Abstract
Background
Coagulopathy is associated with massive transfusion (MT) in trauma, yet most clinical scores to predict this outcome do not incorporate coagulation assays. Previous work has identified that shock increases circulating tissue plasminogen activator(tPA). When tPA levels saturate endogenous inhibitors, systemic hyperfibrinolysis can occur. Therefore, the addition of tPA to a patient’s blood sample could stratify a patients underlying degree of shock and early coagulation changes to predict progression to MT. We hypothesize that a modified thrombelastography (TEG) assay with exogenous tPA, will unmask patients impending risk of MT.
Study Design
Trauma activations were analyzed using rapid TEG(R-TEG) and a modified TEG assay wuth low (Lt-TEG) and high dose of tPA(Ht-TEG). Clinical scores(Shock Index [SI], ABC, and TASH,) were compared to TEG measurements to predict the need for MT, using the areas under the receiver operating characteristic curves.
Results
324 patients were analyzed, 17% required MT. MT patients had a median SI=1.2, ABC score=1, and TASH score=12. R-TEG and tPA TEG parameters were different significantly different in all MT patients compared to non-MT patients (all p<0.02). The Lt-LY30 (lysis at 30 minutes) had the largest AUROC (0.86, 95%CI 0.79–0.93) for prediction of MT similar to INR0.86 (95%CI 0.81–0.91) followed by TASH (0.83, (95%CI 0.77–0.89). Combing INR and tPA-TEG variables results in a positive prediction of MT in 49% of patients with a 98% negative predictive value.
Conclusion
tPA-TEG identifies trauma patients who require MT efficiently, in a single assay that can be completed in a shorter time than other scoring systems, which has improved performance when combined with INR. This new method is consistent with our understanding of the molecular events responsible for trauma-induced coagulopathy.
Introduction
Early identification of patients who require a massive transfusion (MT) after injury remains a challenge. Current clinical tools to predict MT range from a simplified shock index based on heart rate and systolic blood pressure(1) to a more intensive calculation based on these variables in addition to injury mechanism, laboratory values, and imaging(2). Newer prediction models include automated calculations made with phone-based algorithms using similar variables(3) and scores calculated on pre-hospital paramedic assessment(4). The numerous scoring algorithms reflect the difficulty in correctly identifying patients who will require a MT and indicate a need to explore alternative methods, and many have been criticized for a poor positive predictive value(5).
Coagulation abnormalities in trauma are associated with increased blood product use (6–8). Endogenous traumatic coagulopathy(9), is common in patients with a high injury severity and advanced shock(10). This trauma induced coagulopathy (TIC), typically defined as an international normalized ratio of prothrombin time (INR) greater than 1.5, is associated with increased mortality from hemorrhage(11). Within TIC, there are distinct mechanisms that drive impaired thrombin generation versus excessive fibrinolytic activity (12, 13). Clinical studies indicate that hypotension is the driver of hyperfibrinolysis (14). In animals, tPA has been shown to rise during hemorrhagic shock, but not after tissue injury (15). Therefore, as a trauma patient progresses towards decompensated hemorrhagic shock, systemic tPA levels are anticipated to increase.
While elevated circulating tPA appears to drive this process, depletion of circulating inhibitors is necessary before patients develop an overt systemic hyperfibrinolytic phenotype. Whole blood of healthy individuals requires supra-physiologic concentrations of tPA to activate fibrinolysis in vitro due to the buffering capacity of plasma proteases (16). Based on evidence that hemorrhagic shock releases tissue plasminogen activator (tPA), and increases circulating tPA activity due to persistent depletion of its inhibitors, resulting in hyperfibrinolysis, we developed an assay to predict MT. We hypothesize that a modified thrombelastogram (tPA-TEG) with the addition of exogenous tPA (tPA-TEG) predicts the patients’ risk for requiring a MT more efficiently than current scoring systems.
Methods
Patients characteristics
Consecutive adult trauma patients meeting criteria for the highest level of activation at our level 1 trauma center (DHMC: Denver Health Medical Center) from 2014 to 2016 were included in this analysis. All patients had samples collected under protocols approved by the Colorado Multiple Institutional Review Board for prospective evaluation of coagulation in response to trauma. Patient demographics, injury mechanism, laboratory results, and transfusion requirements were recorded by professional research assistants who provide onsite, continuous coverage of the emergency department. Injury severity was measured by the maximum Abbreviated Injury Scale scores (max AIS) for the head/neck, chest, abdomen, and extremities, the Injury Severity Score (ISS) and the Glasgow Coma Scale (GCS).
Blood collection
Blood was collected in 3.5-mL tubes containing 3.2% citrate in the prehospital ambulance or upon arrival to the ED. Prehospital or ED healthcare workers drew study patient blood samples concurrently with the first set of blood samples used for in-hospital laboratory analysis. Professional research assistants performed TEG assays within 2 hours of blood draw. Additional assays were ordered at the discretion of the treating surgeon and performed by the hospital laboratory.
Viscoelastic Assays
All viscoelastic assays were conducted by a team of trained professional research assistants with extensive experience in multiple types of TEG assays. Citrated blood samples were analyzed using the TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles, IL, USA). The following indices were obtained from the tracings of the TEG: Reaction time (R-time min.), angle (°), maximum amplitude (MA [mm]), time to MA (TMA min.) and lysis 30 min after MA (LY30 [%]). Modified assays to quantify sensitivity to fibrinolysis were run in a parallel with traditional TEG assay (r-TEG). The same TEG analyzer was used for this assay, but prior to re-calcification we added exogenous tPA. This tPA challenge of whole blood has been previously validated to quantify fibrinolysis sensitivity and resistance in vitro in the assessment of the effects of different proteins (16, 17), as well as clinically in trauma patients (18). In brief, 500 microliters of whole blood were pipetted into a customized vial containing lyophilized tPA (Molecular Innovation, Novi, MI, USA) to a final concentration of 75 ng/mL or /mL of t-PA, and mixed by gentle inversion. A 340-µL aliquot of this mixture was then transferred to a 37 °C TEG cup, preloaded with 20 µL of 0.2 mol/ L of CaCl2. The same TEG parameters were recorded for the traditional rapid TEG (rTEG), as well as lower dose tPA (Lt-TEG) and higher dose (Ht-TEG). The time to obtain results was defined as the time to maximum amplitude (TMA), while the time to estimated LY30 is, by definition, 30 additional minutes after the TMA is reached.
Massive Transfusion Predictive Scores
Massive transfusion (MT) was defined as >4 units of red blood cells (RBC) per hour (19) or death attributed to hemorrhagic shock during the initial 6 hours postinjury. Clinical scores predicting MT were calculated upon hospital arrival and included the Shock Index (SI= heart rate/systolic blood pressure), assessment of blood consumption (ABC)(21), and trauma associated severe hemorrhage (TASH) (2).
Statistical Analysis
SPSS version 22 (IBM, Armonk, NY, USA) and SAS 9.4 for Windows (SAS Institute Inc., Cary, NC) were used for statistical analysis. TEG measurements are presented as median and interquartile values. Spearman's Rho was used to assess the correlation between LY30 and the other TEG parameters. This comparison aimed at assessing whether TEG measurements obtained earlier than LY30 (which requires 30 minutes post TMA), provide equivalent or better prediction. Comparisons of MT patients versus no MT patients were conducted with the Mann Whitney-U tests and Chi-square tests.
Using the area under the receiver operating characteristic (AUC) curves with 95% confidence intervals (95%CI), we compared the predictive performance for MT of the clinical scores compared to rTEG and tPA-TEG measurements as well as the conventional coagulation test, international normalized ratio (INR). The point with the highest specificity and sensitivity for MT on the receiver operating characteristic (ROC) curve was defined by the Youden index (21). We also assessed predictive power of positive (PV+) and negative (PV−) results, as these are more useful clinically than sensitivity and specificity. We then assessed the time to achieve the best cutoff for TEG variables (i.e. TMA or time to maximum amplitude) using the Friedman’s test to account for correlated data (i.e., values obtained within the same patient). Logistic regression analysis with MT as the dependent variable was used to identify if INR and fibrinolysis variables were independent predictors of MT. The analysis also included an interaction term between the two coagulation parameters in predicting massive transfusion to assess for an effect modification. All tests were two-tailed with alpha set to 0.05.
Results
Study Population
The study included 324 patients, who were predominantly men (81%), with a median age of 33 years (26–48), and injury severity score (ISS) of 16 (5–27). Penetrating injuries occurred in 47% of patients. The most common injury mechanisms were: gunshot wound (25%), motor vehicle crash (24%), and auto-pedestrian accident (12%). Overall, 17% required MT. Mortality rate was 13%, and of these deaths, 33% (n=14) were attributed to uncontrolled hemorrhage or circulatory arrest. A summary of patient demographic is listed in Table 1.
Table 1.
Descriptive Variables of Patient Population
| Variable | Overall population |
|---|---|
| Male sex, % | 81 |
| Age, y, n (%) | 33 (26–48) |
| Emergency department, n (%) | |
| Systolic blood pressure, mmHg | 112 (90–138) |
| Heart rate, beats per minute | 102 (82–118) |
| Glasgow Coma Score | 15 (7–15) |
| Penetrating, % | 47 |
| Injury severity score, n (%) | 16 (5–27) |
| Positive focused assessment with sonography in trauma, % | 22 |
| Massive transfusion, % | 17 |
| Death, % | 13 |
Massive Transfusion (MT) Prediction Scores
Patients requiring MT had a higher median ISS (32, 22–40), compared to patients who did not require MT (12, 4–22) (p<0.001). Patients requiring a MT had higher max AIS of the chest (3 vs. 0), abdomen (2 vs. 0) and extremities (2 vs. 0) compared to non-MT patients (all p<0.001) but not a significantly different rate of penetrating injuries (39% vs. 46% p=0.248). Of MT patients, 33% required greater than 4 RBC units/hour within the first hour postinjury, 19% in the second hour, 28% in the third hour, 10% between the fourth and sixth hours, while an additional 10% died due to uncontrolled hemorrhage within 6 hours postinjury.
The median emergency department (ED) INR in patients undergoing MT was 1.4 (1.2–1.8) vs. 1.1 (1.0–1.2) in non-MT patients (p<0.001). In patients that required a MT, all clinical scores and TEG variables were significantly differed from non-MT patients (Table 2). The predictive performance of these scores and coagulation tests for prediction of massive transfusion is summarized in Table 2. The TASH score had the highest AUC of all clinical scores (0.84 95% CI 0.79–0.90) while INR had an AUC of 0.86 (95%CI 0.81–0.91).
Table 2.
Massive Transfusion Prediction Scores and Coagulation Variables
| Cohort | SHOCK INDEX (n=324) |
ABC (n=324) |
TASH (n=316) |
INR (n=311) |
R-ACT, sec (n=324) |
R- ANGLE, (n=324) |
R-MA, mm (n=324) |
R-LY30 (n=324) |
N-LY30 (n=324) |
Lt- LY30 (n=324) |
Ht- LY30 (n=321) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall, median (25th–75th percentile) | 0.84 (0.67–1.1) | 1 (0–1) | 6 (3–11) | 1.1 (1.0–1.3) | 121 (113–136) | 71 (65–75) | 62 (56–66) | 1.9 (1.0–3.2) | 1.1 (0.4–2.1) | 8.3 (2.3–32.6) | 56 (32–73) |
| Non-massive transfusion, median (25th–75th percentile) | 0.80 (0.66–1.03) | 1 (0–1) | 5 (2–8) | 1.1 (1.0–1.2) | 121 (113–128) | 72 (67–76) | 63 (58–67) | 1.8 (1.0–2.9) | 1.0 (0.4–1.6) | 6.0 (2.0–1.8) | 50 (28–66) |
| Massive transfusion, median (25th–75th percentile) | 1.13 (0.89–1.46) | 1 (0–2) | 12 (10–16) | 1.4 (1.2–1.8) | 128 (117–156) | 65 (57–71) | 54 (43–62) | 2.6 (1.0–19.2) | 3.6 (0.8–22) | 60 (32–78) | 81 (73–92) |
| p Value | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | 0.016 | <0.001 | <0.001 | <0.001 |
p Value is massive transfusion vs non-massive transfusion.
ABC, assessment of blood consumption score; ACT, thrombelastography activated clotting time; Ht, high dose tissue plasminogen activator thrombelastography; INR, internal normalized ratio; Lt, low dose tissue plasminogen activator thrombelastography; LY30, percent clot lysis 30 minutes after reaching maximum amplitude; MA, maximum amplitude; N, native thrombelastography; R, rapid thrombelastography; TASH, trauma associated severe hemorrhage.
Among r-TEG parameters, the r-TEG MA had the greatest AUC (0.79 95%CI 0.73–0.85), while among tPA-TEG, the Lt-TEG LY30 had the greatest AUC (0.86 95%CI 0.79–0.93), which was similar to Ht-TEG LY30 (0.84 95%CI 0.77–0.91).
Optimal Cutoffs for TEG Indices, INR and Clinical Scores
The Youden index for the top three performing TEG variables and INR are listed in Table 2 with their associated sensitivity and specificity for predicting MT in addition to the three clinical scores. Using previously reported cut points for MT based on INR and clinical coagulation scores resulted in low specificity but high specificity with the exception of the TASH score (Table 3).
Table 3.
Predictive Performance of Coagulation Tests and Scores for Massive Transfusion
| Predictor of massive transfusion |
Area under curve (95% CI) |
Youden index |
Sensitivity, % |
Specificity, % |
Positive predictive value, % |
Negative predictive value, % |
|---|---|---|---|---|---|---|
| Lt-LY30 | 0.86 (0.79–0.93) | 27% | 84 | 82 | 50 | 96 |
| Lt-TMA | 0.79 (0.71–0.87) | 23 min | 67 | 85 | 45 | 92 |
| Ht-LY30 | 0.84 (0.77–0.91) | 71% | 80 | 84 | 50 | 95 |
| Ht-TMA | 0.78 (0.71 – 8.5) | 16 min | 84 | 70 | 36 | 94 |
| R-MA | 0.79 (0.73–0.85) | 57mm | 68 | 79 | 41 | 92 |
| INR | 0.86 (0.81–0.91) | 1.1 | 90 | 69 | 33 | 97 |
| Peltan (11)* | 1.5 | 42 | 95 | 58 | 88 | |
| TASH | 0.84 (0.79–0.90) | 8 | 87 | 76 | 38 | 97 |
| Brockamp (5)* | 8.5 | 86 | 74 | 44 | 96 | |
| Shock index | 0.70 (0.61–0.80) | 1.07 | 63 | 77 | 37 | 92 |
| Pottecher (39)* | 0.96 | 68 | 67 | 31 | 92 | |
| ABC | 0.66 (0.58–0.74) | 1 | 85 | 37 | 22 | 92 |
| Nunez (20)* | 2 | 38 | 82 | 30 | 87 |
Represent threshold recommend by prior researchers as thresholds for massive transfusion.
ABC, assessment of blood consumption score; Ht, high dose tissue plasminogen activator thrombelastography; INR, internal normalized ratio; Lt, low dose tissue plasminogen activator thrombelastography; LY30, percent clot lysis 30 minutes after reaching maximum amplitude; MA, maximum amplitude; R, rapid thrombelastography; TASH, trauma associated severe hemorrhage; TMA, time to maximum amplitude.
Expedited Results with Optimal tPA-TEG Indices
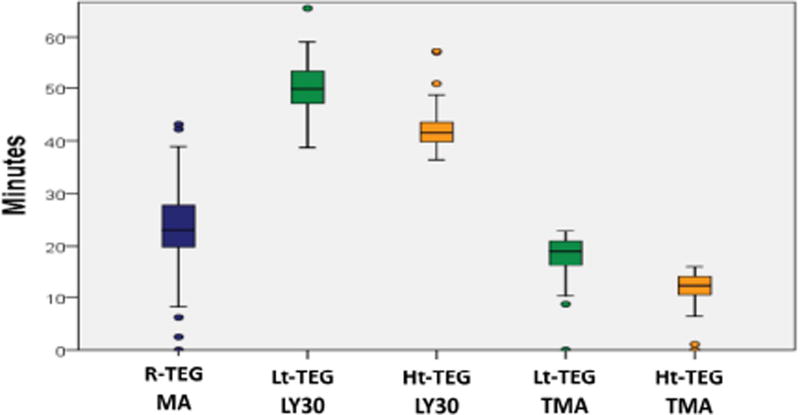
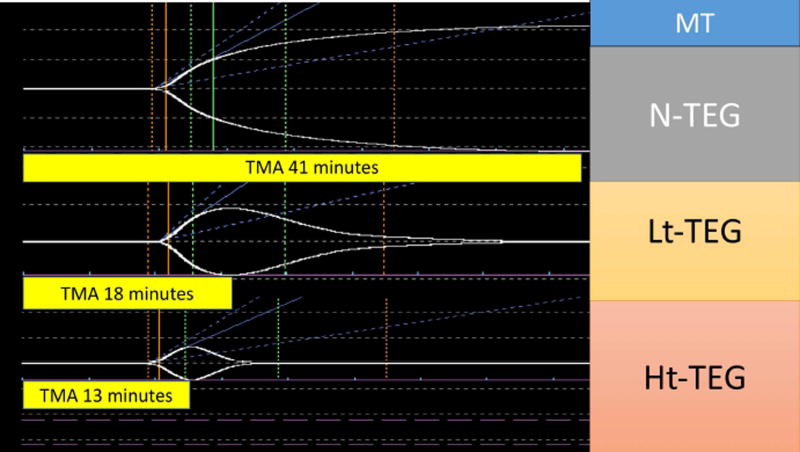
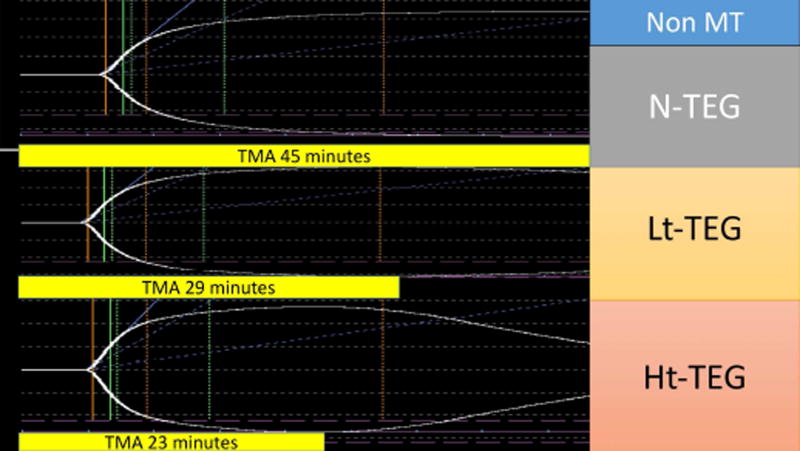
The median time to obtain a Lt-TEG LY30 > 27% was 50 minutes (47–53), compared to 42 minutes (40–44) for the Ht-TEG >71%, and 23 minutes (20–28) for the r-TEG MA < 57mm. Correlation of tpa-TEG indices obtained without the 30-minute delay required for assessment of LY30 are listed in Table 4. In both tPA-TEG tests the TMA had the highest correlation to LY30 and was used as a surrogate predictor of MT. The performance of the TMA of these tests to predict massive transfusion, Youden index, sensitivity and specificity as well as PV+ and PV− are detailed in Table 3. The median time to obtain a Lt-TEG TMA<23min was 19 minutes (16–21). In contrast, the median time with the Ht-TEG was significantly shorter (p<0.001 for all comparisons Figure 1), while retaining good performance (Table 3). Figure 2 provides a visual example of the differences in TMA in paired TEGs with and without tPA in a trauma patient that underwent a massive transfusion versus Figure 3 representing a trauma patient that did not require blood product resuscitation.
Table 4.
Correlation of Percent Clot Lysis 30 Minutes after Reaching Maximum Amplitude to Early LY30 to Early Tissue Plasminogen Activator Thrombelastography Variables
| TEG measurement |
Reaction time, min |
Angle | Maximum amplitude, mm |
Time to maximum amplitude, min |
|---|---|---|---|---|
| Lt-LY30 | Rho 0.06 | Rho −0.14 | Rho −.57 | Rho −0.77 |
| p Value | 0.301 | 0.014 | <0.001 | <0.001 |
| Ht-LY30 | Rho 0.01 | Rho −0.14 | Rho−0.70 | Rho: −0.81 |
| p Value | 0.926 | 0.012 | <0.001 | <0.001 |
Ht, high dose tissue plasminogen activator thrombelastography; Lt, low dose tissue plasminogen activator thrombelastography; LY30, percent clot lysis 30 minutes after reaching maximum amplitude; Rho, Spearman’s Rho correlation; TEG, thrombelastography
Figure 1.
Time to reach optimal cutoff to predict massive transfusion for the most predictive thrombelastography tests. Ht-TEG, high dose thrombelastography; Lt-TEG, low dose thrombelastography; LY30, percent clot lysis 30 minutes after reaching maximum amplitude; MA, maximum amplitude; R-TEG, rapid thrombelastography; TMA, time to maximum amplitude.
Figure 2.
Trauma patient requiring a massive transfusion. The x-axis represents time of the thrombelastography (TEG) tracing and the y-axis represents the clot strength. The figure represents 3 native TEGs that were run in a parallel with no tissue plasminogen activator (tPA), low dose (Lt) tPA, and high dose (Ht) tPA. The yellow bars below each tracing represent the time in minutes required to reach the maximum clot strength. TMA, time to maximum amplitude.
Figure 3.
Trauma patient not requiring a massive transfusion. The x-axis represents time of the thrombelastography (TEG) tracing and the y-axis represents the clot strength. The figure represents 3 native TEGs that were run in a parallel with no tissue plasminogen activator (tPA), low dose (Lt) tPA, and high dose (Ht) tPA. The yellow bars below each tracing represent the time in minutes required to reach the maximum clot strength. TMA, time to maximum amplitude.
Phenotypes of TIC
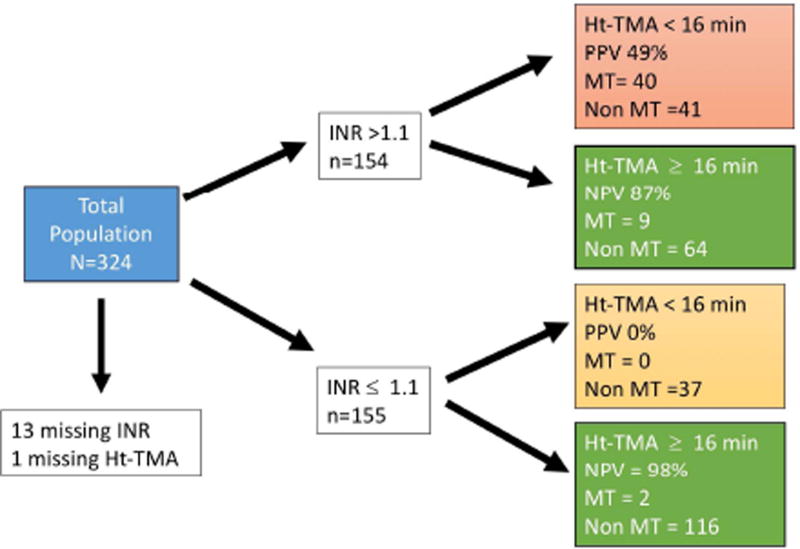
Logistic regression analysis to predict massive transfusion based off of INR versus fibrinolysis variables (LY30 and TMA) demonstrated independence of each variable in predicting massive transfusion. INR (p<0.001) and Ht LY30 (p<0.001) were significantly associated with MT in addition to INR*Ht LY30 (p<0.001) suggesting an effect modification. The same association were appreciated with Ht TMA (INT p=0.006, Ht TMA p<0.001, INR*Ht TMA p<0.001). When stratifying patients, a Ht-TMA < 16 minutes in patient with an INR > 1.1 had a positive predictive value of massive transfusion of 49%. In those patients with an INR ≤ 1.1 and an Ht-TMA > 16 minutes the negative predictive value of a massive transfusion was 98% (Figure 4).
Figure 4.
Stratification of patients by internal normalized ratio (INR) increases predictability of identifying patients that require a massive transfusion using the high dose (Ht) tissue plasminogen activator (tPA) thrombelastography (TEG) time to maximum amplitude (TMA). Roughly half of patients in the study had an INR greater than 1.1. After stratification of these patients the Ht-TMA cut off of less than 16 minutes had a positive predictive value (PPV) of 49% while an INR of less than 1.1 had an overall negative predictive value of 98%. In this schematic, 14 patients were excluded due to the patient not having laboratory assessment of INR or Ht-TEG. MT, massive transfusion.
Discussion
Approximately one in five patients in our trauma activation population underwent a MT or had an early death from exsanguination. Both clinical measurements and laboratory assays stratified the risk of patients who required a MT. In our study, the clinical score using the most variables (TASH), had the greatest AUC compared to other clinical predictors, but requires a combination of radiographs, ultrasound, and laboratory assays. Viscoelastic assays had good performance that was improved with a tPA challenge assay. The low dose tPA challenge LY30 had the best performance of all assays and scores assessed, with a AUC of 0.86 and sensitivity of 84% and specificity of 82% at its optimal cut points. The limitation of the Lt-TEG LY30 is a time delay of 50 min to obtain results. Both tPA challenge TEG LY30s had strong correlations with the time to MA. This TMA variable retained good performance for the Ht-TEG with a Youden index of 16 minutes. The Ht-TEG TMA has the shortest time to provide results for patients at risk of MT with a sensitivity of 80% and specificity of 74%. INR and fibrinolysis coagulation parameters are independently associated with massive transfusion and have an effect modification in predicting the outcome. When stratifying patients with an INR >1.1 the Ht-TMA has a positive predictive value of 49% for MT.
The shock index is a simple score to predict massive transfusion, which only requires a blood pressure and heart rate. The SI was calculated in 100% of patient in this study supporting the user friendly nature of this MT predictor. However, while vital signs are known as poor predictors of impending cardiovascular collapse, they tend to be maintained until advanced shock (22). In addition, vital signs are dynamic and can be altered by treatments and numerous clinical factors other than hemorrhagic shock. For example, alcohol intoxication reduces blood pressure and increases heart rate (23). These confounders impacting SI likely contribute to why this predictor has poor sensitivity in identify patients at risk of MT.
The ABC score adds clinical variables to improve the Shock Index with injury mechanism and the FAST exam, thus not available immediately upon admission. In addition, this score had the lowest performance of all clinical scores assessed. The ABC score has previously been reported to have a high AUC compared to other clinical scores (20), but performed poorly in our study. It is possible that this relatively low performance was due to a high incidence of penetrating trauma that did not result in significant injuries. Interestingly, the ABC score outperformed TASH and other clinical predictor scores in the rural setting (24), and may not be as applicable in a urban trauma center with high rates of penetrating trauma.
The TASH score incorporates all of the ABC variables in addition to specific injury patterns and laboratory values consistent with hemorrhagic shock including base deficit and hemoglobin. The TASH score had a high AUC that is consistent with previously reported studies (5, 25). However, the TASH score requires imaging and arterial blood gas measurements, although the creators of the TASH score claimed these variables are available within 15 minutes of ED arrival compared to the 40 minutes required for an INR test (26). Arguably, risk stratification for massive transfusion can be accomplished with base deficit and hemoglobin (27), but good performance is dependent on a high injury severity, which cannot accurately be predicted by pre hospital and emergency department personnel.
With our growing understanding of the physiologic response to hemorrhagic shock, coagulopathy is a logical indicator of risk for MT(8). Using an INR to identify severely injured patients with hemorrhagic shock is appealing as both pro and anticoagulants have been implicated in the pathogenesis of trauma induced coagulopathy(10). Indeed, INR has recently been shown to have superior predictability for MT than the ABC score(28), which is consistent with our findings. An INR of 1.1 of less is an excellent screening tool to rule out patients at risk of massive transfusion with a negative predictive value of 97%. However, the limitation of an INR is a delay in time to obtain results (26). On the horizon is the use a point of care (POC) test for INR in the pre hospital setting (29). POC- INR test remains to be validated with conventional laboratory plasma-based INR assays. Retrospective data suggested that a POC INR may be as effective for guiding transfusion as a r-TEG (30).
Our findings demonstrate the value of the tPA-TEG in unmasking coagulation changes that indicate a high risk of massive transfusion. Initiation of systemic hyperfibrinolysis appears to be related to the systemic release of tissue plasminogen activator (tPA) causing plasminogen activator inhibitor-1 (PAI-1) depletion, resulting in excessive plasmin activation(31). Animal data indicate that tPA release is related to hemorrhagic shock and not tissue injury(15). Furthermore, shock promotes fibrinolysis from non-trauma patients with pre hospital circulatory collapse, who have a high incidence of hyperfibrinolysis (32). This is also evident in healthy subjects undergoing mimicked shock with lower body blood pooling that increase their tPA levels 10 fold from baseline (33). TEG also provides additional indices of coagulation abnormality, which can guide blood product resuscitation beyond plasma. Goal based TEG resuscitation has been shown to reduce trauma mortality in massively bleeding patients by 50% compared to conventional laboratory assays which include INR (34). The LY30 variable of the Lt-TEG represented a clinically appealing assay which had the highest positive predictive value of all labs and assays assessed with a negative predictive value of 96%. The main limitation of this assay is the requirement of 50 minutes to obtain results.
With our increasing awareness of the unique phenotypes of TIC which are driven by different mechanisms (12, 13), a combination coagulopathy score may be the optimal strategy for risk stratify trauma patients in the future. In this study, INR and fibrinolysis were independent predictors of massive transfusion and interacted with each other to predict this outcome. This is apparent in figure 3, in which a short TMA (<16 min.) in a patient with an INR ≤ 1.1 had a PPV of 0% for MT vs a patient with an INR > 1.1 and short TMA had a PPV of 49%. The use of a pre-hospital POC-INR test to triage patients to an ED TEG assay could optimize resources and lead to an expedited time to identify those patients who are at the highest risk of massive transfusion, and the optimal blood products to deliver. It has previously been demonstrated that using TEG in all comers to the ED has limited utility (35), and TEGs survival advantage has only been demonstrated in patient undergoing a massive transfusion (34). Interestingly, the threshold for INR and r-TEG MA to predict MA are within the normal limites of the assay and would not be indiciative of a blood transfusion. These sutble changes in coagulation early after injury appear to be warning signs of hemorrage that has occurred, and may not reflect if the patient will continue to bleed and require a massive transfusion. Therefor, the clinical implentations of these asssays is to put the trauma surgeon on a high alert for the potential need to activate a massive transfusion protocol, and should not be used blindly to activate a MTP. Ultimately, it is the trauma surgeon obtaining mechanical control of bleeding, which will dictate how much blood products the patient will need during resuscitaiton, which is is a challenging variable to control for in studies focused on massive transfusion.
This study was limited by our current MT definition. The debate on what constitutes MT (36) will likely continue, but a defensible goal is to identify patients who will have transfusion requirements that exceed the first cooler of blood products in the trauma bay. Our trauma activation protocol is initiated with a cooler containing 4 units of RBC and 2 units of plasma, therefore using the >4units RBC per hour was a logical definition for this study, and this definition has been recently validated (19). This study is limited to a single center, and the performance of coagulation assays may differ between institutions. The tPA TEG is not approved for clinical use, and currently requires a trained technician to perform this assay. However, next generation viscoelastic assays are designed to not require a trained technician to conduct the assay, with the added benefit that they can also remotely transmit data and be used as a POC assay (37). Ultimately a simple user-friendly trigger for activation of a massive transfusion protocol is in need for trauma centers in the United States. In a recent survey evaluating blood product utilization in 125 level I and II trauma centers in 2016, 98% reported having a massive transfusion protocol, yet only 7% used a validated scoring mechanism to activate the protocol (38).
Conclusion
Using a high dose tPA TEG to help guide the activation of the massive transfusion protocol provides actionable results within 16 minutes. Combining this assay with an INR can raise its positive predictive value from 36% to 49% while excluding 97% of patients that did not require a massive transfusion. With advances in portability and rapid results of a pre hospital POC INR assay to triage viscoelastic testing in trauma patients, the potential benefit of early identification of patients at risk of massive transfusion can be obtained, while simultaneously enabling the initiation of goal directed resuscitation.
Acknowledgments
Support: This study was supported in part by National Institute of General Medical Sciences grants T32-GM008315 and P50-GM49222; National Heart Lung and Blood Institute grant UM 1HL120877; and Department of Defense Contract Number USAMRAA, W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, National Heart Lung and Blood Institute, National Institutes of Health, or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclaimer: Drs H Moore, E Moore, and Chapman have shared intellectual property with Haemonetics. There is no direct financial relationship. Haemonetics provided reagents and devices to run viscoelastic assays, but has no involvement with data analysis, interpretation, or any contribution to this manuscript.
Presented at the Western Surgical Association 124th Scientific Session, Coronado, CA, November 2016.
References
- 1.Mutschler M, Nienaber U, Munzberg M, et al. The Shock Index revisited - a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Critical care. 2013;17(4):R172. doi: 10.1186/cc12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. The Journal of trauma. 2006 Jun;60(6):1228–36. doi: 10.1097/01.ta.0000220386.84012.bf. discussion 36-7. [DOI] [PubMed] [Google Scholar]
- 3.Mina MJ, Winkler AM, Dente CJ. Let technology do the work: Improving prediction of massive transfusion with the aid of a smartphone application. The journal of trauma and acute care surgery. 2013 Oct;75(4):669–75. doi: 10.1097/TA.0b013e3182a12ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonglet ML, Minon JM, Seidel L, et al. Prehospital identification of trauma patients with early acute coagulopathy and massive bleeding: results of a prospective non-interventional clinical trial evaluating the Trauma Induced Coagulopathy Clinical Score (TICCS) Critical care. 2014;18(6):648. doi: 10.1186/s13054-014-0648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockamp T, Nienaber U, Mutschler M, et al. Predicting on-going hemorrhage and transfusion requirement after severe trauma: a validation of six scoring systems and algorithms on the TraumaRegister DGU. Critical care. 2012;16(4):R129. doi: 10.1186/cc11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. The Journal of trauma. 2011 Aug;71(2):407–14. doi: 10.1097/TA.0b013e31821e1bf0. discussion 14-7. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Seminars in thrombosis and hemostasis. 2010 Oct;36(7):723–37. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion in critical care. 2007 Dec;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 9.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003 Jun;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of surgery. 2007 May;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An International Normalized Ratio-Based Definition of Acute Traumatic Coagulopathy Is Associated With Mortality, Venous Thromboembolism, and Multiple Organ Failure After Injury. Critical care medicine. 2015 Jul;43(7):1429–38. doi: 10.1097/CCM.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. The journal of trauma and acute care surgery. 2013 May;74(5):1223–9. doi: 10.1097/TA.0b013e31828b7fa1. discussion 9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin TL, Moore EE, Moore HB, et al. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014 Sep;156(3):570–7. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. The journal of trauma and acute care surgery. 2014 Dec;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015 Aug;158(2):386–92. doi: 10.1016/j.surg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Gonzalez E, et al. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. Journal of the American College of Surgeons. 2015 May;220(5):872–9. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Gonzalez E, et al. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015 Jan;43(1):39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. Journal of thrombosis and haemostasis : JTH. 2015 Oct;13(10):1878–87. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moren AM, Hamptom D, Diggs B, et al. Recursive partitioning identifies greater than 4 U of packed red blood cells per hour as an improved massive transfusion definition. The journal of trauma and acute care surgery. 2015 Dec;79(6):920–4. doi: 10.1097/TA.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? The Journal of trauma. 2009 Feb;66(2):346–52. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 21.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005 Jan;16(1):73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 22.Wo CC, Shoemaker WC, Appel PL, et al. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Critical care medicine. 1993 Feb;21(2):218–23. doi: 10.1097/00003246-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Binner C, Selinski S, Barysch MJ, et al. Munich Oktoberfest experience: remarkable impact of sex and age in ethanol intoxication. Arch Toxicol. 2008 Dec;82(12):933–9. doi: 10.1007/s00204-008-0373-z. [DOI] [PubMed] [Google Scholar]
- 24.Krumrei NJ, Park MS, Cotton BA, Zielinski MD. Comparison of massive blood transfusion predictive models in the rural setting. The journal of trauma and acute care surgery. 2012 Jan;72(1):211–5. doi: 10.1097/TA.0b013e318240507b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jong A, Deras P, Martinez O, et al. Relationship between Obesity and Massive Transfusion Needs in Trauma Patients, and Validation of TASH Score in Obese Population: A Retrospective Study on 910 Trauma Patients. PloS one. 2016;11(3):e0152109. doi: 10.1371/journal.pone.0152109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maegele M, Lefering R, Wafaisade A, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox sanguinis. 2011 Feb;100(2):231–8. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 27.Hilbert-Carius P, Hofmann GO, Lefering R, et al. Clinical presentation and blood gas analysis of multiple trauma patients for prediction of standard coagulation parameters at emergency department arrival. Anaesthesist. 2016 Apr;65(4):274–80. doi: 10.1007/s00101-016-0150-y. [DOI] [PubMed] [Google Scholar]
- 28.Callcut RA, Cripps MW, Nelson MF, et al. The Massive Transfusion Score as a decision aid for resuscitation: Learning when to turn the massive transfusion protocol on and off. The journal of trauma and acute care surgery. 2016 Mar;80(3):450–6. doi: 10.1097/TA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beynon C, Erk AG, Potzy A, et al. Point of care coagulometry in prehospital emergency care: an observational study. Scandinavian journal of trauma, resuscitation and emergency medicine. 2015;23:58. doi: 10.1186/s13049-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman MD, Makley AT, Hanseman DJ, et al. All the bang without the bucks: Defining essential point-of-care testing for traumatic coagulopathy. The journal of trauma and acute care surgery. 2015 Jul;79(1):117–24. doi: 10.1097/TA.0000000000000691. discussion 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. The journal of trauma and acute care surgery. 2016 Jan;80(1):16–23. doi: 10.1097/TA.0000000000000885. discussion-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schochl H, Cadamuro J, Seidl S, et al. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013 Apr;84(4):454–9. doi: 10.1016/j.resuscitation.2012.08.318. [DOI] [PubMed] [Google Scholar]
- 33.Zaar M, Fedyk CG, Pidcoke HF, et al. Platelet activation after presyncope by lower body negative pressure in humans. PloS one. 2014;9(12):e116174. doi: 10.1371/journal.pone.0116174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Annals of surgery. 2016 Jun;263(6):1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore HB, Moore EE, Chin TL, et al. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014 Sep;156(3):564–9. doi: 10.1016/j.surg.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zatta AJ, McQuilten ZK, Mitra B, et al. Elucidating the clinical characteristics of patients captured using different definitions of massive transfusion. Vox sanguinis. 2014 Jul;107(1):60–70. doi: 10.1111/vox.12121. [DOI] [PubMed] [Google Scholar]
- 37.Gurbel PA, Bliden KP, Tantry US, et al. First report of the point-of-care TEG: A technical validation study of the TEG-6S system. Platelets. 2016 Nov;27(7):642–9. doi: 10.3109/09537104.2016.1153617. [DOI] [PubMed] [Google Scholar]
- 38.Etchill E, Sperry J, Zuckerbraun B, et al. The confusion continues: results from an American Association for the Surgery of Trauma survey on massive transfusion practices among United States trauma centers. Transfusion. 2016 Aug 11; doi: 10.1111/trf.13755. [DOI] [PubMed] [Google Scholar]
- 39.Pottecher J, Ageron FX, Fauche C, et al. Prehospital shock index and pulse pressure/heart rate ratio to predict massive transfusion after severe trauma: Retrospective analysis of a large regional trauma database. The journal of trauma and acute care surgery. 2016 Oct;81(4):713–22. doi: 10.1097/TA.0000000000001191. [DOI] [PubMed] [Google Scholar]