Abstract
Perinatal transmission of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) can result in conjunctivitis in infants. We examined national rates of reported CT/GC conjunctivitis among infants. Surveillance of these infections is heavily affected by the completeness of reported data on specimen source and age. Alternative data sources should be evaluated.
Infection with Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are the 2 most commonly reported sexually transmitted infections in the United States.1 The prevalence of these infections in pregnant women is estimated at 2% to 20% for CT, depending on the population screened,2–4 and less than 1% for GC,5 and are important to monitor because infants born to infected mothers are at high risk for perinatal transmission. The most frequent clinical manifestation of CT/GC infections in infants is conjunctivitis, occurring in 30% to 50% of those born to infected mothers.6–8 The clinical presentation of conjunctivitis can be variable, and if not treated promptly, can lead to visual impairment.9 The risk of blindness due to CT/GC conjunctivitis is dependent on the availability of medical care, especially in developing countries and urban areas of developed countries with less access to healthcare, and occurs in approximately 3% of infants with conjunctival infections as a result of perinatal CT/GC transmission.10
Prophylaxis is used to prevent these infections in infants. Silver nitrate drops were first used in 1880 for GC conjunctival infections in infants; because there was no other available treatment, it quickly became the prophylaxis of choice due to its high effectiveness and low cost.11 Chemical conjunctivitis occurs in 50% to 90% of infants treated with silver nitrate, and as such, it was discontinued in the United States and suspended in other developed countries due to safety concerns.12–14 The current recommendation from the U.S. Preventive Services Task Force suggests prophylaxis with erythromycin ophthalmic ointment immediately after birth and is legally mandated in most states.15
Because of the safety and efficacy concerns related to the use of prophylactic agents against CT/GC conjunctivitis infections in infants, universal prophylaxis has been discontinued in the United Kingdom, Norway, Sweden, and Denmark.10 In 2015, the Canadian Pediatric Society also discontinued prophylaxis and now focuses on improving sexually transmitted infection screening in pregnant women.12 In light of the limited prophylactic options and the limited knowledge on trends in these infections in the United States, we aimed to examine national case report data to estimate the burden and trends in CT/GC conjunctivitis infections in infants younger than 1 year in the United States.
METHODS
We examined national CT/GC case data reported to the Centers for Disease Control and Prevention (CDC).1 A CT/GC conjunctivitis infection in an infant was defined as a CT or GC case reported to CDC (1) with a specimen collection date between January 1, 2010, and December 31, 2015; (2) from the 50 U.S. states and District of Columbia; (3) in infants younger than 1 year; and (4) with a specimen source of either “eye” or “conjunctiva.” This case definition was chosen to increase the specificity of the analyses. Age was determined from 2 variables: “age” and “age type”; only cases with valid data for both variables were included. “Age” is reported as a number to represent the age of the case patient at the time of infection, whereas “age type” represents the units for the “age” field (eg, days, weeks, months, or years). We calculated rates per 100,000 live births using natality data from the Wide-ranging Online Data for Epidemiologic Research public health database developed by the CDC (https://wonder.cdc.gov/natality.html), and describe cases by demographics and over time.
RESULTS
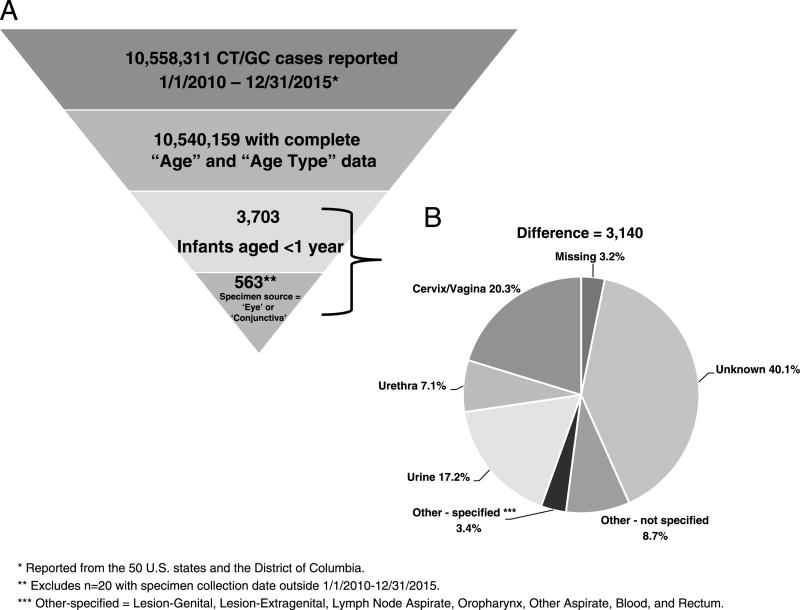
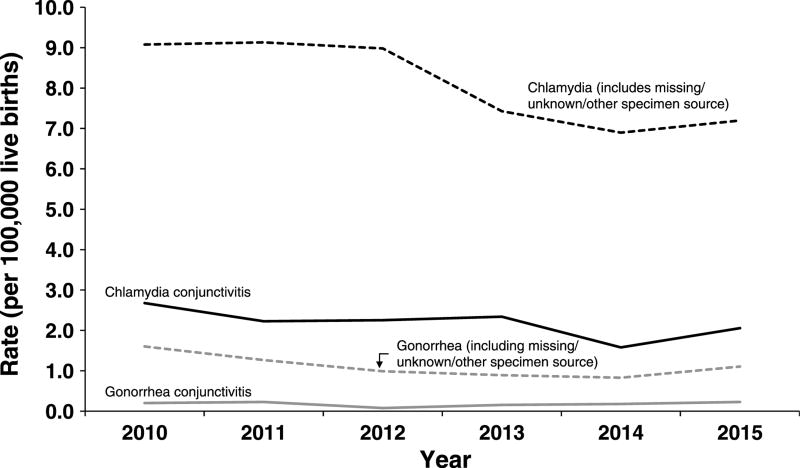
There were 3703 CT/GC cases reported to CDC from the 50 U.S. states and the District of Columbia during 2010 to 2015 with an age less than 1 year. Of those, there were 563 (15.2%) cases with a specimen source of either “eye” or “conjunctiva” (Fig. 1). Cases were similar by gender (48.9% male, 50.8% female) and the plurality of cases were among black, non-Hispanic infants (36.2%), followed by those reporting “other” or “unknown” race (32.0%), white, non-Hispanics (20.1%), and Hispanics (11.7%). This is similar to the racial/ethnic distribution of female CT/GC cases aged 15 to 44 years during 2010 to 2015 (black, non-Hispanic, 32.7%; “other”/“unknown,” 30.1%; white, non-Hispanic, 23.7%; Hispanic, 13.5%). Chlamydia trachomatis accounted for 521 (92.5%) and GC for 42 (7.5%) infections. Rates of reported CT conjunctivitis in infants varied over the study period. During 2010 to 2014, the overall reported rate of CT conjunctivitis decreased by 40.7% (2.7 to 1.6 per 100,000 live births) followed by an increase of 31.2% during 2014 to 2015. The rate of GC conjunctivitis remained relatively constant, at 0.2 cases or less per 100,000 live births each year. Reported rates in 2015 were 2.1 and 0.2 cases per 100,000 live births for CT and GC conjunctivitis, respectively (Fig. 2).
Figure 1.
A, Case definition eligibility criteria for CT/GC conjunctivitis cases among infants younger than 1 year, including the number eligible at each stage. B, Specimen source of excluded cases.
Figure 2.
Rates of reported CT/GC cases among infants younger than 1 year by year and specimen source, United States, 2010–2015.
Nearly 85% of possible cases were excluded due to the restriction of a specimen source of either “eye” or “conjunctiva” and may represent missed cases for surveillance. Of those excluded, 52.0% had a specimen source of “unknown” (40.1%), “other—not specified” (8.7%), or “missing” (3.2%). As a sensitivity analysis, we revised our case definition to include these cases and found the rate of CT infections was higher in all years (eg, in 2010, increase from 2.7 to 9.1 cases per 100,000 live births, Fig. 2). Similar to the CT rates with a specimen source of eye/conjunctiva, during 2010 to 2014, there was a decrease (9.1 to 6.9 per 100,000 live births, 24.1% decrease) followed by a 4.3% increase in 2015. Similarly, the reported rates of GC in infants were higher with the revised case definition, but remained comparatively constant and lower than CT (1.6 to 1.1 cases per 100,000 live births during 2010–2015).
DISCUSSION
Conjunctivitis can result from perinatal transmission of a CT/GC infection from an infected mother to a newborn and is a cause of blindness in affected infants.10 Because prophylactic treatment options for CT/GC conjunctival infections in infants are limited, it is essential to monitor trends associated with these infections. These are the first national estimates of CT/GC conjunctivitis infections in infants younger than 1 year in the United States; national surveillance data indicate rates of reported CT conjunctivitis varied during 2010 to 2015 and GC conjunctivitis remained low.
Few data sources exist to monitor trends in CT/GC conjunctivitis in infants. There are a number of strengths to case report data, including their representation of diagnosed and reported infections from all jurisdictions nationwide, cases are defined using a positive laboratory result, and a standard case definition for CT and GC is used by all jurisdictions for reporting.
However, there are also limitations to case report data. First, many case reports among infants had incorrect or incomplete reporting of specimen source, resulting in a possible underestimate of the true burden of disease. Local monitoring of data quality in cases of CT/GC in infants is prudent to detecting these issues to address them locally (if possible) prior to the report of case data to CDC.
Second, current CT/GC case reports allow age to be reported without “age type” data indicating the units. For instance, a report received with an age of 5 years with a missing “age type” could indicate a person aged 5 days, 5 weeks, 5 months, or 5 years. Without complete “age type” data, which occurred in 0.2% or 18,152 cases during 2010 to 2015, there is insufficient information to determine if a case meets the case definition used. Hence, these analyses were limited to cases with complete age information, which could have resulted in a slight underestimate of these infections.
Third, underreporting may be a consequence of certain CT/GC testing practices. For instance, in the case of a known infected mother, a physician may not collect an ocular specimen for testing and simply provide empiric therapy to the infant. Despite appropriate treatment of the infant, these untested infections would not be reported to CDC, resulting in an underestimate of disease. Additionally, if provider testing and reporting practices changed over time, the number of reported cases could change independent of a true change in incidence.
Two other issues should be considered when evaluating trends in reported cases. In areas where electronic laboratory reporting has been adopted, reporting may be more complete compared to jurisdictions performing manual data entry of paper laboratory reports. This would likely result in trends (perhaps falsely) appearing to increase in those jurisdictions that have adopted electronic laboratory reporting compared with those that have not. Also, nucleic acid amplification tests (NAATs) for the testing of ocular specimens, which have a higher sensitivity than other test technologies, may not be available in all jurisdictions. Nucleic acid amplification tests are not an FDA-approved test for use with ocular specimens, and though many laboratories have received CLIA waivers to use NAATs for testing of ocular specimens, some have not and are still using tests with lower sensitivity. This would likely result in more infections identified in jurisdictions employing NAATs compared with those using tests with lower sensitivity.
In conclusion, these are the first estimates of reported CT/GC conjunctivitis infections in infants younger than 1 year in the United States; however, they are based on case report data and a number of limitations with regards to the surveillance of these infections were identified. As a result, they most likely reflect a minimum burden of disease. Case report data likely underestimate the true burden of disease in the United States because they are influenced by both provider testing and reporting practices. Future work should include evaluating alternative data sources (eg, hospital discharge data) for their utility in monitoring chlamydia and gonorrhea conjunctivitis cases in infants in the United States.
Footnotes
Conflicts of interest and source of funding: None declared.
References
- 1.CDC. Sexually Transmitted Disease Surveillance 2015. Atlanta: U.S. Department of Health and Human Services; 2016. [Google Scholar]
- 2.American Academy of Pediatrics. Chlamydia trachomatis. In: Kimberlin D, editor. Red Book: 2015 Report of the Committee of Infectious Diseases. 30. Elk Grove Village, IL: American Academy of Pediatrics; 2015. p. 288. [Google Scholar]
- 3.FitzSimmons J, Callahan C, Shanahan B, et al. Chlamydial infections in pregnancy. J Reprod Med. 1986;31:19–22. [PubMed] [Google Scholar]
- 4.Much D, Yeh S. Prevalence of Chlamydia trachomatis infection in pregnant patients. Public Health Rep. 1991;106:490–3. [PMC free article] [PubMed] [Google Scholar]
- 5.Laga M, Meheus A, Piot P. Epidemiology and control of gonococcal ophthalmia neonatorum. Bull World Health Organ. 1989;67:471–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis. 2011;53:S99–102. doi: 10.1093/cid/cir699. [DOI] [PubMed] [Google Scholar]
- 7.Laga M, Nzanze H, Brunham RC, et al. Epidemiology of ophthalmia neonatorum in Kenya. Lancet. 1986;2:1145–9. doi: 10.1016/s0140-6736(86)90544-1. [DOI] [PubMed] [Google Scholar]
- 8.Galega FP, Heymann DL, Nasah BT. Gonococcal ophthalmia neonatorum: The case for prophylaxis in tropical Africa. Bull World Health Organ. 1984;62:95–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Kohlhoff SA, Hammerschlag MR. Sexually Transmitted Diseases. 4. New York, NY: McGraw Hill; 2007. Gonococcal and chlamydial infections in infants and children; pp. 1613–27. [Google Scholar]
- 10.Schaller UC, Klauss V. Is Credé's prophylaxis for ophthalmia neonatorum still valid? Bull World Health Organ. 2001;79:262–3. [PMC free article] [PubMed] [Google Scholar]
- 11.Crede CSF. Die Verhütung der Augenentzündung der Neugeborenen [Prevention of inflammatory eye disease in the newborn] Archiv fur Gynaekologie. 1881;18:367–70. [Google Scholar]
- 12.Moore DL, MacDonald NE Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Paediatr Child Health. 2015;20:93–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Darling EK, McDonald H. A meta-analysis of the efficacy of ocular prophylactic agents used for the prevention of gonococcal and chlamydial ophthalmia neonatorum. J Midwifery Womens Health. 2010;55:319–27. doi: 10.1016/j.jmwh.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Nishida H, Risemberg HM. Silver nitrate ophthalmic solution and chemical conjunctivities. Pediatrics. 1975;56:368–73. [PubMed] [Google Scholar]
- 15.USPSTF. Final Recommendation Statement: Ocular Prophylaxis for Gonococcal Ophthalmia Neonatorum: Preventive Medication. 2014 Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ocular-prophylaxis-for-gonococcal-ophthalmia-neonatorum-preventive-medication.