scholar]
- 32.Ardelli BF, Stitt LE, Tompkins JB, Prichard RK. 2009. A comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet Parasitol 165:96–108. doi: 10.1016/j.vetpar.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Tompkins JB, Stitt LE, Ardelli BF. 2010. Brugia malayi: in vitro effects of ivermectin and moxidectin on adults and microfilariae. Exp Parasitol 124:394–402. doi: 10.1016/j.exppara.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Lifschitz A, Virkel G, Imperiale F, Sutra JF, Galtier P, Lanusse C, Alvinerie M. 1999. Moxidectin in cattle: correlation between plasma and target tissues disposition. J Vet Pharmacol Ther 22:266–273. doi: 10.1046/j.1365-2885.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- 35.Haas N, Lindemann U, Frank K, Sterry W, Lademann J, Katzung W. 2002. Rapid and preferential sebum secretion of ivermectin: a new factor that may determine drug responsiveness in patients with scabies. Arch Dermatol 138:1618–1619. doi: 10.1001/archderm.138.12.1618. [DOI] [PubMed] [Google Scholar]
- 36.Medicines Development for Global Health. 2016. Addressing scabies, the neglected of the neglected diseases. Medicines Development for Global Health, Southbank, Australia: http://www.medicinesdevelopment.com/wp-content/uploads/2016/06/MDL-press-release-60615.pdf Accessed 2 August 2016. [Google Scholar]


