Abstract
Maintenance of genomic integrity is critical for adaptive survival in the face of endogenous and exogenous environmental stress. The loss of stability and fidelity in the genome caused by cancer and cancer treatment provides therapeutic opportunities to leverage the critical balance between DNA injury and repair. Blocking repair, and pushing damaged DNA through the cell cycle using therapeutic inhibitors, exemplifies the “pushmi-pullyu” effect of disrupted DNA repair. DNA repair inhibitors can be separated into five bio-functional categories: sensors, mediators, transducers, effectors, and collaborators that recognize DNA damage, propagate injury DNA messages, regulate cell cycle checkpoints, and alter the microenvironment. The result is cancer therapeutics that takes advantage of clinical synthetic lethality, resulting in selective tumor cell kill. This manuscript reviews recent considerations related to DNA repair and new DNA repair inhibition agents and organizes those findings to address future directions and clinical opportunities.
Keywords: DNA damage repair, homologous recombination defects, DNA damage response, genomic instability, mutational burden, synthetic lethality
Opinion
The “pushmi-pullyu” of DNA repair
What allows individuals to be so remarkably different, yet so very similar, is their genomic variability. Such variability, encoding brown versus blue eyes, blond versus brown hair, and other traits, occurs from normal selection for genomic variants. The hallmark of our longevity and responsiveness to environmental and other exposures is our genomic stability, plasticity and genomic vigilance. This stability is the result of ongoing monitoring, recognition, and response to genomic changes, through a series of DNA recognition and repair pathways [3]. Repair is a normal and necessary function in numerous cellular compartments. The ability of our immune cells to respond to and remember antigen stimulation is associated with purposeful real time genomic remodeling. However, such ongoing normal events also have the potential to inadvertently introduce genomic variability and opportunity for genomic injury. It is estimated that cells may have more than 20,000 single strand DNA break (SSB) events per day, with a small fraction going on to double strand breaks (DSB) [4, 5].
Mammalian cells have evolved a number of DNA damage repair pathways with independent, selective and specific purposes, while also interweaving key proteins and interactions [6, 7]. DNA repair pathways are on the lookout for injury and have the responsibility to repair DNA damage or declare futility and trigger apoptosis. This dynamic between DNA damage and repair creates tension within the cell, much like the mythical pushmi-pullyu of Dr. Doolittle [8]:
“Yes,” said the pushmi-pullyu—“to the Abyssinian Gazelles and the Asiatic Chamois—on my mother’s side. My father’s great-grandfather was the last of the Unicorns.”
But you could not do this with the pushmi-pullyu—because, no matter which way you came towards him, he was always facing you.
DNA injury is one head of the pushmi-pullyu, the head opposing DNA damage repair. Genomic stability is the fulcrum between injury and repair.
TP53 has been called “the guardian of the genome” as it is one of the first proteins to be activated with early SSB events [9–11]. Under normal circumstances, its phosphorylation and activation leads to a halt in the cell cycle to allow DNA repair to occur. This stop and wait sign is lost when TP53 is mutated, an early and frequent occurrence in a large proportion of solid tumors. In such cases, the genomic injury is not repaired and is carried into progeny cells. Accumulation of DNA damage is also a trigger for necroptotic death and mitotic catastrophe, depending upon the type of damage [12, 13].
DNA damage and repair pathways
DNA damage and repair pathways have evolved from the less complex prokaryote and lower eukaryote process to a series of distinct and interactive pathways [14]. These DNA repair pathways are the toggles between cell cycle arrest for either repair or apoptosis, and propagation of damage via its conversion into permanent injury. Pathways have specialized to recognize specific subsets of single strand DNA error and repair, such as mismatch repair, limited base errors, and crosslinks [3, 15] (Figure 1). Independent pathways for double strand repair, homologous recombination repair (HR), a high fidelity repair used when there is a sister chromatid available, and the pathways of non-homologous end-joining, which are repair pathways of convenience, where the DNA is just re-linked [15], also exist.
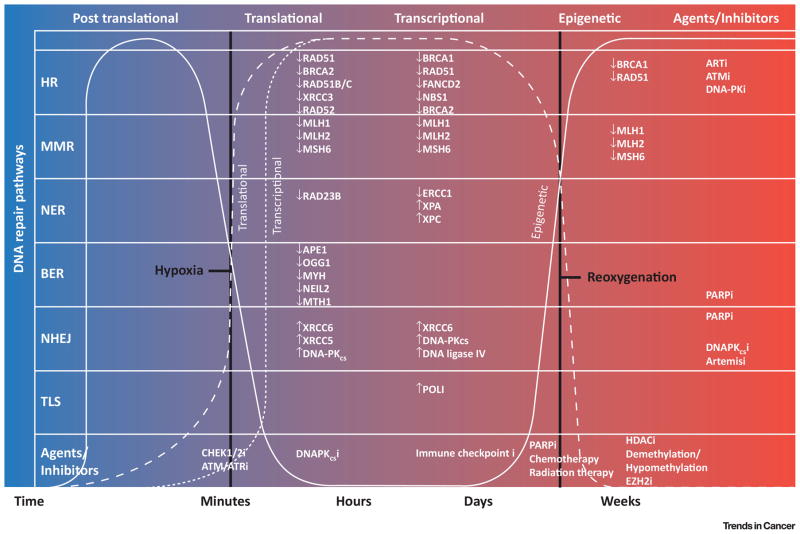
Figure 1. Hypoxia-Regulated DNA Repair.
During DNA replication, translation and transcription, as well as during epigenetic and post-translational modification, DNA is repaired using a number of pathways including HR, MMR, NER, BER, NHEJ, and TLS. DNA repair protein expression is sensitive to and modulated by hypoxia and oxia. In hypoxic conditions, DNA repair pathway protein expression is decreased in many instances, and NER, BER and NHEJ proteins involved in the DNA damage response are increased. The primary point of action for specific agents and inhibitors are indicated by repair pathways, damage response, and as a function of the stage of DNA replication and modification. During replication and repair, the hypoxic microenvironment may predispose to a loss of function phenotype.
Abbreviations: HR, homologous recombination; MMR, mismatch repair; NER, nucleotide excision repair; BER, base excision repair; NHEJ, non-homologous end joining; TLS, translesion synthesis; RAD51, RAD51 recombinase; BRCA2, BRCA2, DNA repair associated; RAD51B/C, RAD51 paralogs B and C; XRCC3, X-ray repair cross complementing 3; RAD52, RAD52 homolog DNA repair protein; MLH1, mutL homolog 1; PMS1, postmeiotic segregation increased 1; MSH6, mutS homolog 6; RAD23B, RAD23 homolog B; APE1, apurinic/apyrimidinic endonuclease 1; OGG1, 8-oxoguanine DNA glycosylase; MYH, mutY DNA glycosylase; NEIL2, nei like DNA glycosylase 2; NUDT1, nudix hydrolase 1; XRCC6, X-ray repair cross complementing 6; XRCC5, XRCC5, X-ray repair cross complementing 5; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; FANCD2, fanconi anemia complementation group D2; NBN, nibrin; ERCC1, excision repair cross-complementation group 1; XPA, xeroderma pigmentosum, complementation group A; XPC, xeroderma pigmentosum, complementation group C; POLI, DNA polymerase iota; i, inhibitor; ATRi, ataxia telangiectasia and Rad3-related kinase inhibitor ; ATMi, ataxia telangiectasia mutated kinase inhibitor ATM serine/threonine kinase inhibitor; DNA-PKi, DNA-dependent protein kinase inhibitor; POLE, DNA polymerase epsilon; ARID1a, AT-rich interaction domain 1A; EZH2i, enhancer of zeste homolog 2 inhibitor; PARPi, poly(ADP-ribose) polymerase inhibitor; CHEK1/2i, checkpoint kinase 1/2 inhibitor; chemo, chemotherapy; RT, radiotherapy; HDACi, histone deacetylase inhibitor
Protein and enzymatic modulators of DNA repair pathways have five roles (Text Box 1 and Table 1) [15, 16]. Sensor proteins recognize and flag injury, to initiate the DNA damage repair and recruit proteins to activate the repair response. Mediators are multifunctional and exert their effects in more than one phase of cell cycle progression. Signal transducers are enzymes that control the activity of the cell cycle checkpoints and DNA repair pathways; transducers relay and amplify these damage signals along adjacent chromatin structures. Effectors permit and/or repair DNA injury and block progression through the cell cycle, and may lead to accumulation of mutations and damage result in genomic instability [5, 17, 18]. The collaborators may be important in modulating the immune and oxygen-regulated angiogenesis of the cellular microenvironment. They harness the microenvironment, angiogenesis and alter the immune environment. The processing of genomic and genetic injury is a therapeutic opportunity. It has been well leveraged over the decades with the development of classical chemotherapies that cause injury to DNA via crosslinking, intercalation, nucleotide-mimetic substitution, or prevention of nucleotide production [19]. Chemotherapy works to drive injury to irreparable levels, preferentially in cancer cells. Now, the category of DNA repair inhibitors, DNARi, is demonstrating clinical activity, especially in the categories of sensors, mediators, transducers, and effectors.
Text Box 1. What defines clinical synthetic lethality and DNA repair inhibitors.
Clinical synthetic lethality: common underlying event that causes a gain- or loss-of-function phenotype or drug that, when combined with a drug targeted to a mutation or dysfunctional pathways augments antitumor effects
-
The “pushmi-pullyu” effect of DNA repair
DNA damage repair pathways collaborate to oversee DNA fidelity and activate damage repair
DNA damage accumulates due to injury and inhibition of repair; this triggers apoptosis or mitotic catastrophe
The push and pull between damage and repair inhibition creates a window of opportunity for therapeutic intervention
-
Bio-functional classification of DNA repair inhibitors (DNARi’s)
Sensor-drugs block recognition of DNA injury
Mediator-drugs block damage recognition and repair
Transducer-drugs block injury message propagation in the cell cycle
Effector-drugs permit and/or repair DNA injury and block cell cycle progression
Collaborator-drugs or microenvironment events cause a loss- or gain- of-function phenotype altering expression of DNA damage repair proteins or cell cycle regulators
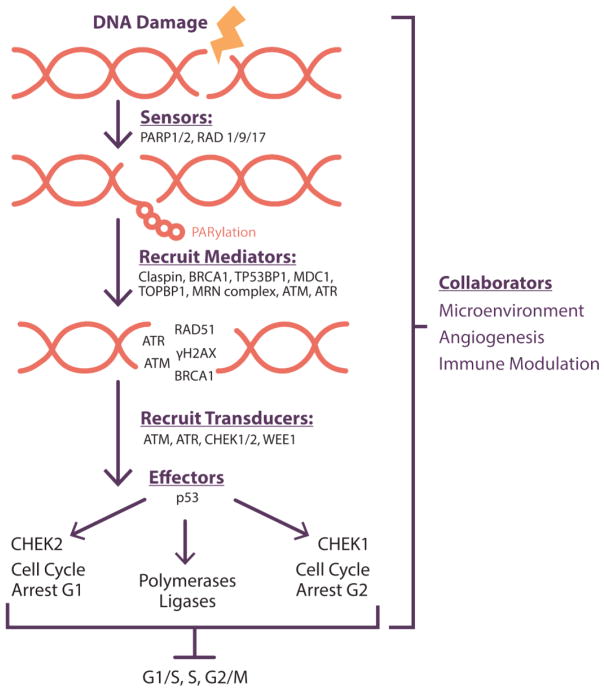
Figure I. Bio-functional Classification of DNA Repair Inhibitors.
The drugs used to block DNA repair can be categorized by their functions in maintaining genomic fidelity. Sensors recognize DNA damage and create flags to signal injury presence. They secondarily participate in recruitment of proteins to mediate activation of the DNA repair pathways. Mediators are multifunctional and exert their effects in more than one phase of the cell cycle progression mediated by the cell cycle checkpoints. Signal transducers are enzymes, such as polymerases, kinases, and phosphatases, that control the activity of the cell cycle checkpoints after DNA damage and/or effect physical change on the DNA, such as exonuclease or ligase functions. Effectors block the progression through the cell cycle under normal conditions, to allow for DNA repair. Dysregulation of such effectors with rapid movement through the cell cycle may lead to accumulation and propagation of deleterious mutations and subsequent damage resulting in genomic instability. The collaborators may be important in modulating the immune and oxygen-regulated cellular microenvironment such as to either augment injury, activate immune response to neoantigens, or provide reactive oxygen species.
Abbreviations: PARP1/2, poly(ADP-ribose) polymerase 1/2; RAD1/9/17, RAD1 checkpoint DNA exonuclease, RAD9 checkpoint clamp component, RAD17 checkpoint clamp loader component; BRCA1, BRCA1, DNA repair associated; TP53BP1, tumor protein p53 binding protein 1;
MRN complex, MRE11-RAD50-NBS1 complex; ATM, ATM serine/threonine kinase inhibitor; ATR, ATR serine/threonine kinase inhibitor; RAD51, RAD51 recombinase; H2AX, H2A histone family member X; CHEK1/2, checkpoint kinase 1/2; WEE1, WEE1 G2 checkpoint kinase
Table 1.
Classes of DNA repair inhibitors, DNARi
| Pathways | Targets | Agents/Inhibitors | |
|---|---|---|---|
| SENSORS | DNA damage recognition | ||
| PARP | olaparib, niraparib, rucaparib, veliparib talazoparib | ||
| MEDIATORS | DNA injury message propagation | ||
| ATM | AZD0156 | ||
| ATR | VX-970, AZD6738 | ||
| DNA-PKcs | CC-115, MSC2490484A | ||
| TRANSDUCERS | DNA injury message propagation | ||
| ATM | |||
| ATR | |||
| DNA-PKcs | |||
| Cell cycle checkpoint regulation | |||
| G1/S | CHEK1 | SCH-900776/MD8776 | |
| CHEK2 | LY2606368 | ||
| CDK4/6/9 | palbociclib, dinociclib, abemaciclib | ||
| G2/M | CHEK1/2 | LY2606368, PD-0332991, prexasertib | |
| WEE1 | AZD1775 | ||
| CDK1/2 | AG-024322, AT7519, flavopirodol, Eisai7070, SCH | ||
| EFFECTORS | Cell cycle checkpoint regulation | ||
| G1/S | CHEK1 | ||
| CHEK2 | |||
| G2/M | CHEK1/2 | ||
| WEE1 | |||
| p53 | Mdm2 | ||
| Antagonists | Nutlin-3, Nutlin-3a, YH239-EE, NVP-CGM097, RG7112, SAR299155, RG7388, AMG232, DS-3032b, MK8242 | ||
| Activators | NSC207895 | ||
| COLLABORATORS | Altered microenvironment (e.g., angiogenesis and immune) | ||
| VEGF ligand blockers | bevacizumab | ||
| VEGFR inhibitors | cediranib, sunitinib, pazopanib, others | ||
| Immune checkpoint inhibitors | pembrolizumab, nivolumab, durvalumab, atezolumab, ipilumamab, tremelimumab | ||
DNA repair inhibition as a therapeutic target
DNA repair is one side of the DNA stability see-saw. The possibility of targeting key elements of DNA repair pathways for therapeutic benefit gained traction after the recognition that disruption of the poly(ADP) ribose polymerase (PARP) sensor signal could selectively injure cells deficient in HR due to mutational loss of BRCA1 and BRCA2 function [20, 21]. This class of agents built on the biology of competitive inhibition of NAD, necessary for function of the PARP enzyme, went from preclinical concept to first international approval for patient use in approximately one decade. It also was the first-in-class of the DNARi class of cancer therapeutics.
Normal cellular homeostasis revolves around the recognition of injury, the first key checkpoint of sensors. TP53 is a central checkpoint protein, responsible for stopping cell cycle activity to allow for activation of repair pathways, functioning as a mediator, transducer, and indirect effector (Figure 2). TP53 is also the most frequently mutated gene in solid tumors, with both gain-of-function and loss-of-function mutations promoting dysfunctional cell cycle progression and pushing conversion of mutational injury into permanent genome injury [16, 22]. Attempts at recovering p53 function, initially by gene therapy introduction of wild type transcripts were unsuccessful [23], and now alternative methods to regulate the DNA repair recognition checkpoints are being developed.
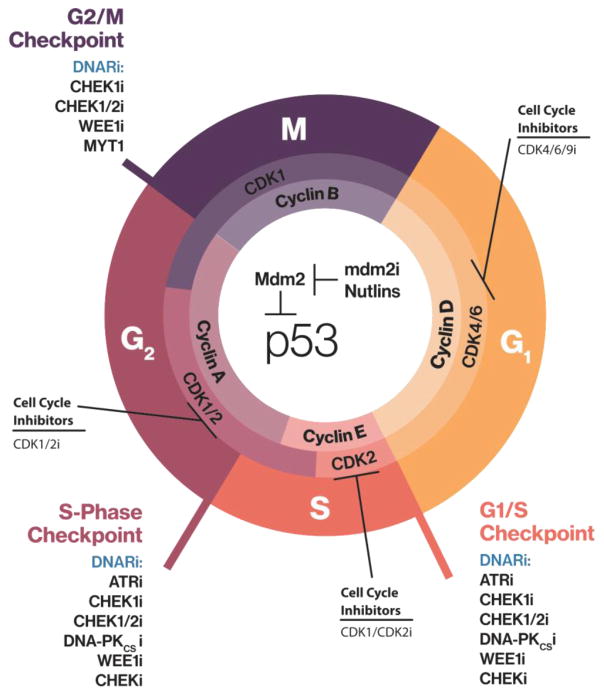
Figure 2. Effect of DNA Damage Repair Inhibitors on Cell Cycle Progression.
TP53 is a sensor that guards the integrity of the genome by altering progression through the cell cycle at the G1/S, S, and G2/M checkpoints. Progression through the cell cycle is tightly regulated by cyclins and cyclin-dependent kinases. Blockade of cell cycle checkpoints propagates DNA damage by permitting the replication of unrepaired DNA and increasing mutational burden. All these events lead to genomic instability and enhanced susceptibility to DNA repair inhibitors, such as those listed on the figure.
Abbreviations: i, inhibitor; DNARi, DNA repair inhibition; CHEK1, checkpoint kinase 1 inhibitor; CHEK1/2i, checkpoint kinases 1/2; WEE1, WEE1 G2 checkpoint kinase; MYT1, myelin transcription factor 1; CDK1/2, cyclin dependent kinases 1/2; CDK4/6/9, cyclin dependent kinases 4/6/9; ATR, ataxia telangiectasia and Rad3-related kinase; DNA-PKCS, DNA-dependent protein kinase catalytic subunit; MDM2, MDM2 proto-oncogene; TP53, tumor protein p53.
The new therapeutic direction of DNARi can be defined functionally by the five categories in Text Box 1 and Table 1. The first class of anticancer drugs targets the sensors, agents that interrupt injury recognition. Agents in this category include inhibitors of pathways that flag DNA injury, exemplified by olaparib, the PARP inhibitor. The second class of drugs block mediators of DNA damage repair and includes, for example, inhibitors of ATM and ATR. These proteins translate the message of PARylation to coordinate generation of a further message. DNARi that block message transducers, the third class, prevent or limit signals that recruit repair proteins, can include inhibitors of CHEK1 and CHEK2, and WEE1, affecting the cell cycle indirectly and linking cell cycle regulation with DNA repair. The fourth group of inhibitors block the effector proteins, such as cell cycle checkpoint regulators, the cyclin dependent kinases, and CHEK1/2 and WEE1, to block the normal cell cycle stop signals allowing cells to focus energy into DNA repair. The direct inhibition of WEE1 kinase and CHEK1/2 kinases and their interaction with DNA injuring agents, and with other DNARi, augments injury and pushes cells through the cell cycle, locking in DNA injury [5, 16]. The final category contains the collaborators, those modulators that promote DNA damage production indirectly. Key examples in this class are the drugs inhibiting angiogenesis and those that modulate immune activation through immune checkpoint inhibitors. Hypoxia promotes DNA injury through multiple potential mechanisms ranging from transcriptional downregulation of DNA repair proteins such as BRCA1 and BRCA2, production of reactive oxygen intermediates, and genomic instability and mutator phenotypes [24, 25]. The latter events may promote neoantigen production, recognized by the awakened immune system in the case of immune checkpoint inhibitor therapy.
Clinical synthetic lethality
Clinical synthetic lethality pushes the concept of synthetic lethality from pairs of Drosophila mutations to treatment paradigms for the cancer clinic. Drugs that cause a loss- or gain-of-function phenotype coupled with cancer specific genomic events, or drugs that are genotypically injurious can result in tumor cell kill. The context of the therapeutic opportunity is important in optimizing selective anti-tumor effects. Examples of six opportunities are described wherein a cellular-endogenous event or an environmental-exogenous event occurs that either initiates or propagates DNA injury. That injury may synergize with DNARi to create clinical synthetic lethality (Text Box 2/Figures 3 and 4).
Text Box 2. Clinical synthetic lethality and therapy in oncology.
Investigators most often factor in synergy and antagonism, or additivity and super-additivity, rather than synthetic lethality, when developing combination therapies in cancer cell lines or animal models. Synthetic lethality was originally described when two or more separate mutations in Drosophila models led to organism death; whereas, as a single mutation was compatible with viability [1]. The concept of synthetic lethality was further described by McLornan et al. to include chemical interactions and contextual changes [2]. We now advance this to clinical synthetic lethality, which focuses on generating anticancer treatments with a wide therapeutic index, sparing normal cells while killing tumor cells. Clinical synthetic lethality either screens for or uses drugs that cause a change of function phenotype, which collaborates clinically with drugs or tumor environments that are injurious. DNA is altered either endogenously by propagation of germline events or somatic mutation, or exogenously through exposures and modifiers that cause DNA and chromosomal damage. Interruption of normal genomic vigilance and repair prevents normal correction of those abnormalities. This can occur through blocking signals of DNA injury, transduction of the signal to the repair complex, or prevention of proper function of the repair complex. Progression through the cell cycle without arrest for repair is one example of prevention of repair complex function; such progression maintains the DNA injury and can promote subsequent mitotic catastrophe and cell death. Complexing DNA injury with dysfunctional or absent DNA repair creates the opportunity for augmenting DNA damage to a lethal level in the tumor cell preferentially. Thus, clinical synthetic lethality translates the ideas of chemical and contextual synthetic lethality into novel therapeutic regimens for cancer patients.
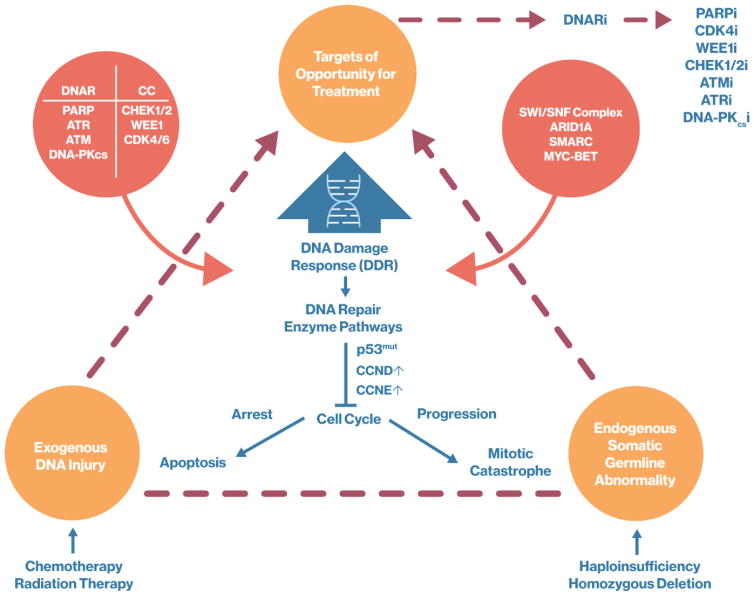
Figure 3. Targets of Opportunity in DNA Damage Response (DDR) and Homologous Recombination Deficiency (HRD).
Endogenous and Exogenous DNA abnormalities and injury result in the DNA damage response and DNA repair, and lead to cell cycle arrest. Arrest of the cell cycle may result in apoptosis or mitotic catastrophe. These events provide targets of opportunity for the treatment of genomically unstable cancer through the inhibition of DNA repair (DNAR) and cell cycle (CC) progression. DNA repair inhibitors (DNARi) block enzymes and proteins critical for DNA replication and repair including PARPi, CDK4i, Wee1i, CHEK1/2i, ATMi, ATRi and DNA-PKcsi.
Abbreviations: DNAR, DNA repair; i, inhibitor; DNARi, DNA repair inhibitor; PARP, poly(ADP-ribose) polymerase; PARPi, poly(ADP-ribose) polymerase inhibitor; ATR, ATR serine/threonine kinase; ATM, ATM serine/threonine kinase; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; CCi, cell cycle; CHEK, checkpoint kinase; CHEK1/2i, checkpoint kinase 1/2 inhibitor; ATMi, ATM serine/threonine kinase inhibitor; ATRi, ATR serine/threonine kinase inhibitor ; DNA-PKcsi, DNA-dependent protein kinase catalytic subunit inhibitor; WEE1, WEE1 G2 checkpoint kinase; WEE1i, WEE1 G2 checkpoint kinase inhibitor; CDK4/6, cyclin dependent kinase 4/6; CDK4/6i, cyclin dependent kinase 4/6 inhibitor; SWI/SNF, switching defective/sucrose nonfermenting; ARID1a, AT-rich interaction domain 1A; SMARC, SWI/SNF related, matrix associated, actin dependent regulator of chromatin; MYC-BETi, MYC and bromodomain and extra-terminal inhibitor; DDR, DNA Damage Response; p53mut, tumor protein 53 mutated; CCND, cyclin D; CCNE, cyclin E
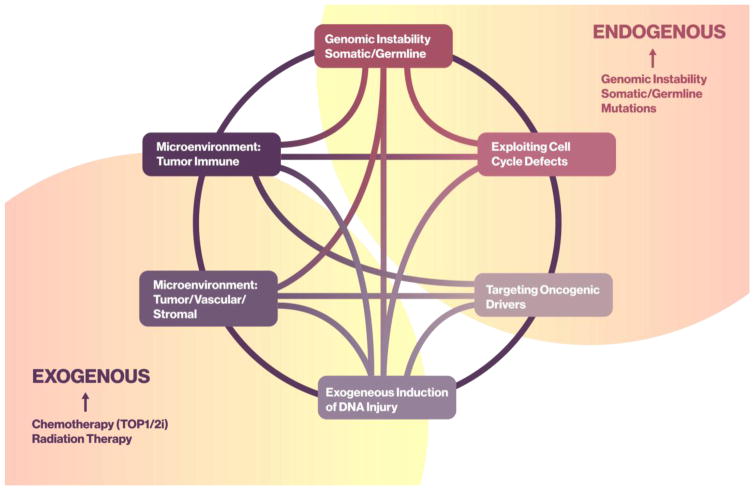
Figure 4. Endogenous and Exogenous Modulators of Clinical Synthetic Lethality.
A circos plot cartoon is used to describe the relationship between endogenous and exogenous inducers or modulators of DNA damage. This DNA damage may collaborate with DNA repair inhibition to create a clinical synthetic lethal event. Endogenous effectors include genomic instability, somatic/germline mutations, cell cycle defects and oncogenic drivers; whereas, exogenous effectors include the events within the cancer cell microenvironment, immune- or tumor/vascular/stromal-mediated DNA injury, and exogenous processes such as ambient or therapeutic radiation and chemotherapeutics.
Abbreviations: TOP1/2i, topoisomerase 1/2 inhibitor; i, inhibitor
Preexisting or endogenous cellular loss- or gain-of-function events (Figure 4) can be leveraged by the introduction of DNARi to maximize injury, drive cell death, and promote neoantigen production while sparing normal cells. Examples of this include accumulated tumor or germline genomic errors; such is seen with deleterious germline mutations in BRCA1 or BRCA2. Tumors arising in this background sustain a second injury and have a loss of function essential to normal HR function. Resultant cancers are more susceptible to DNA injury, such as platinum chemotherapy exposure [26], or inhibition of DNA repair, such as PARPi treatment [27]. Similar data are emerging with somatic mutations in the SWI/SNF complex gene ARID1a [28], with a potential for loss or reduced HR function and potential increased contextual clinical synthetic lethality [29]. Another opportunity for a cooperative interaction with DNARi is somatic amplification or mutation in cell cycle proteins causing dysfunctional cell cycle dynamics and replication stress. This has been demonstrated with amplification of cyclins D and E in breast and ovarian cancers [30–32]. The presence of pluripotent oncogenic drivers, such as mutation in TP53 or Rb, may also result in altered cell cycle dynamics that may confer potential for augmentation of injury in a DNARi background, such as the use of a WEE1 inhibitor [33, 34]. Lastly, patients whose tumors have a high mutational load or burden may be more susceptible to immune checkpoint inhibitors with DNARis, and combinations of these agents are now in clinical trials [5].
Exogenous events also can synergize with DNARi for clinical benefit within the tumor milieu (Figure 4). Radiation and/or chemotherapy create DNA damage; that damage by itself may be insufficient to induce cell death. Preclinical and clinical data indicate that inclusion of DNARi agents, such as PARPi or inhibitors of the G2/M checkpoint kinases, WEE1 or CHEK1, can be additive and/or synergistic with radiation and chemotherapy to improve outcome [33, 35, 36]. The addition of WEE1 kinase inhibition to radiation increases cell injury, with further injury when cisplatin is added [33, 37, 38]. Interestingly, some of the DNARi, such as WEE1 kinase inhibitors, have had limited activity as single agent treatments. Some increase in activity was observed in patients with BRCA1/2 mutations, and/or in combination with DNA damaging agents such as platinums, demonstrating both endogenous and exogenous clinical synthetic lethality with DNARi [39, 40]. Such combinations may, however, result in increased normal cell toxicity and studies optimizing the scheduling of these combinations may be needed to yield optimal patient benefit.
A major exogenous opportunity for therapeutic interaction is altering either the tumor stromal or immune microenvironments. A clinically robust example is found with the collaboration between DNARi and angiogenesis inhibition. Inhibition of VEGF receptors 1, 2, and 3 by the VEGFR tyrosine kinase inhibitor cediranib alters local hypoxia; cediranib markedly improved clinical outcome in ovarian cancer patients when combined with the PARPi, olaparib [41]. The mechanisms behind this successful combination are still being elucidated, and induction of endothelial cell precursors indicate that hypoxic injury was occurring [42]. Hypoxia downregulates the expression of BRCA1, which may in part explain this drug combination’s interaction [24]. There also is anticipated synergistic interaction between DNARi-injured cells and the local tumor immune microenvironment [43, 44]. DNARi propagate any existing DNA injury within the tumor by the dysregulation of DNA repair and/or altered cell cycle kinetics preventing the time for repair necessary or optimal repair (Figure 3).
Stabilizing such mutations creates the opportunity for the presentation of clonal and subclonal neoantigens to tumor infiltrating immune cells [45, 46]. Combining a selective antigen-rich environment with immune checkpoint inhibitors to overcome tumor immune tolerance is a novel clinical synthetic lethality approach currently in early phase clinical development. Here, the DNARi augment a locally rich immune milieu and the immune checkpoint inhibitor revises the local immune microenvironment to reactivate immune surveillance, to recruit CD8+ T cells, and to reduce Treg suppressor cells. This may be further enhanced by the pharmacological manipulation of transcription, driving increased expression of these neoantigens. The presence of such immunogenic neoantigens increases in tumors with a high mutational load, and immune (re)activation may be more likely to occur (Figure 3). Leveraging DNA damage repair inhibition while pushing cancer cells toward cell cycle arrest, apoptosis and mitotic catastrophe leads to the perfect storm in the tumor microenvironment and synthetic lethality in clinical settings.
Selection paradigms to optimize clinical synthetic lethality
Selection of patients for treatment with DNARi’s using markers of homologous repair defects have started. At present, only deleterious germline BRCA1/2 mutations are considered predictive, prognostic, and fit-for-purpose when selecting patients for treatment with PARPi. Controversy still surrounds the definition of tumor BRCA1/2 mutation as the requirement for homozygosity has not been clarified. This selective/enrichment biomarker has been validated in clinical trials in patients with breast and ovarian cancer [47, 48]. A variety of commercial laboratory assays are available for identifying deleterious BRCA mutations. NGS or whole exome/genome sequencing panels to be used to identify mutations in DNA repair genes are easily in reach, though have not been comprehensively or prospectively validated in large prospective clinical trials to confirm their predictive or prognostic significance, except in men with prostate cancer where germline screening is being recommended [49, 50].
The development of selection and enrichment biomarkers for HRD and other mutations is a high priority in clinical trials with combinations of drugs selected to target synthetic lethality. Approaches using bioinformatics analyses of tumor genomics may identify synthetic lethal interactions. Exceptional responders should be evaluated using exome/transcriptome and whole genome including functional studies to determine the unique nature of response [51]. For example, response to platinum therapy is associated with nucleotide excision repair defects in ovarian cancer [52]. The development of targeted screening tools, prevention treatment strategies for germline carriers and identification of agents that create loss of function phenotypes that selectively decrease DNA repair in tumor with limited or no impact in normal cells are just three examples of strategies to implicate clinical synthetic lethality.
A hybrid homologous recombination deficiency (HRD) score was built using weighted values from measures of loss of heterozygosity (LOH), telomeric imbalance (TAI), and large-scale state transitions (LST) [41, 53–55]; validation of this HRD score is anticipated soon. Evaluation of this HRD score was included as a prospectively planned objective of the NOVA study of niraparib, a PARPi, for treatment of high grade ovarian cancer patients. Recent developmental applications of this HRD score have suggested it may be applicable to triple negative breast cancer [55], and endometrial cancers.
Concluding Remarks
Clinical synthetic lethality can be broadly defined as an underlying event often seen in cancer that causes a gain- or loss-of-function phenotype or drug that, when combined with a drug targeted to a mutation or dysfunctional pathways augments antitumor effects. The dynamic interplay between DNA damage and repair inhibition, “pushmi-pullyu”, creates a window of opportunity for therapeutic intervention resulting in more than incremental improvement in clinical outcome (see Outstanding Questions box). Categorizing DNA repair inhibitors bio-functionally based on the role they play in DNA repair as sensors, mediators, transducers, effectors and collaborators, allows clinical cancer researchers to directly evaluate the inhibitors role in DNA repair and its associated interactions with other DNARi’s and the tumor microenvironment. Leveraging clinical synthetic lethality hopefully will lead to improved patient outcomes and survival.
Acknowledgments
To Kristie Magee, PhD and Peter Thielen at Technical Resources International for valuable assistance in the editing, proofreading and assembly, and the development of the figures that accompany this manuscript, respectively. Thank you.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Percy Ivy, Associate Chief, Investigational Drug Branch, Program Director, Experimental Therapeutics Clinical Trials Network, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, 9609 Medical Center Drive, Room 5W458, MSC 9739, Bethesda, MD 20852, Phone 01 240 276 6565.
Johann de Bono, Regius Professor of Cancer Research, Professor of Experimental Cancer Medicine and Honorary Consultant in Medical Oncology, Head of Division of Clinical Studies, Director of Drug Development Unit and Head of Prostate Cancer Targeted Therapy Group, ICR | Royal Marsden NHS Foundation Trust | Sycamore House | Downs Road | Sutton | Surrey | UK I SM2 5PT, T +44 (0) 208 722 4029 | F +44 (0) 208 642 7979.
Elise C. Kohn, Head, Gynecological Cancers Section, Clinical Investigations Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, 9609 Medical Center Drive, Room 5W436, MSC 9737, Bethesda, MD 20852.
References
- 1.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nature reviews Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 2.McLornan DP, et al. Applying synthetic lethality for the selective targeting of cancer. The New England journal of medicine. 2014;371:1725–1735. doi: 10.1056/NEJMra1407390. [DOI] [PubMed] [Google Scholar]
- 3.Dietlein F, et al. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends in genetics : TIG. 2014;30:326–339. doi: 10.1016/j.tig.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Lindahl T, et al. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995;20:405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor MJ. Targeting the DNA Damage Response in Cancer. Molecular cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 7.Willis NA, et al. Deciphering the Code of the Cancer Genome: Mechanisms of Chromosome Rearrangement. Trends in cancer. 2015;1:217–230. doi: 10.1016/j.trecan.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lofting H. The Story of Doctor Dolittle: Being the History of His Peculiar Life at Home and Astonishing Adventures in Foreign Parts Never Before Printed. Yearling Books; 1920. [Google Scholar]
- 9.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes & development. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 11.Williams AB, Schumacher B. p53 in the DNA-Damage-Repair Process. Cold Spring Harbor perspectives in medicine. 2016:6. doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, et al. Human Chk1 expression is dispensable for somatic cell death and critical for sustaining G2 DNA damage checkpoint. Molecular cancer therapeutics. 2003;2:543–548. [PubMed] [Google Scholar]
- 13.Nghiem P, et al. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood RD, et al. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 15.Sancar A, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annual review of biochemistry. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 16.Porro A. Editorial: Grappling with the Multifaceted World of the DNA Damage Response. Frontiers in genetics. 2016;7:91. doi: 10.3389/fgene.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Current opinion in cell biology. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Harper JW, Elledge SJ. The DNA damage response: ten years after. Molecular cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Cheung-Ong K, et al. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chemistry & biology. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 21.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Swisher SG, et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. Journal of the National Cancer Institute. 1999;91:763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 24.Glazer PM, et al. Hypoxia and DNA repair. The Yale journal of biology and medicine. 2013;86:443–451. [PMC free article] [PubMed] [Google Scholar]
- 25.Scanlon SE, Glazer PM. Multifaceted control of DNA repair pathways by the hypoxic tumor microenvironment. DNA repair. 2015;32:180–189. doi: 10.1016/j.dnarep.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottenberg S, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. The New England journal of medicine. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 28.Kim KH, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nature medicine. 2015;21:1491–1496. doi: 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitler BG, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nature medicine. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courjal F, et al. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. International journal of cancer. 1996;69:247–253. doi: 10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 32.Keyomarsi K, et al. Cyclin E and survival in patients with breast cancer. The New England journal of medicine. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 33.Morgan MA, et al. Improving the efficacy of chemoradiation with targeted agents. Cancer discovery. 2014;4:280–291. doi: 10.1158/2159-8290.CD-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller S, Haas-Kogan DA. WEE1 Kinase As a Target for Cancer Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3485–3487. doi: 10.1200/JCO.2015.62.2290. [DOI] [PubMed] [Google Scholar]
- 35.Sarcar B, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Molecular cancer therapeutics. 2011;10:2405–2414. doi: 10.1158/1535-7163.MCT-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nature reviews Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 37.Pouliot LM, et al. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer research. 2012;72:5945–5955. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowley R, et al. The wee1 protein kinase is required for radiation-induced mitotic delay. Nature. 1992;356:353–355. doi: 10.1038/356353a0. [DOI] [PubMed] [Google Scholar]
- 39.Leijen S, et al. Phase II study with Wee1 inhibitor AZD1775 plus carboplatin in patients with p53 mutated ovarian cancer refractory or resistant (<3 months) to standard first line therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(suppl) doi: 10.1200/JCO.2016.67.5942. abstr 2507. [DOI] [PubMed] [Google Scholar]
- 40.Oza AM, et al. An international, biomarker-directed, randomized, phase II trial of AZD1775 plus paclitaxel and carboplatin (P/C) for the treatment of women with platinum-sensitive, TP53-mutant ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(suppl) doi: 10.1158/1078-0432.CCR-20-0219. abstr 5506. [DOI] [PubMed] [Google Scholar]
- 41.Timms KM, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast cancer research : BCR. 2014;16:475. doi: 10.1186/s13058-014-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JM, et al. CECs and IL-8 Have Prognostic and Predictive Utility in Patients with Recurrent Platinum-Sensitive Ovarian Cancer: Biomarker Correlates from the Randomized Phase-2 Trial of Olaparib and Cediranib Compared with Olaparib in Recurrent Platinum-Sensitive Ovarian Cancer. Frontiers in oncology. 2015;5:123. doi: 10.3389/fonc.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi T, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaquin N, et al. DDR-mediated crosstalk between DNA-damaged cells and their microenvironment. Front Genet. 2015;6:94. doi: 10.3389/fgene.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGranahan N, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tutt A, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 48.Ledermann J, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. The New England journal of medicine. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 49.Mateo J, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pritchard CC, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. NEJM. 2016 doi: 10.1056/NEJMoa1603144. ( http://www.nejm.org/doi/pdf/10.1056/NEJMoa1603144) [DOI] [PMC free article] [PubMed]
- 51.Iyer G, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3133–3140. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ceccaldi R, et al. A unique subset of epithelial ovarian cancers with platinum sensitivity and PARP inhibitor resistance. Cancer research. 2015;75:628–634. doi: 10.1158/0008-5472.CAN-14-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abkevich V, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. British journal of cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birkbak NJ, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer discovery. 2012;2:366–375. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telli ML, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]