ABSTRACT
Combination therapy including colistin and a carbapenem has been found to be associated with lower mortality in the treatment of bloodstream infections (BSI) due to KPC-producing Klebsiella pneumoniae when the isolates show a meropenem or imipenem MIC of <16 mg/liter. However, the optimal treatment of BSI caused by colistin- and high-level carbapenem-resistant KPC-producing K. pneumoniae is unknown. A prospective cohort study including episodes of bacteremia caused by colistin-resistant and high-level meropenem-resistant (MIC ≥ 64 mg/liter) KPC-producing K. pneumoniae diagnosed from July 2012 to February 2016 was performed. The impact of combination therapy on crude 30-day mortality was analyzed by Cox regression using a propensity score as a covariate to control for indication bias and in an inverse probability of treatment weighting (IPTW) cohort. The study sample comprised 104 patients, of which 32 (30.8%) received targeted monotherapy and 72 (69.2%) received targeted combination therapy; none of them received either colistin or a carbapenem. The 30-day crude mortality rate was 30.8% (43.8% in patients treated with monotherapy and 25% in patients receiving combination therapy). In the Cox regression analysis, 30-day mortality was independently associated with septic shock at BSI onset (hazard ratio [HR], 6.03; 95% confidence interval [CI], 1.65 to 21.9; P = 0.006) and admission to the critical care unit (HR, 2.87; 95% CI, 0.99 to 8.27; P = 0.05). Targeted combination therapy was associated with lower mortality only in patients with septic shock (HR, 0.14; 95% CI, 0.03 to 0.67; P = 0.01). These results were confirmed in the Cox regression analysis of the IPTW cohort. Combination therapy is associated with reduced mortality in patients with bacteremia due to colistin-resistant KPC-producing K. pneumoniae with high-level carbapenem resistance in patients with septic shock.
KEYWORDS: Klebsiella pneumoniae, bacteremia, carbapenems, colistin, mortality
INTRODUCTION
Carbapenem-resistant Klebsiella pneumoniae infections are a public health problem in many areas due to the difficulties involved in treating these infections and their high associated mortality (1–8). Colistin, gentamicin, tigecycline, and fosfomycin are the most frequently used active antibiotics that are currently available to treat KPC-producing K. pneumoniae (9). For bacteremic infections, combination treatment regimens that include meropenem (at least when the MIC is ≤8 mg/liter) are recommended (6, 10); however, recent data suggest that combination therapy is needed only in patients at high risk of death (11). In recent years, outbreaks of colistin-resistant KPC-producing K. pneumoniae infections with high-level meropenem resistance (MIC ≥ 64 mg/liter) have been reported. For these isolates, the usefulness of colistin (a keystone in the treatment of carbapenem-resistant organisms) and the potential benefits of associating meropenem are reduced or lost (12). In such settings, carbapenem-sparing treatment regimens using the few available active drugs can be designed. To date, the best treatment regimen for bloodstream infections due to colistin-resistant KPC-producing K. pneumoniae with high-level carbapenem resistance is unknown. Additionally, avoiding the use of carbapenems may also reduce the selecting pressure in centers with ongoing transmission of KPC-producing K. pneumoniae.
This study, which is based on an analysis of daily clinical practice during a KPC-producing K. pneumoniae outbreak, examines the variables associated with mortality due to colistin-resistant KPC-producing K. pneumoniae bacteremia with high-level carbapenem resistance. Particular attention is given to the impact of targeted combination therapy not including carbapenems.
RESULTS
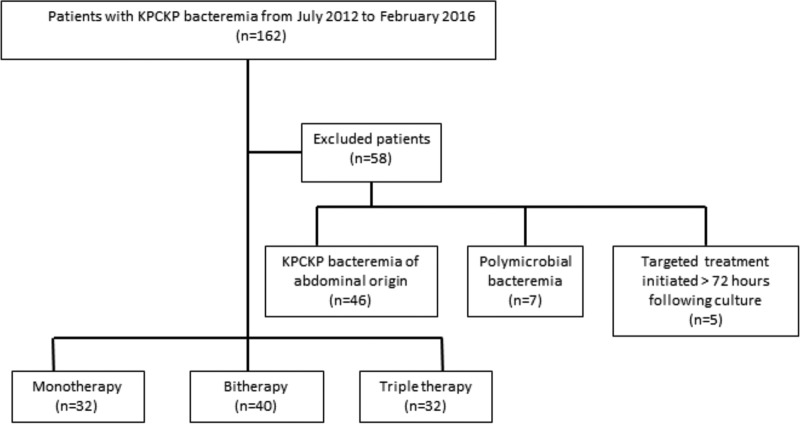
Of the 162 KPC-producing K. pneumoniae BSIs occurring during the study period, 104 met all the inclusion criteria (Fig. 1). Of these, 93.3% were considered nosocomial and the rest were health care associated (6.7%). The main characteristics of the patients with colistin-resistant KPC-producing K. pneumoniae bacteremia with high-level meropenem resistance receiving monotherapy or combination therapy are shown in Table 1.
FIG 1.
Study flow diagram.
TABLE 1.
Characteristics of patients with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance receiving monotherapy or combination therapyd
| Variable | Monotherapy patients (n = 32) | Combination therapy patients (n = 72) | P value |
|---|---|---|---|
| Male, n (%)a | 14 (43.8) | 43 (59.7) | 0.13 |
| Median age (yr) (IQR)c | 72 (78–61.25) | 62 (74.25–47) | 0.17 |
| Comorbidities | |||
| Chronic renal disease, n (%)a | 7 (21.9) | 20 (27.8) | 0.53 |
| Diabetes mellitus, n (%)a | 16 (50) | 20 (27.8) | 0.03 |
| Chronic obstructive pulmonary disease, n (%)a | 4 (12.5) | 11 (15.3) | 0.7 |
| Active solid tumor, n (%)a | 8 (25) | 22 (30.6) | 0.4 |
| Transplant, n (%)b | 2 (6.2) | 9 (12.5) | 0.50 |
| Median Charlson index (IQR)c | 6 (7–2.5) | 3 (6–2) | 0.5 |
| Admission from a care facility, n (%)b | 3 (9.4) | 7 (9.7) | 0.9 |
| Prior known colonization, n (%)a | 8 (25) | 23 (31.9) | 0.5 |
| Admission in previous 3 mo, n (%)a | 18 (56.3) | 29 (40.3) | 0.13 |
| Surgery in previous week, n (%)a | 10 (31.3) | 26 (36.1) | 0.63 |
| Invasive procedures in previous wka | |||
| Central venous catheter, n (%) | 19 (59.4) | 53 (73.6) | 0.15 |
| Urinary catheter, n (%) | 26 (81.3) | 63 (87.5) | 0.4 |
| Mechanical ventilation, n (%) | 10 (31.3) | 40 (56.3) | 0.02 |
| Admission warda | 0.01 | ||
| Medical, n (%) | 9 (28.1) | 20 (27.8) | |
| Surgical, n (%) | 11 (34.4) | 8 (11.1) | |
| Critical care, n (%) | 12 (37.5) | 44 (61.1) | |
| Renal failure at time of diagnosis, n (%)a | 11 (34.4) | 33 (45.8) | 0.28 |
| Source of infectiona | 0.12 | ||
| Pneumonia, n (%) | 8 (25) | 31 (43.1) | |
| Primary bacteremia, n (%) | 16 (50) | 22 (30.6) | |
| Urinary tract infection, n (%) | 8 (25) | 19 (26.4) | |
| Median length of hospitalization (days) during follow-up (IQR)c | 16.5 (14.25–22.75) | 17 (12–24) | 0.92 |
| Septic shock, n (%)a | 8 (25) | 40 (55.6) | 0.004 |
| Median Pitt score (IQR)c | 3 (5–2) | 4 (5–2) | 0.30 |
| Appropriate empirical therapy, n (%)a | 11 (34.4) | 44 (61.1) | 0.01 |
| Mortality at 30 days of follow-up, n (%)a | 14 (43.8) | 18 (25) | 0.05 |
P values were determined using Pearson's χ2 test.
P values were determined using Fisher's exact test.
P values were determined using the Mann-Whitney test.
Abbreviations: IQR, interquartile range; HR, hazard ratio; n (%), number and percentage of patients.
Phenotypic characteristics of the isolated strains.
The 104 isolates were resistant to colistin, penicillins, cephalosporins, ertapenem, meropenem, ciprofloxacin, and co-trimoxazole. Some of the isolates were susceptible to tigecycline (n = 84, 80.8%), fosfomycin (n = 30, 28.8%) and gentamicin (n = 49, 47.1%); 45 strains (43.3%) showed intermediate sensitivity to gentamicin (MIC = 4 mg/liter). At the time of this study, ceftazidime-avibactam was not available in Spain.
Antimicrobial therapy.
All patients received empirical therapy with anti-Gram-negative antibiotics (alone or in combination with other antibiotics) at currently recommended doses. Empirical therapy was classified as inappropriate in 49 patients (47.1%) according to the antimicrobial sensitivity test. When the isolate was classified as KPC-producing K. pneumoniae (typically after 24 to 72 h of culture), the patients were treated with at least one active drug against the KPC-producing K. pneumoniae clone circulating in the hospital. A definitive treatment regimen was designed according to the results of the antimicrobial sensitivity test.
Variables associated with mortality.
Table 2 shows the variables associated with 30-day mortality in the univariate analysis. Thirty-two patients died (30.8%) at day 30 of follow-up. Mortality was 43.8% (14/32 patients) in patients receiving monotherapy and 25% (18/72 patients) in patients receiving combination therapy (P = 0.05). The different treatment regimens and their crude associated mortality rates are shown in Table 3. The Kaplan-Meier curves for targeted treatment with monotherapy or with combination therapy are presented in Fig. 2. In the Cox regression multivariate analysis using propensity score as a covariate (Table 2), 30-day mortality was independently associated with septic shock at BSI onset (hazard ratio [HR], 6.03; 95% confidence interval [CI], 1.65 to 21.9; P = 0.006) and admission to the critical care unit (HR, 2.87; 95% CI, 0.99 to 8.27; P = 0.05). Combination therapy was not associated with decreased mortality by itself, but its interaction with septic shock was statistically significant (HR, 0.14; 95% CI, 0.03 to 0.65; P = 0.01), meaning that combination therapy has a protective effect for mortality among patients with septic shock. In the Cox regression analysis of the IPTW cohort, similar results were obtained; in this model, other variables such as urinary tract source (HR, 2.97; 95% CI, 1.42 to 6.24; P = 0.004), pneumonia (HR, 4.75; 95% CI, 2.34 to 9.67; P < 0.001), and appropriate empirical therapy (HR, 0.48; 95% CI, 0.26 to 0.88; P = 0.02) were also associated with mortality. Combined treatment alone was not significant, but its interaction with septic shock was also statistically significant (HR, 0.10; 95% CI, 0.03 to 0.32; P < 0.001) (Table 2).
TABLE 2.
Univariate and multivariate analysis of variables associated with 30-day mortality rate in patients with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistancea
| Variable | No. (%) of patients |
Univariate analysis |
Multivariate analysis with propensity score as covariable |
Multivariate analysis using the inverse probability weighted-cohort |
||||
|---|---|---|---|---|---|---|---|---|
| Survivors (n = 72) | Nonsurvivors (n = 32) | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (yr), median (IQR) | 65.0 (51–73.2) | 63.5 (47–72.25) | 0.99 (0.98–1.02) | 0.87 | ||||
| Male sex | 39 (54.2) | 18 (56.2) | 1.16 (0.58–2.3) | 0.68 | ||||
| Diabetes mellitus | 23 (31.9) | 13 (40.6) | 1.32 (0.65–2.67) | 0.45 | ||||
| Charlson score, Median (IQR) | 4 (2–6) | 4 (2–6) | 1.00 (0.9–1.11) | 0.9 | ||||
| Pitt score, Median (IQR) | 3 (2–5) | 4 (2–6) | 1.1 (0.95–1.28) | 0.22 | ||||
| Septic shock | 31 (43.1) | 17 (53.1) | 1.59 (0.79–3.2) | 0.19 | 6.03 (1.65–21.9) | 0.006 | 6.53 (3.00–14.24) | <0.001 |
| Source of bacteremia | ||||||||
| Primary bacteremia | 29 (40.3) | 9 (28.1) | Reference | Reference | Reference | Reference | ||
| Urinary tract infection | 19 (26.4) | 8 (25) | 1.16 (0.45–3) | 0.76 | 1.9 (0.71–5.33) | 0.2 | 2.97 (1.42–6.24) | 0.004 |
| Pneumonia | 24 (33.3) | 15 (46.9) | 1.45 (0.64–3.3) | 0.37 | 2.5 (0.9–6.97) | 0.08 | 4.75 (2.34–9.67) | <0.001 |
| Ward of admission when the bacteremia was diagnosed | ||||||||
| Medical | 24 (33.3) | 5 (15.6) | Reference | Reference | Reference | Reference | ||
| Surgical | 13 (18.1) | 6 (18.8) | 1.86 (0.57–6.11) | 0.3 | 2.04 (0.5–8.9) | 0.34 | 2.10 (0.74–5.97) | 0.17 |
| Critical care | 35 (48.6) | 21 (65.6) | 2.61 (0.98–6.92) | 0.05 | 2.87 (0.99–8.27) | 0.05 | 4.22 (1.97–9.03) | <0.001 |
| Mechanical ventilation | 34 (47.9) | 16 (50) | 1.1 (0.54–2.18) | 0.81 | ||||
| Appropriate empirical therapy | 41 (57.7) | 14 (43.8) | 0.6 (0.28–1.12) | 0.1 | 0.53 (0.26–1.1) | 0.09 | 0.48 (0.26–0.88) | 0.02 |
| Targeted treatment | ||||||||
| Monotherapy | 18 (25) | 14 (43.8) | Reference | Reference | Reference | Reference | Reference | |
| Combination therapy | 54 (75) | 18 (56.2) | 0.52 (0.26–1.05) | 0.07 | 0.81 (0.27–2.42) | 0.71 | 0.62 (0.27–1.41) | 0.25 |
| Interaction of septic shock and combination therapy | 0.14 (0.03–0.65) | 0.01 | 0.10 (0.03–0.32) | <0.001 | ||||
| Propensity score | 1.1 (0.26–4.56) | 0.63 | 0.54 (0.04–6.59) | 0.93 | ||||
Abbreviations: IQR, interquartile range; HR, hazard ratio; CI, confidence interval.
TABLE 3.
Outcome of patients with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance according to treatment regimen
| Treatment regimen | No. dead/treated | Mortality (%) |
|---|---|---|
| Monotherapy | ||
| Tigecycline | 8/15 | 53.3 |
| Gentamicin | 4/9 | 44.4 |
| Fosfomycin | 2/8 | 25 |
| Total for monotherapy | 14/32 | 43.8 |
| Combination therapy | ||
| Tigecycline + gentamicin | 3/13 | 23.1 |
| Tigecycline + fosfomycin | 6/16 | 37.5 |
| Gentamicin + fosfomycin | 3/11 | 27.3 |
| Tigecycline + fosfomycin + gentamicin | 6/32 | 18.8 |
| Total for combination therapy | 18/72 | 25 |
FIG 2.
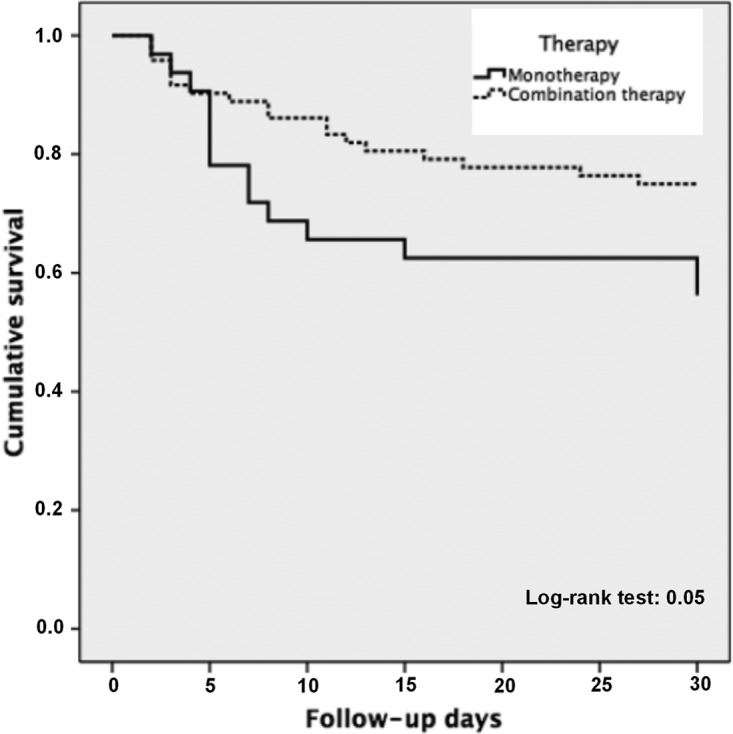
Kaplan-Meier curves for targeted treatment with monotherapy or with combination therapy.
The Kaplan-Meier curves for targeted treatment with monotherapy or with combination therapy in patients with and without septic shock are presented in Fig. 3.
FIG 3.
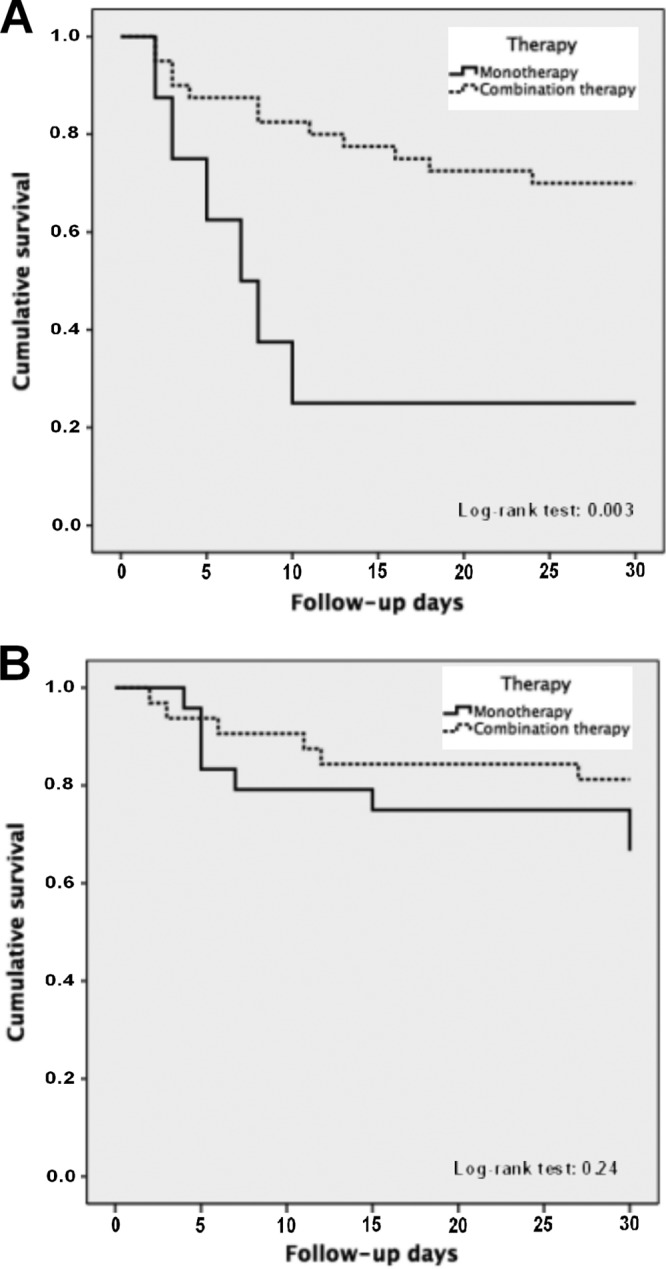
Kaplan-Meier curves for targeted treatment with monotherapy or combination therapy in patients with septic shock (A) and without septic shock (B).
DISCUSSION
The reported mortality among patients with KPC-producing K. pneumoniae bacteremia is very high, ranging from 20 to 70% depending on the prescribed treatment regimen and the underlying severity of disease in the patients (6–8, 10). This series, which includes only colistin-resistant KPC-producing K. pneumoniae with high-level meropenem resistance, confirms these findings (overall crude mortality, 30.8%). No randomized clinical trials published to date have defined the most effective therapeutic regimen for these patients. Current recommendations are based on the results of retrospective cohort studies (4, 6, 10), which suggest that (i) combination therapy is superior to monotherapy and (ii) combination therapy should include a carbapenem. However, recent data suggest that combination therapy is probably not needed in low-risk patients (11) and information to guide therapy for colistin-resistant and high-level meropenem-resistant isolates is lacking. The availability of new drugs such as ceftazidime-avibactam is promising but will probably not solve all the problems (13).
We found that the interaction between combination therapy and septic shock was statistically significant; that means that combination therapy was protective for mortality only among patients with septic shock, but the protective effect could not be proven in patients without septic shock. However, the confidence interval was wide, and therefore, we cannot discard a protective effect that is not apparent due to a presumably high beta error. Of note, appropriate empirical therapy was less frequent among patients receiving monotherapy; however, the effect of this variable was controlled in the multivariate analyses.
As stated, carbapenems were not used in our patients. The use of meropenem has been recommended for strains with moderate resistance to meropenem (MICs of up to 8 mg/liter), for which an optimized dose of meropenem would theoretically allow a high probability of attaining the pharmacodynamics target (4, 6, 10, 14, 15). Tumbarello et al. (6) and Daikos et al. (10) observed that the efficacy of combination regimens including meropenem is lower when the MIC of this antibiotic increases. In these studies, mortality rates were 18.7% and 19.3% when the MIC was <16 mg/liter and 35.2% and 35.9% when the MIC was ≥16 mg/liter, respectively. These data suggest that meropenem would be ineffective when the strain exhibits high-level resistance. In fact, meropenem (2 g every 8 h in extended infusion) did not reach any pharmacokinetic-pharmacodynamic target in 19 patients with infections due to KPC-producing K. pneumoniae with meropenem MICs of >16 mg/liter in a recent study (16). Additionally, a recent study found a higher rate of failure for the carbapenem-colistin combination in patients with KPC-producing K. pneumoniae bacteremia caused by isolates highly resistant to colistin and doripenem (17). Finally, the combination of ertapenem and doripenem was also shown to be efficacious only against isolates with a doripenem MIC of <32 mg/liter (18).
Data about the efficacy of carbapenem-spare regimens are scarce. To the best of our knowledge, two case series with a low number of cases have been reported. Sbrana et al. showed a high clinical response rate (92%) in 22 polytrauma patients (19), and Papadimitriou-Olivgeris et al. (20) observed a 43.4% mortality in 53 patients. The mortality rate observed in combination regimens not including carbapenems found in this study (25%) is more similar to that reported in the literature for strains with low resistance when regimens including carbapenems were used (4, 6, 10). However, the figures might not be comparable despite the similarities in the features of the patients across the studies. Therefore, despite the lack of data, it seems reasonable to avoid the use of carbapenems in infections caused by highly resistant isolates in order to avoid further selection pressure.
Because of the limited therapeutic options, the combined regimens in this series included different combinations of tigecycline, gentamicin, and fosfomycin. The low number of patients in each group precludes elaborate comparisons among them. It is also necessary to determine whether the use of gentamicin in susceptible or intermediate strains improves the prognosis of patients with severe sepsis, as previously reported by our group (21). However, due to the small sample size of this study, we could not analyze this. The most frequent combination in this study was tigecycline and gentamicin (45 patients, 32 of which also received fosfomycin). Mortality in this group was 20%; interestingly, the combination of tigecycline and gentamicin was more efficacious than each of them combined with colistin and meropenem in a murine model (22).
Our article has some limitations. We were unable to compare the efficacy of combination therapy with and without carbapenems and with or without colistin, as these drugs were not used. The study was not randomized, and despite attempts to control for confounders by using a propensity score and multivariate analysis, residual confounding might have occurred. The study was performed in the context of an outbreak. Finally, the sample size, which was constrained by the available cases, is a further limitation of the analysis.
In conclusion, combination regimens were associated with improved outcomes in patients with bacteremia due to KPC-producing K. pneumoniae presenting with septic shock and showing colistin resistance and high-level carbapenem resistance. Further studies are required to determine whether combination therapy is needed in patients without septic shock.
MATERIALS AND METHODS
Study design and patients.
This prospective cohort study includes patients with BSI caused by KPC-producing K. pneumoniae strains belonging to the ST512 clone in the setting of a nosocomial outbreak in a single teaching hospital with 1,233 beds. Approximately 40,000 patients are admitted to the center yearly. The patients were recruited from July 2012 to February 2016. All blood cultures collected at the center were revised daily, and medical infectious disease consultants (I.M., C.N., and J.T.-C.) evaluated the prescribed treatment and followed each case. During the study period, screening cultures were routinely performed in the intensive care unit (ICU) and the hematology unit (weekly rectal swab sampling). The analyses were performed following STROBE recommendations (23) (data not shown).
Patients were required to meet the following inclusion criteria: (i) age, ≥18 years; (ii) episode of clinically significant bacteremia due to colistin-resistant KPC-producing K. pneumoniae with high-level meropenem resistance (see below); (iii) targeted treatment initiated in the first 72 h after blood cultures were taken, which included at least 1 active antibiotic in vitro. Patients with polymicrobial bacteremia or with an intra-abdominal source (which are usually polymicrobial), patients who survived <48 h after initiating active antibiotic treatment, those under palliative care or with nonresuscitation orders, and previously included patients who suffered subsequent episodes were excluded from this study. Bacteremia due to KPC-producing K. pneumoniae was defined as the presence of at least one set of positive blood cultures for colistin-resistant KPC-producing K. pneumoniae with high-level meropenem resistance in patients with evidence of systemic inflammatory response. The day of onset of infection (day 0) was defined as the day of collection of the blood culture in which the microorganism was isolated.
The study was approved by the Spanish Agency for Medicines and Health Products (AEMPS, code FIC-KPC-2015-01) and by the Ethics Committees of the participating hospitals (code 2848), which exempted the need to seek written informed consent due to the observational nature of the study. All the data collected were anonymized.
Variables.
The main outcome variable was crude mortality at 30 days following the diagnosis of bacteremia.
The variables collected for each patient were the following: sex; age; underlying chronic diseases; disease severity measured by the Charlson comorbidity index (24); admission from a care facility; prior known KPC-producing K. pneumoniae colonization; admission in the previous 3 months; surgery in the previous week; invasive procedures performed in the week prior to the diagnosis of infection (need for mechanical ventilation, use of central venous catheter, urinary catheter); ward of admission when the bacteremia was diagnosed (medical, surgical, or intensive care); presence of renal failure at the time of diagnosis of infection; source of bacteremia (pneumonia or urinary or primary bacteremia); overall length of hospital stay; presentation with septic shock (25); Pitt bacteremia score (26); appropriate empirical therapy, targeted monotherapy, or targeted combination therapy; and antimicrobial susceptibility.
Treatment regimens.
In vitro-active antibiotics were included in the therapeutic regimen and selected according to the clinical judgment of the treating physician. Patients whose therapeutic regimen included a single in vitro-active drug were considered to be undergoing monotherapy. Patients whose regimen included 2 or 3 in vitro-active drugs were considered to be undergoing combination therapy. In patients with nosocomial pneumonia or septic shock, tigecycline was administered at a loading dose of 200 mg followed by 100 mg every 12 h. In all other cases, a loading dose of 100 mg followed by 50 mg every 12 h was administered. Gentamicin was given intravenously in a single daily dose of 5 mg/kg of body weight/day, and the dose was adjusted according to concentrations in blood. Fosfomycin was administered at an intravenous dose of 4 g every 6 h and corrected according to renal function. The duration of treatment ranged from 10 to 14 days upon judgment of the attending physician.
Definitions.
The definitions were established prior to the data collection and statistical analysis. Crude mortality was defined as death by any cause. The strain was considered to have high-level meropenem resistance when the MIC was ≥64 mg/liter. The source of bacteremia was defined according to the criteria of the Centers for Disease Control and Prevention (CDC/NHS) for nosocomial infections (27) irrespective of the acquisition type. Infection was considered nosocomial when the index blood culture was collected >48 h after admission and there was no clinical evidence of infection at the time of admission. The rest were classified as health care- or community-associated infections in accordance with previous definitions (28). Primary bacteremia was defined as any catheter-related bacteremia and bacteremia of unknown source. Septic shock was defined according to the most recent criteria when the study began in 2012 (29). The Cockroft-Gault formula was used to calculate creatinine clearance (CLCR). The presence of a CLCR of <60 ml/min was considered renal failure.
Empirical therapy was defined as treatment administered within the first 24 h following the collection of blood cultures and prior to determining the susceptibility of the isolate. Empirical therapy was considered appropriate when the isolate was susceptible in vitro to at least one of the prescribed antibiotics. Treatment that was initiated or maintained after receiving the susceptibility results was considered targeted therapy. A targeted antibiotic treatment regimen was considered active when including at least one antibiotic to which the isolate was susceptible in vitro (for gentamicin, intermediate isolates were also considered; see below). To classify patients as receiving a specific regimen, the regimen should have been initiated in the first 72 h following the index blood culture and maintained for at least 50% of the duration of treatment (or at least 48 h if the patient died before) in order to guarantee a minimum exposure time to the regimen.
Microbiological variables and antibiotic susceptibility studies.
Blood cultures were performed using the Bactec 9240 automatic blood culture detection system (Becton Dickinson, USA). Blood culture bottles flagged as positive and with Gram-negative stains were processed as follows: (i) direct inoculation of microorganisms from positive-blood-culture bottles into the matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) system for identification combined with inoculation in chromogenic media (bioMérieux) and/or PCR (GeneXpert Carba-R; Cepheid, USA) for carbapenemase detection; (ii) standard culture involving overnight agar medium subcultures from the positive-blood-culture bottles for identification using standard microbiological techniques and antibiotic susceptibility testing by microdilution using the Gram-negative REV.2 Wider panel (Siemens Healthcare Diagnostics, Camberley, UK) or Etest strips when needed (Liofilmchem, Italy). Colistin susceptibility was performed by microdilution and was checked, in selected strains, using a broth dilution method. Those selected strains yielded a negative screening for mcr-1 and mcr-2 genes using PCR with specific primers. MICs were interpreted following the breakpoint recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (30) and the U.S. Food and Drug Administration (FDA) recommendations for tigecycline. Meropenem was not considered active against any isolate because the MIC was ≥64 mg/liter in all cases. Colistin was not considered active treatment because the MIC was always ≥2 mg/liter. Tigecycline, gentamicin, and fosfomycin were considered active when the MIC was ≤2 mg/liter, ≤4 mg/liter, and ≤32 mg/liter, respectively.
The K. pneumoniae index isolates in this outbreak were characterized as belonging to the ST512 clone by the reference laboratory of the Virgen Macarena University Hospital of Seville, Seville, Spain. The characteristics of the strain have been previously reported (31).
Statistical analysis.
The results were expressed as medians and interquartile ranges (IQR) for the continuous variables or as percentages for the categorical variables. Comparisons for continuous variables were performed using the Mann-Whitney U test; for categorical variables, the Pearson's χ2 test with Yates' continuity correction or Fisher's exact test was used as appropriate. Survival curves were obtained using the Kaplan-Meier method and compared using the log rank test. A propensity score for receiving active combination treatment was calculated using a multivariate logistic regression analysis that included the following variables: age, sex, prior known colonization, admission from a care facility, hospital admission in the previous 3 months, surgery in the previous week, invasive procedures in the previous week (mechanical ventilation, central venous catheter, urinary catheter), admissions unit following diagnosis (medical, surgical, ICU), focus of infection (pneumonia, primary bacteremia, urinary tract infection), septic shock, appropriate empirical therapy, Charlson comorbidity index, Pitt score on the day of bacteremia, and renal failure at time of diagnosis of infection. The model obtained had an area under the receiver operating characteristic (ROC) curve of 0.90. The propensity score was used in 2 ways: (i) as a covariate in multivariate Cox regression analysis and (ii) to form an inverse probability of treatment weighting (IPTW) cohort.
The variables associated with mortality were studied using the Cox regression. The scale of the continuous variables was assessed using the Box-Tidwell test. Possible interactions between variables were studied. Variables with a P value of <0.05 were studied as potential confounders and considered to be confounders if the percentage change in the coefficients was greater than 20%. To assess the goodness of fit of the model, the likelihood ratio test was performed. The condition of proportional hazards was verified by the Kleinbaum-Klein method. Analyses were performed using R software (version 3.0.1) and SPSS 15.0 (SPSS Inc.).
ACKNOWLEDGMENTS
This study was funded by Planes Nacionales de I+D+i 2008-2011 and 2013-2016, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0010 and REIPI RD16/0016/0001; RD16/0016/0001; RD16/00167008) and cofinanced by European Development Regional Fund “A way to achieve Europe” and operative program Intelligent Growth 2014-2020.
I.M., B.G.-G., I.G.-A., A.C., E.P.-N., C.N., J.J.C., F.R.-L., J.R.-B., and J.T.-C. are members of the Spanish Network for Research in Infectious Diseases (REIPI).
Conflict of interest: J.R.-B. served as scientific advisor for a research project for AstraZeneca, Pfizer, and InfectoPharm and has been a speaker in unrestricted accredited educational activities funded by Merck. All other authors declare no conflict of interest.
REFERENCES
- 1.Endimiani A, Depasquale JM, Forero S, Perez F, Hujer AM, Roberts-Pollack D, Fiorella PD, Pickens N, Kitchel B, Casiano-Colon AE, Tenover FC, Bonomo RA. 2009. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J Antimicrob Chemother 64:1102–1110. doi: 10.1093/jac/dkp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuner EA, Yeh JY, Hall GS, Sekeres J, Endimiani A, Bonomo RA, Shrestha NK, Fraser TG, van Duin D. 2011. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 69:357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira S, Yousef B, Smolykov R, Codish S, Borer A. 2012. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol 33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 7.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Bano J, Cisneros JM, Cobos-Trigueros N, Fresco G, Navarro-San Francisco C, Gudiol C, Horcajada JP, Lopez-Cerero L, Martinez JA, Molina J, Montero M, Pano-Pardo JR, Pascual A, Pena C, Pintado V, Retamar P, Tomas M, Borges-Sa M, Garnacho-Montero J, Bou G, Study Group of Nosocomial Infections of the Spanish Society of Infectious Diseases. 2015. Diagnosis and antimicrobial treatment of invasive infections due to multidrug-resistant Enterobacteriaceae. Guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology. Enferm Infecc Microbiol Clin 33:337.e1–337.e21. doi: 10.1016/j.eimc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez-Gutierrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Pano-Pardo JR, Venditti M, Tumbarello M, Daikos G, Canton R, Doi Y, Tuon FF, Karaiskos I, Perez-Nadales E, Schwaber MJ, Azap OK, Souli M, Roilides E, Pournaras S, Akova M, Perez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodriguez-Bano J, REIPI/ESGBIS/INCREMENT Investigators. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 12.Giani T, Arena F, Vaggelli G, Conte V, Chiarelli A, Henrici De Angelis L, Fornaini R, Grazzini M, Niccolini F, Pecile P, Rossolini GM. 2015. Large nosocomial outbreak of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae traced to clonal expansion of an mgrB deletion mutant. J Clin Microbiol 53:3341–3344. doi: 10.1128/JCM.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 15.Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. 2012. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect 18:439–448. doi: 10.1111/j.1469-0691.2012.03823.x. [DOI] [PubMed] [Google Scholar]
- 16.Del Bono V, Giacobbe DR, Marchese A, Parisini A, Fucile C, Coppo E, Marini V, Arena A, Molin A, Martelli A, Gratarola A, Viscoli C, Pelosi P, Mattioli F. 2017. Meropenem for treating KPC-producing Klebsiella pneumoniae bloodstream infections: should we get to the PK/PD root of the paradox? Virulence 8:66–73. doi: 10.1080/21505594.2016.1213476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields RK, Nguyen MH, Potoski BA, Press EG, Chen L, Kreiswirth BN, Clarke LG, Eschenauer GA, Clancy CJ. 2015. Doripenem MICs and ompK36 porin genotypes of sequence type 258, KPC-producing Klebsiella pneumoniae may predict responses to carbapenem-colistin combination therapy among patients with bacteremia. Antimicrob Agents Chemother 59:1797–1801. doi: 10.1128/AAC.03894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiskirchen DE, Crandon JL, Nicolau DP. 2013. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int J Antimicrob Agents 41:582–585. doi: 10.1016/j.ijantimicag.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Sbrana F, Malacarne P, Viaggi B, Costanzo S, Leonetti P, Leonildi A, Casini B, Tascini C, Menichetti F. 2013. Carbapenem-sparing antibiotic regimens for infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit. Clin Infect Dis 56:697–700. doi: 10.1093/cid/cis969. [DOI] [PubMed] [Google Scholar]
- 20.Papadimitriou-Olivgeris M, Marangos M, Christofidou M, Fligou F, Bartzavali C, Panteli ES, Vamvakopoulou S, Filos KS, Anastassiou ED. 2014. Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand J Infect Dis 46:642–648. doi: 10.3109/00365548.2014.923106. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, Lopez-Cerero L, Pascual A, Natera C, Rodriguez M, Salcedo I, Rodriguez-Lopez F, Rivero A, Rodriguez-Bano J. 2015. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 22.Michail G, Labrou M, Pitiriga V, Manousaka S, Sakellaridis N, Tsakris A, Pournaras S. 2013. Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 57:6028–6033. doi: 10.1128/AAC.00891-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. 1989. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 87:540–546. doi: 10.1016/S0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 27.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 30.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. EUCAST definitive document E.DEF 3.1, June 2000: determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect 6:509–515. doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Cerero L, Egea P, Gracia-Ahufinger I, Gonzalez-Padilla M, Rodriguez-Lopez F, Rodriguez-Bano J, Pascual A. 2014. Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneumoniae (ST512) in Spain. Int J Antimicrob Agents 44:538–540. doi: 10.1016/j.ijantimicag.2014.08.006. [DOI] [PubMed] [Google Scholar]