ABSTRACT
Pyrazinamide (PZA) is a critical drug used for the treatment of tuberculosis (TB). PZA is a prodrug that requires conversion to the active component pyrazinoic acid (POA) by pyrazinamidase (PZase) encoded by the pncA gene. Although resistance to PZA is mostly caused by pncA mutations and less commonly by rpsA, panD, and clpC1 mutations, clinical strains without these mutations are known to exist. While efflux of POA was demonstrated in Mycobacterium tuberculosis previously, the efflux proteins involved have not been identified. Here we performed POA binding studies with an M. tuberculosis proteome microarray and identified four efflux proteins (Rv0191, Rv3756c, Rv3008, and Rv1667c) that bind POA. Overexpression of the four efflux pump genes in M. tuberculosis caused low-level resistance to PZA and POA but not to other drugs. Furthermore, addition of efflux pump inhibitors such as reserpine, piperine, and verapamil caused increased susceptibility to PZA in M. tuberculosis strains overexpressing the efflux proteins Rv0191, Rv3756c, Rv3008, and Rv1667c. Our studies indicate that these four efflux proteins may be responsible for PZA/POA efflux and cause PZA resistance in M. tuberculosis. Future studies are needed to assess their roles in PZA resistance in clinical strains.
KEYWORDS: M. tuberculosis proteome microarray, Mycobacterium tuberculosis, efflux pump, overexpression, pyrazinamide resistance
INTRODUCTION
Tuberculosis (TB) remains a major global public health problem despite the availability of current chemotherapy and the Mycobacterium bovis BCG vaccine. In 2015, there were an estimated 10.4 million new TB cases and 1.4 million deaths worldwide (1). The number of new cases of multidrug-resistant tuberculosis (MDR-TB) has reached 480,000. PZA is an important TB drug that shortens the duration of therapy from the previous 9 to 12 months to 6 months (2) due to its ability to kill a population of persister bacilli that are not killed by other TB drugs (3, 4). However, clinically, resistance to PZA is becoming an increasing problem (5–8), and its mechanisms of resistance are not completely understood.
PZA is a prodrug that requires conversion to its active form, POA, by pyrazinamidase (PZase) encoded by the pncA gene (9). Mutations in pncA leading to the loss of PZase activity are the major mechanism of PZA resistance (4, 10, 11). PZA is known to interfere with multiple functions in Mycobacterium tuberculosis, including cytoplasmic acidification (12), disruption of membrane energy and transport function (12, 13), inhibition of the protein degradation pathway via RpsA involved in trans-translation (14) and also ClpC1 protease (15, 16), and energy production via PanD (17–19). Although resistance to PZA is mostly caused by pncA mutations and less commonly by rpsA, panD, and clpC1, clinical strains without these mutations such as 9739 (20) are known to exist.
Efflux pumps are known to play a major role in drug resistance in other bacteria (21, 22). More recently, efflux proteins were shown to be involved in resistance to bedaquiline and clofazimine (CFZ) (23–25). The efflux pump systems can be divided into the ATP-binding cassette (ABC) superfamily, the major facilitator superfamily (MFS), the multidrug and toxic compound extrusion (MATE) family, the small multidrug resistance (SMR) family, and the resistance nodulation division (RND) superfamily. So far, the efflux pumps reported in M. tuberculosis have belonged to the ABC, MFS, and SMR superfamilies (26). It has been demonstrated that M. tuberculosis has a weak POA efflux activity that can be inhibited by reserpine and energy inhibitors, but in contrast, Mycobacterium smegmatis has a very efficient efflux system (12, 13). However, despite many studies, the efflux proteins involved in PZA/POA extrusion have not been identified.
In this study, we took a different approach by looking at proteins that bind POA from the M. tuberculosis proteome microarray and identified four putative efflux proteins: Rv0191, Rv3756c, Rv3008, and Rv1667c. We demonstrate that overexpression of the genes coding for these four proteins in M. tuberculosis caused resistance to PZA but not to other TB drugs and that inhibitors of efflux pumps caused increased susceptibility to PZA.
RESULTS
POA binding study with M. tuberculosis proteome microarray.
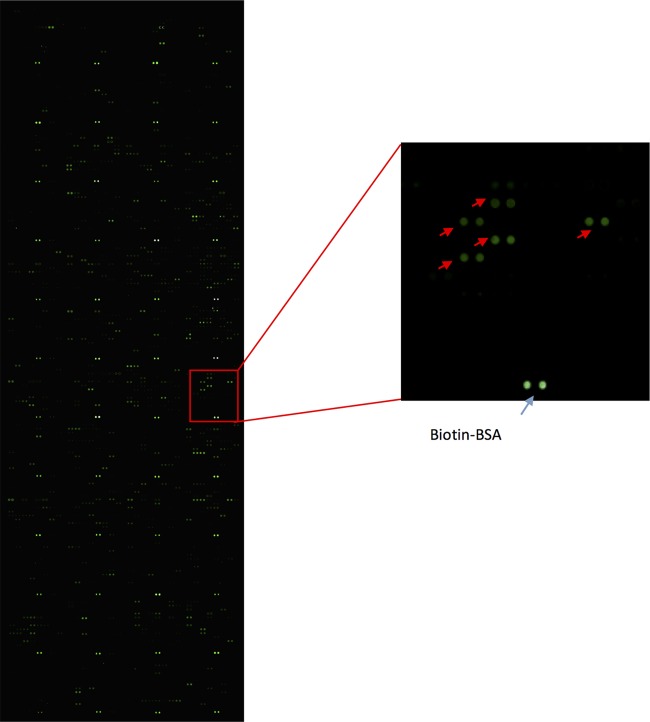
The Mycobacterium tuberculosis proteome microarray contains 4,262 recombinant proteins, covering more than 95% of the coding genes (27). The candidate list was generated by calculating the signal-to-noise ratio (SNR). The SNR of each protein was averaged for the two duplicated spots on each microarray to ensure reproducibility. Here the positive criterion for binding was determined as an SNR of >3. We identified 85 positive proteins that bound POA (Fig. 1), and in this study, we focused on four proteins that are functionally related to drug efflux/transport for further study: Rv0191 (a predicted arabinose efflux permease), Rv3756c (glycine betaine/carnitine/choline/l-proline ABC transporter permease), Rv3008 (uncharacterized membrane protein YhiD, involved in acid resistance), and Rv1667c (macrolide-transport ATP-binding ABC transporter).
FIG 1.
POA binding study with the M. tuberculosis proteome microarray. Each array contained biotin-labeled BSA as a positive control. Positive proteins are marked with an arrow.
Overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c caused PZA and POA resistance in M. tuberculosis.
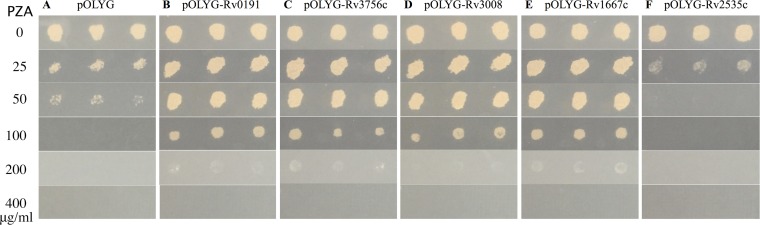
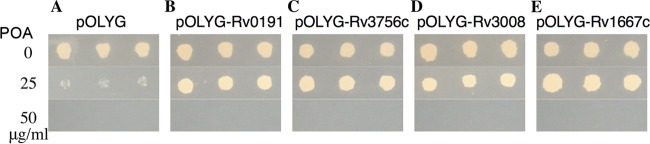
To determine if Rv0191, Rv3756c, Rv3008, and Rv1667c are involved in PZA resistance, we overexpressed the genes coding for these four proteins from M. tuberculosis in M. tuberculosis strain H37Ra. Results showed that overexpression of the Rv0191, Rv3756c, Rv3008, and Rv1667c genes caused PZA resistance (MIC of >200 μg/ml at pH 6.8 [Fig. 2B, C, D, and E, respectively]) in M. tuberculosis strain H37Ra compared with the pOLYG vector control (MIC of <100 μg/ml at pH 6.8 [Fig. 2A]). In addition, as an irrelevant control, the strain overexpressing Rv2535c involved in clofazimine (CFZ) resistance was sensitive to PZA (MIC of <50 μg/ml at pH 6.8 [Fig. 2F]). These results suggested that overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c was responsible for the increased PZA MIC of M. tuberculosis H37Ra. Results of POA susceptibility testing showed that Rv0191, Rv3756c, Rv3008, and Rv1667c overexpression strains were all resistant to POA at 25 μg/ml, while the pOLYG vector control was sensitive at this concentration (Fig. 3).
FIG 2.
PZA susceptibility testing of strains overexpressing Rv0191, Rv3756c, Rv3008, and Rv1667c. (A) pOLYG vector control. (B) pOLYG-Rv0191. (C) pOLYG-Rv3756c. (D) pOLYG-Rv3008. (E) pOLYG-Rv1667c. (F) pOLYG-Rv2535c.
FIG 3.
POA susceptibility testing of strains overexpressing Rv0191, Rv3756c, Rv3008, and Rv1667c. (A) pOLYG vector control. (B) pOLYG-Rv0191. (C) pOLYG-Rv3756c. (D) pOLYG-Rv3008. (E) pOLYG-Rv1667c.
Overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c did not cause resistance to other drugs.
To determine whether Rv0191, Rv3756c, Rv3008, and Rv1667c are specific to PZA or can transport multiple unrelated drugs, susceptibility to other drugs, including other first-line and second-line drugs, was performed in the same way. We found that overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c caused no resistance to frontline drugs isoniazid (INH) and ethambutol (EMB) (INH, < 0.05 μg/ml; EMB, <1.0 μg/ml) or second-line drugs CFZ, amikacin (AMK), streptomycin (STR), and levofloxacin (LEV) (CFZ, <0.5 μg/ml; AMK, <1.0 μg/ml; STR, <0.25 μg/ml; LEV, <0.25 μg/ml) (data not shown). These findings suggest that Rv0191, Rv3756c, Rv3008, and Rv1667c do not seem to transport the other TB drugs and instead participate in extruding PZA specifically.
Validation of Rv0191, Rv3756c, Rv3008, and Rv1667c overexpression in H37Ra by real-time RT-PCR.
Overexpression of the Rv0191, Rv3756c, Rv3008, and Rv1667c genes in the H37Ra/pOLYG-Rv0191, H37Ra/pOLYG-Rv3756c, H37Ra/pOLYG-Rv3008, and H37Ra/pOLYG-Rv1667c strains was confirmed by real-time reverse transcription (RT)-PCR. As shown in Fig. 4, 77.1 ± 12.8-, 64.0 ± 19.0-, 71.3 ± 0.3-, and 12.3 ± 0.8-fold increases in expression of Rv0191, Rv3756c, Rv3008, and Rv1667c were detected in strains containing pOLYG-Rv0191, pOLYG-Rv3756c, pOLYG-Rv3008, and pOLYG-Rv1667c, respectively, compared to the strain containing only the pOLYG vector.
FIG 4.
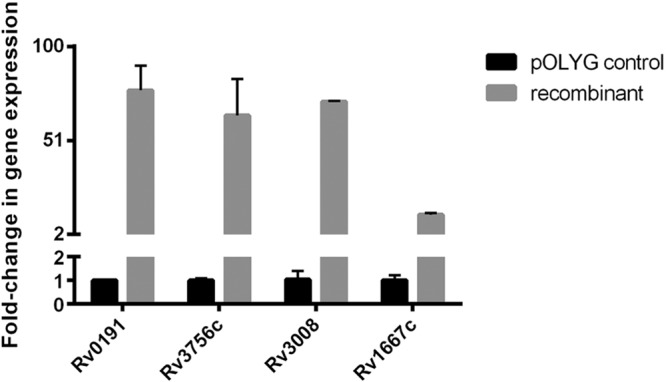
Relative quantitation of efflux pump genes overexpressed by real-time reverse transcription-PCR. M. tuberculosis 16S rRNA was used as a reference gene.
Mutations of Rv0191, Rv3756c, Rv3008, and Rv1667c in PZA-resistant clinical strains in the GMTV database.
The results of a search of the Genome-wide Mycobacterium tuberculosis Variation (GMTV) database showed that mutations of Rv0191, Rv3756c, Rv3008, and Rv1667c are associated with PZA resistance in clinical strains (Table 1). Among them, mutations of Rv0191 were the most common, where 406 mutations in the Rv0191 gene are associated with PZA resistance in the GMTV database, most of which occurred in the 213th and 66th codons. These findings suggest that mutations in Rv0191, Rv3756c, Rv3008, and Rv1667c may play important roles in PZA resistance in clinical strains of M. tuberculosis.
TABLE 1.
Mutations in Rv0191, Rv3756c, Rv3008, and Rv1667c in PZA-resistant strains in the GMTV database
| Gene | No. of SNPsa | Most common nucleotide variation (no.) | Codon no. | Effectb |
|---|---|---|---|---|
| Rv0191 | 406 | GCT/ACT (299) | 213 | Nonsynon |
| GCC/GCT (87) | 66 | Synon | ||
| Rv3756c | 58 | GTT/GTG (42) | 78 | Synon |
| Rv3008 | 16c | |||
| Rv1667c | 19 | CCG/CCT (9) | 136 | Syn |
SNPs, single nucleotide polymorphisms.
Nonsyn, nonsynonymous; Syn, synonymous.
All 16 SNPs are distinct.
Efflux inhibition decreased PZA resistance in strains overexpressing Rv0191, Rv3756c, Rv3008, and Rv1667c.
In the presence of the efflux pump inhibitors resperine, piperine, and verapamil, PZA resistance caused by overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c was reduced (Fig. 5). The efflux inhibition by reserpine and verapamil in overexpression strains showed a 2-fold decrease in MICs of PZA (Fig. 5A and C, respectively). Piperine also had an inhibitory effect on the resistance levels (Fig. 5B). The PZA MIC of the overexpression strains was decreased, which provides further evidence that the function of these genes is indeed encoding efflux pumps for POA.
FIG 5.
Efflux inhibitors decreased PZA resistance caused by overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c. (A) PZA plus resperine. (B) PZA plus piperine. (C) PZA plus verapamil.
DISCUSSION
PZA resistance is mainly caused by mutations in the pncA gene, encoding PZase involved in conversion of prodrug PZA to the active form POA (8, 9). Mutations in potential drug targets PanD, RpsA, and ClpC1, involved in energy metabolism and protein degradation, respectively (14, 17, 18, 28) are less common. However, there are still some clinical strains, such as strain 9739 (20), that do not have any of the known resistance gene mutations in pncA, rpsA, panD, and clpC1 (unpublished observation). Although mutations of drug targets are thought to be the primary mechanism of drug resistance, efflux pumps are also found to be an important resistance mechanism in many bacteria (21, 29). It has been reported that M. tuberculosis has a weak or deficient POA efflux mechanism that can be inhibited by efflux pump inhibitors such as reserpine or the energy inhibitor valinomycin (13, 30). However, the efflux pump involved in PZA/POA extrusion in M. tuberculosis has never been identified. Here, we used a novel approach to identify possible POA binding proteins from the entire M. tuberculosis proteome, identified 4 potential efflux proteins (Rv0191, Rv3756c, Rv3008, and Rv1667c), and demonstrated that their overexpression specifically caused resistance to PZA and POA but not to other TB drugs.
We found that recombinant strains showed a >60-fold increase in expression of Rv0191, Rv3756c, and Rv3008 and >12-fold increases in expression of Rv1667c as confirmed by real-time PCR analysis. Our experiments showed that MICs of PZA in recombinant strains were increased (4-fold) compared with that of the control strain. In addition, a strain overexpressing Rv2535c involved in clofazimine resistance as an irrelevant control was still susceptible to PZA, suggesting that overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c is specifically involved in PZA resistance. Furthermore, the PZA MICs for H37Ra strains carrying pOLYG-Rv0191, pOLYG-Rv3756c, pOLYG-Rv3008, and pOLYG-Rv1667c were also decreased in the presence of the efflux inhibitors reserpine, piperine, and verapamil. These findings with efflux inhibitors provide further support for the involvement of the 4 newly identified PZA efflux proteins in PZA resistance in M. tuberculosis.
Results of POA susceptibility testing showed that overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c caused a low level of POA resistance. PZA is a prodrug that enters the bacilli by passive diffusion and is converted by PZase into POA, which exits cells via passive diffusion and a deficient efflux mechanism in M. tuberculosis (12). This mode of action of PZA provides explanation for the low-level resistance caused by strains overexpressing Rv0191, Rv3756c, Rv3008, and Rv1667c. In addition, a small amount of PZA may be extruded by efflux pumps before activation, which can explain a higher level of PZA resistance but lower level of resistance to POA.
To further confirm Rv0191, Rv3756c, Rv3008, and Rv1667c are involved in the efflux of PZA, mutations of these four genes were analyzed in clinical strains resistant to PZA in the GMTV database. The results showed that there were indeed many mutations in Rv0191, Rv3756c, Rv3008, and Rv1667c, where mutations of Rv0191 were the most common. In addition, 299 of the 406 strains carried nonsynonymous single nucleotide polymorphisms at the 213th codon (GCT/ACT) of Rv0191, suggesting that this mutation may be related to PZA resistance clinically. There existed high-frequency synonymous mutations in the 66th codon (GCC/GCT [87 of 406]) of Rv0191, in the 78th codon (GTT/GTG [42 of 58]) of Rv3756c, and in the 136th codon (CCG/CCT [9 of 19]) of Rv1667c. Synonymous mutations usually seem not to affect the final amino acid sequences of proteins, but several studies have shown that synonymous mutations are associated with carcinogenesis, indicating a functional role of synonymous mutations (31, 32). Changes in biases of codon and mRNA secondary structure may affect mRNA stability, ribosomal translation, or protein folding (33). It remains to be determined whether these mutations in Rv0191, Rv3756c, and Rv1667c are associated with PZA resistance in clinical strains in future studies.
The finding that overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c conferred a resistance phenotype to PZA and POA but not to other drugs suggests that these efflux proteins are specifically responsible for resistance to PZA. However, we observed a consistent higher susceptibility to INH in the overexpression strains, suggesting Rv0191, Rv3756c, Rv3008, and Rv1667c may participate in INH transport or uptake. Structurally, PZA and INH are structural analogs of nicotinamide, and studies in mice showed a negative interaction of INH and PZA (34). Hence, PZA and the structurally related molecule INH may be identified by the same transport protein. Future studies are needed to address this possibility and the substrate specificity of the efflux proteins by detailed uptake and biochemical studies.
In summary, we identified four new PZA/POA efflux proteins (Rv0191, Rv3756c, Rv3008, and Rv1667c) that are involved in PZA resistance in M. tuberculosis. Our findings are significant as they offer a possible new mechanism of PZA resistance via efflux in M. tuberculolsis. Future studies are needed to determine their contribution in PZA resistance in clinical isolates.
MATERIALS AND METHODS
POA binding study using the M. tuberculosis proteome microarray.
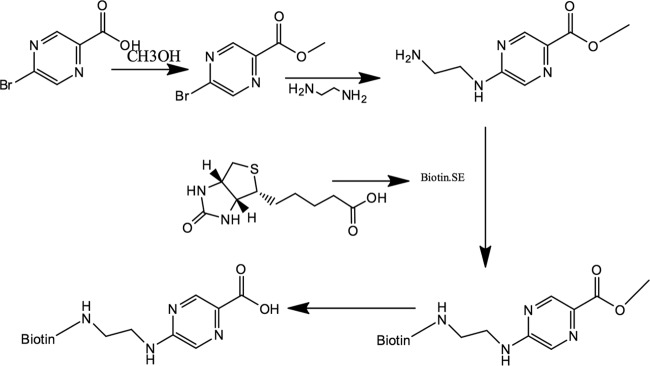
POA was biotinylated (Fanbo Biochemicals, China) as shown in Fig. 6. First, the M. tuberculosis proteome microarray (BC Biotech, China) was blocked in blocking buffer (3 ml 10% bovine serum albumin [BSA], 7 ml 1× phosphate-buffered saline [PBS]) with shaking 50 to 60 rpm at room temperature for 1 h. Then, 0.5 mM biotin-POA and 0.5 mM biotin-BSA as a control (Sigma-Aldrich) were prepared in incubation buffer (3 ml 10% BSA, 7 ml 1× PBS-Tween 20 [PBST]) and added to cover the microarray after removal of the blocking buffer. After incubation at room temperature with shaking for 1 h, the microarray was washed with 1× PBST for three times with shaking. Then 3 ml Cy3-streptavidin (Sigma-Aldrich) was added, followed by incubation at room temperature with shaking in the dark for 1 h. After being washed three times in 1× PBST and twice in double-distilled water (ddH2O), the microarray was dried and scanned using a LuxScan 10Κ-A scanner. Data were analyzed with GenePix Pro 6.0 software.
FIG 6.
Structure of biotin-labeled POA.
Candidate gene overexpression.
Rv0191, Rv3756c, Rv3008, and Rv1667c were amplified by PCR from M. tuberculosis strain H37Ra. The primers used are listed in Table 2. Since the vector pOLYG we used was a noninducible vector, the forward primers of genes we designed were taken about 150 to 300 bp upstream of the start codon to include their own promoters (35). The PCR products were digested with the restriction enzymes XbaI and HindIII (Thermo Fisher) and ligated to the plasmid pOLYG digested with the same enzymes. Recombinant constructs were confirmed by DNA sequencing and electroporated into M. tuberculosis H37Ra (36). The complete pOLYG vector alone was electroporated into M. tuberculosis H37Ra as a control. Simultaneously we overexpressed Rv2535c, which encodes cytoplasmic peptidase PepQ involved in clofazimine resistance (25) rather than an efflux pump as another control.
TABLE 2.
Primers used for candidate gene overexpression
| Gene | Primer orientation | Primer sequence (5′→3′)a |
|---|---|---|
| Rv0191 | Forward | GCTCTAGACAGATCAGCGCCGTCAC |
| Reverse | CCCAAGCTTTTAGCCGTCGCCGGG | |
| Rv3756c | Forward | GCTCTAGAACTGCCGCTGTCTATCCC |
| Reverse | CCCAAGCTTCTACCGTAGGGCGTGTC | |
| Rv3008 | Forward | GCTCTAGAGCCTGGGTTGTCACCAC |
| Reverse | CCCAAGCTTTTAGTTGCCGTCCGCGG | |
| Rv1667c | Forward | GCTCTAGACGTGGAGCTGGCCAAG |
| Reverse | CCCAAGCTTTCATTCGAGCATCTCCGAAAG |
Primers were flanked with recognition sequences of restriction enzymes XbaI and HindIII at their 5' end with 2 or 3 additional bp at the extreme 5' end in both primers. The forward primers of genes were taken about 300 bp upstream of the start codon to include their own promoters. The reverse primers of genes were taken 15 to 25 bp from the stop codon.
RNA preparation.
Total RNA from M. tuberculosis cultures was isolated by TRI reagent. Briefly, 1.0 ml of TRI reagent was added to the cells and the mixture was vortexed. Suspensions were incubated at room temperature for 5 min, and 200 μl chloroform was added. Treated samples were shaken vigorously for 15 s and incubated at room temperature for 2 to 3 min. The samples were centrifuged at no more than 12,000 × g for 15 min at 4°C. The upper aqueous phase was collected and precipitated with 500 μl isopropyl alcohol. Samples were incubated at room temperature for 10 min and centrifuged at no more than 12,000 × g for 10 min at 4°C. The RNA pellet was washed with 75% ethanol twice and centrifuged at no more than 7,500 × g for 5 min at 4°C. The pellet was air dried and dissolved in RNase-free water. RNA (0.5 μg) was taken for cDNA by PrimeScript RT master mix (TaKaRa, Japan). The cDNA was diluted 5-fold.
Real-time RT-PCR.
Overexpression of Rv0191, Rv3756c, Rv3008, and Rv1667c in H37Ra was confirmed by real-time reverse transcription-PCR in a Light Cycler 480 II (Roche Diagnostics, Germany) using LightCycler 480 SYBR green I master mix (Roche Diagnostics, Germany). The primers for RT-PCR are listed in Table 3. 16S rRNA was used as a normalization control. The annealing temperatures of all primers were 60°C. Crossing point (Cp) values of controls and samples were recorded in each case. Mean ΔCp values were calculated and normalized to that of 16S rRNA by the threshold cycle (2−ΔΔCT) method. Relative quantification was done to determine overexpression of the Rv0191, Rv3756c, Rv3008, and Rv1667c genes in H37Ra/pOLYG-Rv0191, H37Ra/pOLYG-Rv3756c, H37Ra/pOLYG-Rv3008, and H37Ra/pOLYG-Rv1667c compared to that of H37Ra/pOLYG, respectively.
TABLE 3.
Primers for real-time RT-PCR to confirm gene overexpression
| Gene | Primer orientation | Primer sequence (5′→3′) |
|---|---|---|
| Rv0191 | Forward | TGCTGTCCTGGTATGCCCTTGT |
| Reverse | GCCGAGACGAGTTGCGAGA | |
| Rv3756c | Forward | GGGCATGACCGAGTCCCA |
| Reverse | CATCATCGCACCGACCAGA | |
| Rv3008 | Forward | CAACGCAGATCAGTGCCG |
| Reverse | GACAGATTGCTCGGTGGTGA | |
| Rv1667c | Forward | GAGGTCGCCACCACAACAT |
| Reverse | CTGCCGCTTGGCTTCG |
Drug susceptibility testing.
For PZA susceptibility testing, PZA (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water and filter sterilized. 7H11 agar plates (pH 5.8, 6.0, and 6.8) with increasing concentrations of PZA (i.e., 25, 50, 100, 200, 400, 800, and 1,600 μg/ml) were prepared. For the controls, 7H11 agar plates without PZA were used. PZA susceptibility testing was performed for different M. tuberculosis strains (wild-type H37Ra, H37Ra/pOLYG, H37Ra/pOLYG-Rv0191, H37Ra/pOLYG-Rv3756c, H37Ra/pOLYG-Rv3008, H37Ra/pOLYG-Rv1667c, and H37Ra/pOLYG-Rv2535c) in 7H11 agar. Cultured M. tuberculosis strains (1 × 107 CFU/ml), were transferred from 96-well plates onto 7H11 agar plates in triplicate followed by incubation at 37°C for 2 to ∼4 weeks. The MIC of PZA was determined based on growth or no growth on the PZA-containing plates.
POA, isoniazid (INH), ethambutol (EMB), clofazimine (CFZ), amikacin (AMK), streptomycin (STR) and levofloxacin (LEV) were purchased from Sigma-Aldrich (St. Louis, MO). INH, EMB, AMK, and STR were dissolved in ddH2O. POA and CFZ were dissolved in dimethyl sulfoxide (DMSO). LEV was dissolved in hydrochloric acid. All of these drugs were filtered for sterilization. 7H11 agar plates were prepared with increasing concentrations of drugs: POA, 25, 50, 100, 200, 400, and 800 μg/ml; INH, 0.0125, 0.025, 0.05, 0.1, and 0.2 μg/ml; EMB, 0.1, 0.25, 0.5, 1.0, and 2.0 μg/ml; CFZ, 0.1, 0.25, 0.5, 1.0, and 2.0 μg/ml; AMK, 0.2, 0.5, 1.0, 2.0, and 5.0 μg/ml; STR, 0.1, 0.25, 0.5, 1.0, and 2.0 μg/ml; and LEV, 0.1, 0.25, 0.5, 1.0, 2.0, and 5.0 μg/ml. The bacterial suspension was transferred from 96-well plates, imprinted on 7H11 agar in triplicate, and incubated at 37°C for 2 to ∼4 weeks.
Database analysis for mutations in Rv0191, Rv3756c, Rv3008, and Rv1667c.
Genome-wide Mycobacterium tuberculosis Variation (GMTV) is a database that covers the whole-genome sequencing information of 1,084 M. tuberculosis isolates from various data sets in different regions of Russia (37). The GMTV database contains data involving molecular biology, epidemiology, tuberculosis clinical data, year, region, and drug resistance information for Mycobacterium tuberculosis, which can be used to identify the genetic variation of drug resistance, clinical outcomes, or pathogens related to geographical distribution. PZA resistance was selected in the database, and Rv0191, Rv3756c, Rv3008, and Rv1667c were input to identify mutations of these genes in the clinical strains.
Efflux inhibition study.
Resperine, piperine, and verapamil (potency of >99%) were purchased from Sigma-Aldrich (St. Louis, MO), dissolved in DMSO, and filtered for sterilization. Resperine (3 μg/ml), piperine (10 μg/ml), and verapamil (40 μg/ml) were added, respectively, to 7H11 agar plates at increasing concentrations as efflux inhibitors (38, 39). The final concentration of DMSO was <1%, and this concentration had no effect on M. tuberculosis growth. PZA susceptibility testing was performed for different M. tuberculosis strains (pOLYG-Rv0191, pOLYG-Rv3756c, pOLYG-Rv3008, and pOLYG-Rv1667c) in 7H11 agar as described above.
Supplementary Material
ACKNOWLEDGMENTS
We thank T.X., J.Z.C., and J.W. for helpful advice on the experiments.
This work was supported by the National Natural Science Foundation of China (grant no. 81572046). Ying Zhang was supported in part by NIH grants AI99512 and AI108535.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00940-17.
REFERENCES
- 1.WHO. 2016. World Health Organization Report on Global Tuberculosis 2016. Reference no. WHO/HTM/TB/201613. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 3.Heifets L, Lindholm-Levy P. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis 145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Shi W, Zhang W, Mitchison D. 2013. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2:1–12. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield MG, Streicher EM, Dolby T, Simpson JA, Sampson SL, Van Helden PD, Van Rie A, Warren RM. 2016. Prevalence of pyrazinamide resistance across the spectrum of drug resistant phenotypes of Mycobacterium tuberculosis. Tuberculosis (Edinb) 99:128–130. doi: 10.1016/j.tube.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitfield MG, Soeters HM, Warren RM, York T, Sampson SL, Streicher EM, van Helden PD, van Rie A. 2015. A global perspective on pyrazinamide resistance: systematic review and meta-analysis. PLoS One 10:e0133869. doi: 10.1371/journal.pone.0133869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobelens F, Gebhard A, van den Hof S. 2016. No evidence that pyrazinamide resistance is acquired after fluoroquinolone resistance. Int J Tuberc Lung Dis 20:282. doi: 10.5588/ijtld.15.0628. [DOI] [PubMed] [Google Scholar]
- 8.Miotto P, Cabibbe AM, Feuerriegel S, Casali N, Drobniewski F, Rodionova Y, Bakonyte D, Stakenas P, Pimkina E, Augustynowicz-Kopec E, Degano M, Ambrosi A, Hoffner S, Mansjo M, Werngren J, Rusch-Gerdes S, Niemann S, Cirillo DM. 2014. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. mBio 5:e01819-14. doi: 10.1128/mBio.01819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorpio A, Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med 2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 10.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 41:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreevatsan S, Pan X, Zhang Y, Kreiswirth BN, Musser JM. 1997. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob Agents Chemother 41:636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Scorpio A, Nikaido H, Sun ZH. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol 181:2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Wade MM, Scorpio A, Zhang H, Sun Z. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother 52:790–795. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- 14.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE III, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yee M, Gopal P, Dick T. 2017. Missense mutations in the unfoldase ClpC1 of the caseinolytic protease complex are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 61:e02342-16. doi: 10.1128/AAC.02342-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Chen J, Shi W, Cui P, Zhang J, Cho S, Zhang W, Zhang Y. 2017. Mutation in clpC1 encoding an ATP-dependent ATPase involved in protein degradation is associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect 6:e8. doi: 10.1038/emi.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Chen J, Shi W, Liu W, Zhang W, Zhang Y. 2013. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect 2:e34. doi: 10.1038/emi.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi W, Chen J, Feng J, Cui P, Zhang S, Weng X, Zhang W, Zhang Y. 2014. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis. Emerg Microbes Infect 3:e58. doi: 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopal P, Yee M, Sarathy J, Low JL, Sarathy JP, Kaya F, Dartois V, Gengenbacher M, Dick T. 2016. Pyrazinamide resistance is caused by two distinct mechanisms: prevention of coenzyme A depletion and loss of virulence factor synthesis. ACS Infect Dis 2:616–626. doi: 10.1021/acsinfecdis.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng SJ, Thibert L, Sanchez T, Heifets L, Zhang Y. 2000. pncA mutations as a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis: spread of a monoresistant strain in Quebec, Canada. Antimicrob Agents Chemother 44:528–532. doi: 10.1128/AAC.44.3.528-532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alibert S, N′Gompaza Diarra J, Hernandez J, Stutzmann A, Fouad M, Boyer G, Pages JM. 2017. Multidrug efflux pumps and their role in antibiotic and antiseptic resistance: a pharmacodynamic perspective. Expert Opin Drug Metab Toxicol 13:301–309. doi: 10.1080/17425255.2017.1251581. [DOI] [PubMed] [Google Scholar]
- 22.Kuete V, Ngameni B, Tangmouo JG, Bolla JM, Alibert-Franco S, Ngadjui BT, Pages JM. 2010. Efflux pumps are involved in the defense of Gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob Agents Chemother 54:1749–1752. doi: 10.1128/AAC.01533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Chen J, Cui P, Shi W, Zhang W, Zhang Y. 2015. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 70:2507–2510. doi: 10.1093/jac/dkv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues L, Parish T, Balganesh M, Ainsa JA. 2017. Antituberculosis drugs: reducing efflux = increasing activity. Drug Discov Today 22:592–599. doi: 10.1016/j.drudis.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Deng J, Bi L, Zhou L, Guo SJ, Fleming J, Jiang HW, Zhou Y, Gu J, Zhong Q, Wang ZX, Liu Z, Deng RP, Gao J, Chen T, Li W, Wang JF, Wang X, Li H, Ge F, Zhu G, Zhang HN, Gu J, Wu FL, Zhang Z, Wang D, Hang H, Li Y, Cheng L, He X, Tao SC, Zhang XE. 2014. Mycobacterium tuberculosis proteome microarray for global studies of protein function and immunogenicity. Cell Rep 9:2317–2329. doi: 10.1016/j.celrep.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Liu Y, Bi J, Cai Q, Liao X, Li W, Guo C, Zhang Q, Lin T, Zhao Y, Wang H, Liu J, Zhang X, Lin D. 2015. Structural basis for targeting the ribosomal protein S1 of Mycobacterium tuberculosis by pyrazinamide. Mol Microbiol 95:791–803. doi: 10.1111/mmi.12892. [DOI] [PubMed] [Google Scholar]
- 29.Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. 2009. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother 53:3181–3189. doi: 10.1128/AAC.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade MM, Zhang Y. 2006. Effects of weak acids, UV and proton motive force inhibitors on pyrazinamide activity against Mycobacterium tuberculosis in vitro. J Antimicrob Chemother 58:936–941. doi: 10.1093/jac/dkl358. [DOI] [PubMed] [Google Scholar]
- 31.Gartner JJ, Parker SC, Prickett TD, Dutton-Regester K, Stitzel ML, Lin JC, Davis S, Simhadri VL, Jha S, Katagiri N, Gotea V, Teer JK, Wei X, Morken MA, Bhanot UK, NISC Comparative Sequencing Program, Chen G, Elnitski LL, Davies MA, Gershenwald JE, Carter H, Karchin R, Robinson W, Robinson S, Rosenberg SA, Collins FS, Parmigiani G, Komar AA, Kimchi-Sarfaty C, Hayward NK, Margulies EH, Samuels Y. 2013. Whole-genome sequencing identifies a recurrent functional synonymous mutation in melanoma. Proc Natl Acad Sci U S A 110:13481–13486. doi: 10.1073/pnas.1304227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supek F, Minana B, Valcarcel J, Gabaldon T, Lehner B. 2014. Synonymous mutations frequently act as driver mutations in human cancers. Cell 156:1324–1335. doi: 10.1016/j.cell.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 33.Brule CE, Grayhack EJ. 2017. Synonymous codons: choose wisely for expression. Trends Genet 33:283–297. doi: 10.1016/j.tig.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosset J, Truffotpernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaora PO. 1998. Expression of genes in mycobacteria. Methods Mol Biol 101:261–273. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Garbe T, Young D. 1993. Transformation with katG restores isoniazid-sensitivity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol Microbiol 8:521–524. doi: 10.1111/j.1365-2958.1993.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 37.Chernyaeva EN, Shulgina MV, Rotkevich MS, Dobrynin PV, Simonov SA, Shitikov EA, Ischenko DS, Karpova IY, Kostryukova ES, Ilina EN, Govorun VM, Zhuravlev VY, Manicheva OA, Yablonsky PK, Isaeva YD, Nosova EY, Mokrousov IV, Vyazovaya AA, Narvskaya OV, Lapidus AL, O'Brien SJ. 2014. Genome-wide Mycobacterium tuberculosis Variation (GMTV) database: a new tool for integrating sequence variations and epidemiology. BMC Genomics 15:308. doi: 10.1186/1471-2164-15-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida D, Ioerger T, Tyagi S, Li SY, Mdluli K, Andries K, Grosset J, Sacchettini J, Nuermberger E. 2016. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:4590–4599. doi: 10.1128/AAC.00753-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Kumar M, Sharma S, Nargotra A, Koul S, Khan IA. 2010. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother 65:1694–1701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.