ABSTRACT
We explored if baseline CD4/CD8 T-cell ratio is associated with immunodiscordant response to antiretroviral therapy in HIV-infected subjects. Comparing immunodiscordant and immunoconcordant subjects matched by pretreatment CD4 counts, we observed a lower pretreatment CD4/CD8 T-cell ratio in immunodiscordant subjects. Furthermore, pretreatment CD4/CD8 T-cell ratio, but not CD4 counts, correlated with the main immunological alterations observed in immunodiscordants, including increased regulatory T-cell (Treg) frequency and T-cell turnover-related markers. Then, in a larger cohort, only baseline CD4/CD8 T-cell ratio was independently associated with immunodiscordance, after adjusting by the viral CXCR4-tropic HIV variants. Our results suggest that the CD4/CD8 T-cell ratio could be an accurate biomarker of the subjacent immunological damage triggering immunodiscordance.
KEYWORDS: CD4/CD8 T-cell ratio, delayed cART, low CD4 recovery, T-cell turnover, thymic function, CD4/CD8 ratio, antiretroviral therapy, human immunodeficiency virus, immunodiscordant
INTRODUCTION
Approximately one out of four HIV-infected subjects with delayed combination antiretroviral therapy (cART) initiation persistently maintains low CD4 counts despite suppressive cART (subjects immunodiscordant to cART) and shows increased risk of non-AIDS-related events and mortality (1, 2). Traditional associated risk factors for such anomalous immune responses to cART include age, male sex, low CD4 nadir, injection drug use, and hepatitis C virus (HCV) coinfection (reviewed in references 3 and 4). More recently, a predictive value for baseline CXCR4-tropic HIV variants has also been proposed for both the low CD4 recovery (5) and the emergence of non-AIDS-related events (6).
The HIV infection leads to an inversion of the CD4/CD8 T-cell ratio (7), and globally, the CD4/CD8 T-cell ratio has been associated with clinical progression, independently of CD4 counts (7–9). However, whereas a low baseline CD4 count is undoubtedly associated with immunodiscordance, a potential different meaning of the baseline CD4/CD8 T-cell ratio has not yet been explored. Nevertheless, we have recently reported that CD4/CD8 T-cell ratio is associated with thymic function in antiretroviral-naive subjects (10), and a low thymic function is a possible trigger of immunodiscordance (11). Thus, our objective was to analyze if the baseline CD4/CD8 T-cell ratio is associated with immunodiscordant response to cART.
RESULTS
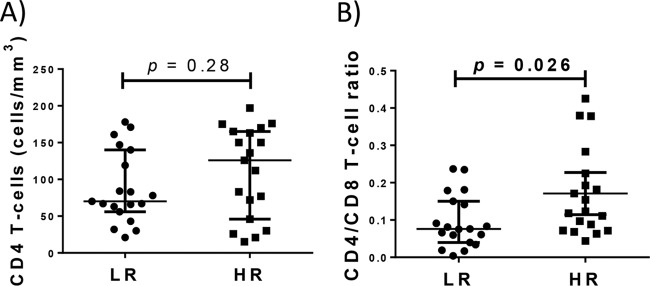
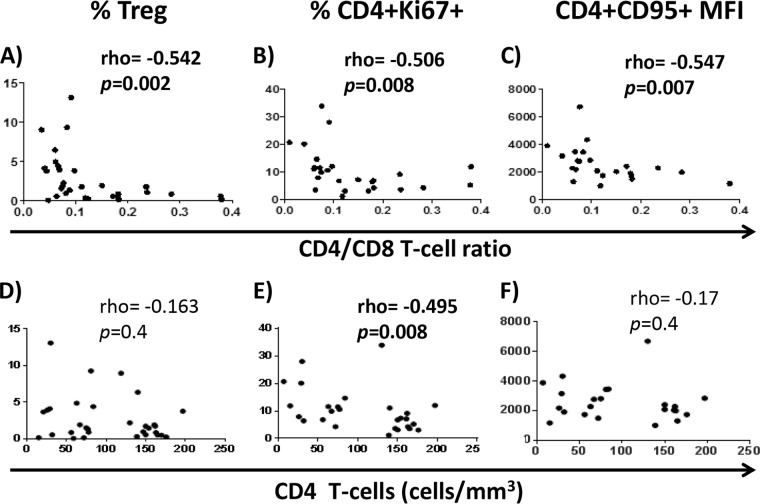
We have analyzed pre-cART samples of HIV-infected subjects who had started cART with <200 CD4 T cells/mm3 and, after 24 months of successful therapy, did not reach 250 CD4 T cells/mm3 (low-CD4-recovery [LR] subjects) or overcame this threshold (high-CD4-recovery [HR] subjects). We first compared the CD4/CD8 T-cell ratio in pre-cART samples of LR and HR subjects from a previous study where both groups had been frequency matched by sex, age, baseline CD4 counts, and viral load (12). Data for CD8 counts were not available from the source cohort (CoRIS), but CD8 and CD4 T-cell frequencies had been determined by flow cytometry in most samples (19 LR and 19 HR subjects), and such frequencies were used to calculate the CD4/CD8 T-cell ratio. Interestingly, despite similar baseline CD4 T-cell absolute counts between LR subjects and HR subjects, baseline CD4/CD8 T-cell ratio was lower in LR subjects (Fig. 1A and B, respectively; see also Table S1 in the supplemental material). Moreover, the CD4/CD8 T-cell ratio was strongly associated inversely with the T-regulatory cell (Treg) frequency, the percent CD4+ Ki67+ cells, and the expression of CD95 (mean fluorescence intensity) on CD4 T cells (Fig. 2A to C). However, only the percent CD4+ Ki67+ cells was associated with CD4 counts (Fig. 2D to F).
FIG 1.
CD4 T-cell counts and CD4/CD8 T-cell ratios in matched LR and HR subjects. Despite similar baseline CD4 T-cell absolute counts between LR subjects and HR subjects (A), baseline CD4/CD8 T-cell ratio was lower in LR subjects (B). The Mann-Whitney U test was used for comparison.
FIG 2.
Correlations between CD4/CD8 T-cell ratio or CD4 T-cell counts and different immunological parameters in subjects from the case-control study. Correlations between CD4/CD8 T-cell ratio or CD4 counts and the percentage of Tregs (A and D, respectively), the percentage of CD4 T cells expressing Ki67 (B and E, respectively), and the mean fluorescence intensity (MFI) of CD95 expression on CD4 T cells (C and F, respectively) are shown. The Spearman rank test was used for correlations.
In a subgroup of these matched LR and HR subjects, we could also determine the HIV tropism (16 LR versus 17 HR), and we observed a trend of higher frequency of CXCR4-tropic HIV variants in LR subjects (31.3% versus 11.8%, respectively; P = 0.17). To better address the associations between baseline CD4/CD8 T-cell ratio and viral tropism with immunodiscordance, we extended our analysis to include more subjects. Thus, an additional 58 subjects (12 LR and 46 HR), with available plasma samples before cART initiation, were included. After assembling the two groups of LR and HR subjects, we finally analyzed a total of 91 subjects (28 LR and 63 HR), and their baseline characteristics are shown in Table 1. Of note, the CD4/CD8 T-cell ratios from the two groups of subjects (the initial matched group and the additional subjects) were comparable despite different calculations (Fig. S1a and b).
TABLE 1.
Predictive value of baseline clinical and demographical variables for the low-CD4-recovery subjects despite suppressive cART
| Variable | Subject group (n)g |
Odds ratio (95% CI); Ph |
|||
|---|---|---|---|---|---|
| LR (28) | HR (63) | Univariate analysis | Multivariate model 1e | Multivariate model 2f | |
| Male sex, no./total no. (%) | 25/28 (89) | 56/63 (89) | 1.04 (0.25–4.36); 0.9 | ||
| Age (yr), median (IQR) | 42 (33–49) | 37 (30–48) | 0.97 (0.94–1.02); 0.2 | ||
| CD4 count (cells/μl), median (IQR) | 68 (32–126) | 121 (47–165) | 1.01 (1.00–1.02); 0.022 | 1.01 (1.00–1.02); 0.065 | |
| CD8 count (cells/μl),a median (IQR) | 631 (301–872) | 529 (317–660) | 1.00 (0.99–1.00); 0.7 | ||
| CD4/CD8 T-cell ratio,b median (IQR) | 0.08 (0.05–0.18) | 0.16 (0.10–0.30) | 2.12 (1.25–3.59); 0.005 | 1.94 (1.14–3.29); 0.014 | |
| Viral load (log HIV RNA copies/ml), median (IQR) | 4.8 (4.2–5.3) | 4.9 (4.7–5.4) | 1.42 (0.84–2.42); 0.2 | ||
| Time to HIV diagnosis (mo), median (IQR) | 1 (0–16) | 2 (0–21) | 1.00 (0.99–1.01); 0.8 | ||
| Injection drug use, no./total no. (%) | 4/28 (14) | 12/63 (19) | 0.71 (0.21–2.43); 0.6 | ||
| Previous stage C event, no./total no. (%)c | 9/28 (32) | 21/63 (33) | 0.95 (0.37–2.45); 0.9 | ||
| Anti-HCV+, no./total no. (%) | 6/27 (22) | 9/61 (14) | 1.68 (0.53–5.31); 0.4 | ||
| Anti-HBV+, no./total no. (%)d | 4/24 (17) | 7/61 (12) | 1.54 (0.41–5.84); 0.5 | ||
| CXCR4-tropic HIV variants, no./total no. (%) | 10/28 (36) | 11/63 (17) | 2.63 (0.95–7.21); 0.061 | 2.24 (0.76–6.60); 0.15 | 1.71 (0.57–5.07); 0.3 |
| PI-containing regimen, no./total no. (%) | 17/28 (54) | 22/63 (35) | 2.15 (0.87–5.32); 0.097 | 2.20 (0.85–5.70); 0.11 | 2.13 (0.81–5.66); 0.14 |
| Highly toxic NRTI-containing regimen, no./total no. (%)i | 2/28 (7) | 2/28 (3) | 2.35 (0.31–17.56); 0.4 | ||
Data available from only 58 subjects.
In 58 subjects, the CD4/CD8 T-cell ratio was calculated from the absolute T-cell counts, whereas in 33 subjects, it was calculated from the flow cytometry immunophenotyping; CD4/CD8 T-cell ratio values were multiplied by 10 for univariate and multivariate analyses to minimize the range of the 95% CI.
CDC stage classification. Hepatitis C virus (HCV) serology data were available from 88 subjects (27 LR subjects and 61 HR subjects).
Data available from only 85 subjects.
Model 1 included CD4 counts and PI-containing regimen and CXCR4-tropic HIV variants.
Model 2 included CD4/CD8 T-cell ratio and PI-containing regimen and CXCR4-tropic HIV variants.
Continuous variables are expressed as median value (IQR), and categorical variables are expressed as number of cases (%).
Italic or boldface P values indicate trends or statistical significance, respectively.
Zidovudine (AZT), stavudine (d4T), didanosine (ddi), and zalcitabine (ddc).
Comparing all LR and HR subjects, lower baseline CD4 counts and CD4/CD8 T-cell ratio but a trend of higher frequency of CXCR4-tropic HIV variants and of subjects exposed to protease inhibitor (PI)-containing regimens were found in LR subjects. Two alternative multivariate model analyses were performed due to the colinearity between CD4 counts and CD4/CD8 T-cell ratio (Table 1). Thus, in the adjusted model including baseline CD4 counts, this variable showed a trend of independent association, whereas in the alternative model, the baseline CD4/CD8 T-cell ratio was independently associated with the poor CD4 recovery. In both models, frequencies of CXCR4-tropic HIV variants and PI-containing regimen exposure were displaced.
DISCUSSION
We report here that a lower baseline CD4/CD8 T-cell ratio characterizes subjects with delayed cART initiation, who will undergo low CD4 recovery despite suppressive cART. Adjusted analysis revealed a predictive value for the baseline CD4/CD8 T-cell ratio. Furthermore, baseline CD4/CD8 T-cell ratio, more than baseline CD4 count, was inversely associated with CD4 T-cell turnover-related markers and the frequency of Tregs.
To the best of our knowledge, this is the first time that the baseline CD4/CD8 T-cell ratio has been analyzed as a potential risk factor for immunodiscordant response to cART. Our data interconnect recent findings regarding the higher risk of morbidity and mortality associated with both low-CD4-recovery subjects (1, 2) and low-CD4/CD8-T-cell-ratio subjects (7, 8). More importantly, although the CD8 T-cell pool has not been classically considered relevant in this scenario, our data suggest that homeostasis of the CD8 T-cell pool, probably interacts with the homeostasis of CD4 T cells, also contributing to this scenario. Indeed, Casetti et al. have recently described a role of MIP1α-producing CD8 T cells in the compromised immune reconstitution after cART (13).
The subjacent meaning of the CD4/CD8 T-cell ratio in this scenario is unknown. We have recently reported a positive association between the baseline CD4/CD8 T-cell ratio and the thymic function in antiretroviral-naive HIV-infected subjects (10). Such an association would gain particular relevance in the context of immunodiscordance to cART, where a lower thymic function could be a primary immunological mechanism (11). This hypothesis still needs to be demonstrated but is also supported by the strong inverse associations between CD4/CD8 T-cell ratio and turnover-related markers (Ki67 and CD95) as well as the Treg frequency. Note that a CD4/CD8 T-cell ratio lower than 0.1 is associated with an abrupt increase in Treg frequency, and consistently, we have reported a higher Treg frequency and T-cell turnover in LR subjects before cART instauration (12).
Classical studies on immunodiscordance to cART suggested a deleterious effect of CXCR4-tropic HIV variants on CD4 recovery (14). Accordingly, we also found a higher baseline of CXCR4-tropic HIV variants in low-CD4-recovery subjects. However, in the adjusted analyses, the baseline CD4/CD8 T-cell ratio was the only variable independently associated with immunodiscordant responses to cART. This suggests that CD4/CD8 T-cell ratio could reflect immunological damage, probably involving a lower thymic function and an enhanced T-cell turnover, which could eventually favor the emergence of CXCR4-tropic HIV variants. In accordance, the switch from CCR5-tropic to CXCR4-tropic HIV variants has been proposed to occur in the context of enhanced cellular turnover of CD4 T cells (15) and has also been demonstrated to occur in macaques infected with simian immunodeficiency virus (SIV) CCR5-tropic variants during their progression (16).
Future research focused on our findings could help in early diagnosis of immunodiscordants to therapy among late presenters. However, as noted, CD4/CD8 T-cell ratios were very low even in immunoconcordant subjects, which could make it difficult to identify a clinically relevant threshold. Classical risk factors, such as male sex or older age, associated with immunodiscordant response to cART were not observed in our cohort, probably due to the inclusion of a group of LR and HR subjects matched by these and other variables. This fact prevented us from studying the potential contribution of such risk factors to the effect of the CD4/CD8 T-cell ratio on the immunodiscordance phenomenon. On the other hand, we did not have available data of CD8 counts from a subgroup of subjects, but the CD4/CD8 T-cell ratio calculated by using immunophenotyping data proved to be equivalent to the values calculated by using absolute T-cell numbers.
In summary, our study indicates that the baseline CD4/CD8 T-cell ratio is a potent risk factor, beyond the CD4 counts, associated with immunodiscordance among subjects with delayed cART initiation. Additionally, the CD4/CD8 T-cell ratio seems to reflect, better than the CD4 counts, the deep immunological damage of the T-cell homeostasis triggering such an anomalous response to cART.
MATERIALS AND METHODS
Subjects and study design.
We selected pre-cART samples of LR subjects and HR subjects from two different sources: (i) from a previous case-control study (12) with LR and HR subjects from CoRIS-BioBank (see the supplemental material for details) and (ii) consecutive LR and HR subjects visiting the Infectious Diseases Service of Virgen del Rocío University Hospital. First, we included LR and HR subjects from a previous study (12) where both groups had been frequency matched by sex, age, baseline CD4 counts, and viral load. For these subjects, peripheral blood mononuclear cell (PBMC) samples were available and flow cytometry had been performed for immunophenotyping, including CD4, CD8, Ki67, CD95, and Treg cells (see the supplemental material for details). Additionally, for those with available plasma samples, the HIV tropism was determined. Second, consecutive LR and HR subjects were additionally selected from the Infectious Diseases Service of the Virgen del Rocío Hospital (see the supplemental material for details) including those fulfilling criteria for definitions and having available plasma samples for the determination of the HIV tropism.
Factors predictive of becoming an LR subject were analyzed among baseline data after assembling both groups of LR and HR subjects. Thus, baseline clinical and demographic data, including age, sex, CD4 and CD8 count and ratio, viral load, HCV and HBV coinfection, time from HIV diagnosis, transmission route, and previous AIDS diagnosis were considered. Baseline HIV tropism was also included, analyzed by a genotypic method (geno2pheno; see the supplemental material for details). We also analyzed different types of antiretroviral treatment, such as protease inhibitor (PI) exposure and highly toxic nucleotide analogue (nucleoside reverse transcriptase inhibitor [NRTI]) exposure (including zidovudine [AZT], stavudine [d4T], didanosine [ddi], and zalcitabine [ddc]). Written informed consent had been obtained for every patient at the patient's entry in the cohorts.
Statistical analysis.
Data are expressed as median and interquartile range (IQR) and numbers and percentages as required. Following univariate analyses, multivariate logistic regression was fitted to evaluate the adjusted effect of baseline variables on becoming an LR subject. Variables presenting P values of <0.15 in the univariate analysis were introduced into the multivariate analysis. Odds ratios with 95% confidence intervals (CIs) are shown. A P value of <0.05 was considered statistically significant. The statistical analysis was performed and graphs were made using the Statistical Package for the Social Sciences software (SPSS 21.0; USA) and Prism version 5.0 (GraphPad Software, USA), respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Mar Rodriguez, Marta de Luna, and the Cytometry Service of IBiS, especially M. José Castro, for their technical assistance and Juan Manuel Praena for statistical assistance. We also acknowledge the HIV BioBank integrated in the Spanish AIDS Research Network and collaborating centers for the generous gifts of clinical samples used in this work. This study would not have been possible without the collaboration of all the patients, medical and nursing staff, and data managers who have taken part in the project.
The HIV BioBank, integrated in the Spanish AIDS Research Network, is supported by Instituto de Salud Carlos III, Spanish Health Ministry (grants RD06/0006/0035 and RD12/0017/0037), as part of the Plan Nacional R + D + I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER) and Fundación para la investigación y prevención del SIDA en España (FIPSE). The RIS Cohort (CoRIS) is funded by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en SIDA by the RD12/0017/0018 project, as part of the Plan Nacional R + D + I, and cofinanced by ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER). The project number is RIS_EPICLIN 20/2015. This work was supported by grants from the Fondo de Investigación Sanitaria, ISCIII (FIS; PI14/01693), and the Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía (Proyecto de Investigación de Excelencia; CTS2593), and cofunded by Fondos Europeos para el Desarrollo Regional (FEDER). I. Rosado-Sánchez was supported by the Spanish AIDS Research Network of Excellence (RIS; RD12/0017/0029). Y. M. Pacheco was supported by the Fondo de Investigación Sanitaria through the Miguel Servet program (CPII13/00037) and by the Servicio Andaluz de Salud through the Nicolás Monardes program (C-0010/13).
F.P., J.G.-G., M.M., and E.B.-M. worked on behalf of CoRIS and the HIV BioBank integrated in the Spanish AIDS Research Network. See the supplemental material for a list of clinical centers and individuals contributing to the HIV BioBank and CoRIS.
We declare no conflicts of interest.
I.R.-S. contributed to the study design, to the analysis and interpretation of data, and to the writing and performed flow cytometry. I.H.-F., A.I.Á.-R., and M.G. contributed to the flow cytometry and/or to the collection of data. M.A.A.-C. and E.R.-M. contributed to the viral tropism determinations. F.P., J.G.-G., M.M., and E.B.M. represent the contribution to sample and data collection by RIS BB/CoRIS. F.V. contributed to data interpretation and discussion. M.L. and Y.M.P. contributed to the study design and to the analysis and interpretation of data. Y.M.P. conceived the study and drafted the manuscript. All the authors critically revised the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00605-17.
REFERENCES
- 1.Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, Rivero M, Muñoz-Medina L, Bernal-Morell E, Gutiérrez F, Leal M, CoRIS. 2015. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res 117:69–74. doi: 10.1016/j.antiviral.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, Porter K, Sabin C, Riordan A, Fätkenheuer G, Gutiérrez F, Raffi F, Kirk O, Mary-Krause M, Stephan C, de Olalla PG, Guest J, Samji H, Castagna A, d'Arminio Monforte A, Skaletz-Rorowski A, Ramos J, Lapadula G, Mussini C, Force L, Meyer L, Lampe F, Boufassa F, Bucher HC, De Wit S, Burkholder GA, Teira R, Justice AC, Sterling TR, Crane MH, Gerstoft J, Grarup J, May M, Chêne G, Ingle SM, Sterne J, Obel N, Antiretroviral Therapy Cohort Collaboration (ART-CC), Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. 2014. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 58:1312–1321. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. 2012. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012:670957. doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massanella M, Negredo E, Clotet B, Blanco J. 2013. Immunodiscordant responses to HAART—mechanisms and consequences. Expert Rev Clin Immunol 9:1135–1149. doi: 10.1586/1744666X.2013.842897. [DOI] [PubMed] [Google Scholar]
- 5.Casadellà M, Manzardo C, Noguera-Julian M, Ferrer E, Domingo P, Pérez-Álvarez S, Podzamczer D, Plana M, Clotet B, Gatell JM, Miró JM, Paredes R, ADVANZ and ADVANZ-3 Investigators. 2015. Clinical value of ultradeep HIV-1 genotyping and tropism testing in late presenters with advanced disease. AIDS 29:1493–1504. doi: 10.1097/QAD.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 6.Maffongelli G, Alteri C, Gentilotti E, Bertoli A, Ricciardi A, Malagnino V, Svicher V, Santoro MM, Dori L, Perno CF, Andreoni M, Sarmati L. 2016. Impact of HIV-1 tropism on the emergence of non-AIDS events in HIV-infected patients receiving fully suppressive antiretroviral therapy. AIDS 30:731–741. doi: 10.1097/QAD.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrano-Villar S, Pérez-Elías MJ, Dronda F, Casado JL, Moreno A, Royuela A, Pérez-Molina JA, Sainz T, Navas E, Hermida JM, Quereda C, Moreno S. 2014. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 9:e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S, Deeks SG. 2014. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilho JL, Shepherd BE, Koethe J, Turner M, Bebawy S, Logan J, Rogers WB, Raffanti S, Sterling TR. 2016. CD4/CD8 ratio, age, and risk of serious non-communicable diseases in HIV-infected adults on antiretroviral therapy. AIDS 30:899–908. doi: 10.1097/QAD.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosado-Sánchez I, Herrero-Fernández I, Genebat M, Ruiz-Mateos E, Leal M, Pacheco YM. 2017. Thymic function impacts the peripheral CD4/CD8 ratio of HIV-infected subjects. Clin Infect Dis 64:152–158. doi: 10.1093/cid/ciw711. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Pinelo S, Vallejo A, Díaz L, Soriano-Sarabia N, Ferrando-Martínez S, Resino S, Muñoz-Fernández MA, Leal M. 2009. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother 64:579–588. doi: 10.1093/jac/dkp248. [DOI] [PubMed] [Google Scholar]
- 12.Rosado-Sánchez I, Jarrín I, Pozo-Balado MM, de Pablo-Bernal RS, Herrero-Fernández I, Rodríguez-Gallego E, Genebat M, Vera M, Berenguer J, Martín ML, Bernal E, Vidal F, Blanco J, Leal M, Pacheco YM. 2017. Higher levels of IL-6, CD4 turnover and Treg frequency are already present before cART in HIV-infected subjects with later low CD4 recovery. Antiviral Res 142:76–82. doi: 10.1016/j.antiviral.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Casetti R, Pinnetti C, Sacchi A, De Simone G, Bordoni V, Cimini E, Tumino N, Besi F, Viola D, Turchi F, Mazzotta V, Antinori A, Martini F, Ammassari A, Agrati C. 2017. HIV-specific CD8 T cells producing CCL-4 are associated with worse immune reconstitution during chronic infection. J Acquir Immune Defic Syndr doi: 10.1097/QAI.0000000000001392. [DOI] [PubMed] [Google Scholar]
- 14.Delobel P, Nugeyre MT, Cazabat M, Sandres-Sauné K, Pasquier C, Cuzin L, Marchou B, Massip P, Cheynier R, Barré-Sinoussi F, Izopet J, Israël N. 2006. Naïve T-cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. J Virol 80:10229–10236. doi: 10.1128/JVI.00965-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro RM, Hazenberg MD, Perelson AS, Davenport MP. 2006. Naïve and memory cell turnover as drivers of CCR5-to-CXCR4 tropism switch in human immunodeficiency virus type 1: implications for therapy. J Virol 80:802–809. doi: 10.1128/JVI.80.2.802-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho SH, Tasca S, Shek L, Li A, Gettie A, Blanchard J, Boden D, Cheng-Mayer C. 2007. Coreceptor switch in R5-tropic simian/human immunodeficiency virus-infected macaques. J Virol 81:8621–8633. doi: 10.1128/JVI.00759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.