Abstract
Background
Outcomes for children with relapsed and refractory neuroblastoma are dismal. The combination of irinotecan and temozolomide (I/T) has activity in these patients, and the toxicity profile of I/T makes it an excellent backbone for study of new agents. Temsirolimus (TEM) and dinutuximab (DIN) were selected for testing with I/T in subjects with relapsed or refractory neuroblastoma.
Methods
Children’s Oncology Group (COG) ANBL1221, a randomised Phase II selection design trial, compared response and toxicity in subjects treated with I/T and either temsirolimus (I/T/TEM) or dinutuximab with granulocyte-macrophage colony stimulating factor (I/T/DIN). Patients were eligible at first relapse/progression or first designation of refractory disease, provided organ function requirements were met. Patients had to have histologic verification of neuroblastoma and/or demonstration of tumour cells in bone marrow with increased urinary catecholamines at diagnosis. Patients were eligible at first designation of relapse (defined as recurrence after response to treatment), or first designation of refractory disease (defined as inadequate response to treatment that included at least 4 cycles of ≥2 chemotherapeutic agents, including an alkylator and a platinum-containing compound. Patients previously treated for refractory or relapsed disease were ineligible. Computer-based randomisation with sequence generation defined by permuted block randomisation, with blocks of size 2, was used to assign patients 1:1 to I/T/TEM or I/T/DIN. Randomisation was stratified to ensure equal distribution of disease category (measurable vs. evaluable), prior exposure to anti-GD2 antibody therapy, and tumour MYCN amplification status. Patients on both regimens received oral temozolomide (100 mg/m2/dose) and intravenous (IV) irinotecan (50 mg/m2/dose) on days 1–5 of 21-day cycles. TEM (35 mg/m2/dose IV) was given on days 1 and 8. DIN (17·5 or 25 mg/m2/day IV) was administered on days 2–5. The primary endpoint was objective (complete or partial) response; responses were centrally reviewed by an independent panel of radiologists. Response was analysed on an intent-to-treat basis. Toxicity was assessed in all participants who received at least one dose of protocol therapy. Follow up of the initial cohort is ongoing. This study is registered at ClinicalTrials.gov (NCT01767194).
Findings
Thirty-five eligible subjects were enrolled from February 22, 2013-March 23, 2015. Median age was 5.7 years (range 2·1–16·2 years; interquartile range (IQR) 4·5–9·1 years). Among 18 subjects randomised to I/T/TEM, 1 PR was observed (5·6%, 95% confidence interval (CI): [0·0%, 16·1%]). Among 17 patients randomised to I/T/DIN, 9 (53%, 95% CI: [29·2%, 76·7%]) had objective responses (4 PR, 5 CR), including responses in 5/10 patients with relapsed/progressive disease and 4/7 with refractory disease. I/T/DIN met protocol-defined criteria for selection as the combination meriting further study.
The most common ≥Grade 3 toxicities among I/T/TEM patients were neutropenia (8/18; 44%), anemia (6/18; 33%), thrombocytopenia (5/18; 28%), increased alanine aminotransferase (5/18; 28%), and hypokalemia (4/18; 22%). The most common ≥Grade 3 toxicities among I/T/DIN patients were pain (7/17; 44%), hypokalemia (6/17; 38%), neutropenia (4/17; 25%), thrombocytopenia (4/17; 25%), anemia (4/17; 25%), fever/infection (4/17; 25%), and hypoxia (4/17; 25%). One I/T/DIN patient experienced Grade 3 peripheral motor neuropathy. Deaths during protocol therapy included an I/T/DIN patient who had PD in the chest and died of respiratory failure during Cycle 2 and an I/T/DIN patient who achieved CR after cycle 6 and died unexpectedly after 14 cycles of treatment. One I/T/DIN patient developed Grade 4 hypoxia possibly related to therapy and met protocol-defined criteria for unacceptable toxicity.
Interpretation
I/T/DIN shows significant anti-tumour activity in patients with relapsed or refractory neuroblastoma. Further evaluation of biomarkers in a larger cohort of patients may identify those most likely to respond to this chemo-immunotherapeutic regimen.
Funding
USA National Cancer Institute (NCI)
Keywords: Neuroblastoma, Relapse, Phase II Clinical Trial, Dinutuximab
Introduction
Despite maximally intensive treatment, survival rates for children with newly diagnosed high-risk neuroblastoma remain ~50%.(1) Molecularly targeted therapies are being studied, and the combination of targeted agents with chemotherapy may be advantageous. Irinotecan and temozolomide (I/T) can be safely administered to patients with relpased or refractory neuroblastoma, providing a backbone onto which targeted agents can be integrated.(2, 3) The Children’s Oncology Group ANBL1221 trial (NCT01767194) was designed to study responses to I/T with one of two targeted agents.
Temsirolimus (TEM) inhibits the mammalian target of rapamycin (mTOR), which plays a role in regulation of protein synthesis and cell proliferation.(4) Neuroblastoma cells are sensitive to mTOR inhibitors in vitro and in vivo.(5, 6) Although single agent activity was modest in some preclinical studies,(7, 8) data suggest that mTOR inhibitors may be effective in subsets of neuroblastoma tumours.(9) In addition, mTOR inhibitors have synergistic or additive effects when combined with chemotherapeutics.(10, 11) Previous studies provided temsirolimus dosing information,(12, 13) and a COG trial demonstrated that I/T/TEM could be delivered safely to children with relapsed/refractory solid tumours.(14) Among 15 evaluable subjects with neuroblastoma, 2 had objective responses, supporting further study of this combination.(14)
Dinutuximab (DIN), a chimeric antibody targeting the disialoganglioside GD2, was also combined with I/T during this trial. GD2 is expressed on neuroblastoma cells, but expression in normal tissues is restricted to cerebellar neurons, skin melanocytes and peripheral pain fibers.(15–17) Due to this expression pattern, anti-GD2 antibodies have been studied as targeted immunotherapy for neuroblastoma.(18) DIN became a standard component of high-risk therapy after a randomised COG trial demonstrated an improvement in event-free survival (EFS) for patients assigned to receive DIN with GM-CSF and interleukin-2 following myeloablative therapy.(19) GM-CSF was selected for use in this study rather interleukin-2 because the latter has been associated with more significant capillary leak syndrome and more frequent renal dysfunction when given in combination with DIN. Because monoclonal antibodies in combination with chemotherapy were shown to be effective beyond the setting of minimal residual disease in adults,(20–27) the combination of I/T/DIN with GM-CSF was evaluated.
The primary objective of ANBL1221 was to determine whether TEM or DIN merits testing in a frontline trial for children with high-risk neuroblastoma. A randomised Phase II selection design (28–30) was used; tumour response was the primary endpoint.
METHODS
Study Design and Participants
ANBL1221 was an open label, prospective randomised Phase II COG trial with a selection (“Pick-the-Winner”) design. (30) Within each treatment regimen, a Simon’s 2-stage “Activity” design was used to determine if a given regimen failed to meet the minimum required level of activity and would be eliminated. This level was defined as ≥4 responders out of 17 subjects and ≥7 responders out of 25 subjects randomised to a given regimen. If both regimens met the minimum activity level, the Selection Design would be applied. In the Selection Design, the winner would be the regimen with ≥3 responders above the number of responders to the other regimen. If the winner could not be identified by these criteria, other criteria [toxicity, feasibility, progression-free survival (PFS)] were to be used to select the winner.
Patients had to have histologic verification of neuroblastoma and/or demonstration of tumour cells in bone marrow with increased urinary catecholamines at diagnosis. Patients were eligible at first designation of relapse (defined as recurrence after response to treatment), or first designation of refractory disease (defined as inadequate response to treatment that included at least 4 cycles of ≥2 chemotherapeutic agents, including an alkylator and a platinum-containing compound. Patients previously treated for refractory or relapsed disease were ineligible (including those previously treated with I/T), as were patients with bone marrow as the only site of disease.
Patients of any age were eligible for this trial. Patients must have had histologic verification of neuroblastoma or ganglioneuroblastoma or demonstration of neuroblastoma cells in the bone marrow with elevated urinary catecholamines at the time of initial diagnosis. Patients were required to have Karnofsky/Lansky scores ≥50%. Other requirements included: recovery from acute toxic effects of prior therapies, negative pregnancy test for females of child-bearing potential, adequate organ function [serum creatinine ≤ upper limit of normal (ULN) or glomerular filtration rate (GFR) ≥70 ml/min/1·73 m2, alanine aminotransferase (ALT) ≤5 times ULN, bilirubin ≤1·5 times ULN, prothrombin time ≤1·2 times ULN, serum triglycerides ≤300 mg/dL, serum cholesterol ≤300 mg/dL, shortening fraction ≥27%, no symptoms of pulmonary dysfunction]. Patients had to have an absolute neutrophil count ≥750/mm3 and an unsupported platelet count ≥75,000/mm3. Patients who had undergone stem cell transplantation (SCT) or high dose 131I-MIBG therapy during frontline treatment were eligible ≥6 weeks after these therapies if other criteria were met. Subjects previously treated with anti-GD2 antibodies were eligible unless they had PD during anti-GD2 therapy. Chemotherapy was not permitted within 2 weeks of enrollment. Biological agents (including anti-GD2 antibodies), retinoids, or growth factors were not permitted within 7 days of enrollment. Four weeks had to have elapsed since radiation to target lesions; progression in such lesions was required. Palliative radiation to non-target lesions was permitted without timing restrictions. Patients with diarrhea or uncontrolled illnesses and those taking enzyme-inducing anti-convulsants were ineligible. Patients with a history of significant allergic reactions to anti-GD2 antibodies or compounds similar to TEM were ineligible, as were those who had previously received an mTOR inhibitor with chemotherapy. Written informed consent was obtained from parents/guardians of minor participants. Data were entered at COG treating centres and aggregated at the COG Statistics and Data Centre (Gainesville, FL, USA). The study was approved by the NCI Pediatric Central Institutional Review Board (IRB) and local IRBs.
Randomisation and Masking
Computer-based randomisation with sequence generation defined by permuted block randomisation, with blocks of size 2, was used to assign patients 1:1 to I/T/TEM or I/T/DIN. Randomisation was stratified to ensure equal distribution of disease category (measurable vs. evaluable), prior exposure to anti-GD2 antibody therapy, and tumour MYCN amplification status. The COG RandoNode web service (integrated with the NCI OPEN system) assigned treatment such that the allocation sequence was not known at the site when treatment arm assignment occurred. Participants/families and those administering assigned therapy were aware of the treatment assignment. However, radiology central review was conducted without information as to arm assignment.
Procedures
All subjects received oral temozolomide (100 mg/m2/dose) and intravenous (IV) irinotecan (50 mg/m2/dose given over 90 minutes) on days 1–5. I/T/TEM patients received TEM (35 mg/m2/dose) IV over 30 minutes on days 1and 8. I/T/DIN patients initially received DIN (25 mg/m2/day over 10 hours) IV on Days 2–5. The infusion could be extended to 20 hours if patients experienced pain, fever, tachycardia, tachypnea, or hypotension unresponsive to supportive measures. A change in manufacturing of DIN and use of a calculated rather than theoretical extinction coefficient led to revision of the prescribed dose to 17·5 mg/m2/day. I/T/DIN subjects also received GM-CSF (250 mcg/m2/dose) subcutaneously on days 6–12. Treatment cycles were repeated every 21 days with a maximum 17 cycles of therapy. Prophylactic cefixime was recommended to reduce irinotecan-associated diarrhea. Loperamide was used to treat diarrhea occurring ≥24 hours post-irinotecan. On-therapy patients had evaluations of renal, hepatic, and hematologic function weekly. Adverse events were graded according to NCI Common Terminology Criteria for Adverse Events v4.0.
For patients experiencing neutropenia or thrombocytopenia causing a delay of ≥14 days between treatment cycles, temozolomide doses were to be reduced by 25% for subsequent cycles. If a patient were to experience Grade 4 therapy-associated diarrhea despite maximal use of anti-diarrheal medications and appropriate use of prophylactic antibiotics, irinotecan doses were to be reduced by 25% for subsequent cycles. DIN was to be held in patients with hypotension or capillary leak syndrome unresponsive to standard interventions, those with severe allergic reactions to DIN, and those with persistent elevation in creatinine to ≥2× the upper limit of normal for age/gender persisting despite optimized fluid management. DIN was also to be held for patients with pain unresponsive to narcotics and those with Grade 4 neurotoxicity.
Pre-treatment disease evaluations were performed within 3 weeks of study enrollment and response was assessed after cycles 2, 4, and 6, and every 4 cycles thereafter. RECIST criteria were used for response assessment in patients with disease measurable by CT/MRI.(31) Responses based on anatomic imaging were centrally reviewed. For patients with MIBG-avid lesions, Curie scoring determined response during central review.(32) Marrow involvement was assessed using routine staining; bilateral evaluations were required.
Criteria for removal from protocol therapy included disease progression, inability to tolerate study therapy, refusal of further therapy, development of a second malignant neoplasm, or completion of 17 cycles of therapy. Patients could also be removed from therapy if the treating physician or patient/family determined that removal would be in the patient’s best interest. Patients who did not meet criteria to start the next treatment cycle within 21 days after the planned subsequent cycle start date (ie, a ≥3 week delay in start of next cycle) and those whose repeat eligibility studies were outside of required parameters would also be removed from protocol therapy. Patients who met off protocol therapy criteria due to toxicity before attaining an objective response were considered non-responders.
Outcomes
The primary endpoint was best overall response based on results of CT/MRI imaging, MIBG scans and bone marrow aspirates/biopsies, determined after completion of six cycles of protocol therapy. Modified International Neuroblastoma Response Criteria (INRC) were used to integrate the results of disease evaluation procedures and permit assessment of overall response.(33) Responders were defined as those with best overall response of complete (CR) or partial response (PR). Patients with overall CR had no evidence of tumour and normal urinary catecholamines. Patients with PR in soft tissue disease (per RECIST) had to have ≥50% reduction in Curie score (if MIBG-avid lesions were present at study entry) and resolution of marrow disease (if present at study entry) to be designated as having an overall PR. Patients with stable disease (SD) in any site category had SD overall. Such patients were considered non-responders but could continue protocol therapy. PD was defined as any of the following: development of a new lesion, doubling of the percentage of tumour cells in bone marrow (with minimum 25% marrow involvement), or a ≥20% increase in longest dimension of a soft tissue mass. Patients with PD were removed from protocol therapy. Patients who met off protocol therapy criteria due to toxicity before attaining an objective response were considered non-responders. For a given patient, the response endpoint was binary (responder, non-responder). Progression-free and overall survival (OS) were secondary endpoints of the study. For PFS, an event is defined as a relapse, disease progression, or death attributable to tumour or treatment. For OS an event was a death due to any cause.
Statistical Analysis
Seventeen subjects were to be assigned to each arm during the first stage of the study. All eligible, randomised patients were considered evaluable for the intent-to-treat analysis of response. If a second stage in the “Activity” design were to be required, 25 patients were to be assigned to each treatment. This optimal two-stage design has 91.1% power to detect a 25% difference (15% under the null, 40% under the alternative hypothesis) in response rate with a Type I error of 0.064.
Any eligible patient who received at least one dose of TEM or DIN was considered evaluable for toxicity. The protocol included a 3-stage stopping rule for unacceptable toxicity. Toxicity rates and categorical patient characteristics in the two groups were compared using a Fisher’s exact test. Age was compared using a two-sided Wilcoxon rank sum test. A 95% Wald confidence interval was placed on the response rate for each regimen.
Survival curves were constructed according to Kaplan-Meier, with standard errors according to Peto.(34),(35) Survival was analysed on an intent-to-treat basis; all eligible patients were considered assessable for survival endpoints. Survival curves were compared using a two-sided log-rank test. For PFS, time to event was calculated from enrollment to first occurrence of relapse, PD, or death related to disease or its treatment, or time of last patient contact if no event occurred. Patients were censored at time of death not due to disease. For overall survival (OS), time to event was time from enrollment until death from any cause, or time to last contact if the patient was alive. PFS and OS are presented as 1-year point estimates ± standard errors. Hazard ratios (HR) for the difference between treatment groups are derived from Cox proportional hazards models. P-values <0·05 were considered statistically significant.
Analyses were performed using SAS (SAS/STAT User’s Guide, Version 9·4; SAS Institute, Cary, NC). Survival curves were created using R (R Project for Statistical Computing; https://www.r-project.org/). This trial was registered on clinicaltrials.gov (NCT01767194).
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Patients were enrolled from February 22, 2013 to March 23, 2015. In total, 36 patients were enrolled. One patient was ineligible due to ALT above the required range. The remaining 35 subjects form the basis of this report. Eighteen patients were randomised to I/T/TEM and 17 to I/T/DIN (Table 1, web appendix pages 1–4). Age at enrollment ranged from 2·1 to 16·2 years (median 5·7 years, interquartile range (IQR) 4·5–9·1 years). Time from diagnosis of high-risk disease to enrollment ranged from 0·27 to 5·03 years (median 0·82 years, IQR 0·51–2·68 years). Most patients (34/35; 97%) had INSS Stage 4 disease at diagnosis; a single patient had relapsed INSS Stage 3 disease. MYCN status was known for 32 patients; 8 had MYCN amplified tumours (5 randomised to I/T/TEM, 3 randomised to I/T/DIN). Twenty-two subjects had measurable disease (12 I/T/TEM, 10 I/T/DIN) and 13 had evaluable disease (6 I/T/TEM, 7 I/T/DIN). Nineteen patients had a first episode of relapsed neuroblastoma (10 I/T/TEM, 9 I/T/DIN), 16 had disease that was refractory to initial therapy (8 I/T/TEM, 8 I/T/DIN). Prior treatment included high dose chemotherapy with autologous stem cell transplant in 19 subjects (9 I/T/TEM, 10 I/T/DIN), and prior anti-GD2 therapy in 10 (4 I/T/TEM, 6 I/T/DIN). The treatment groups were well-balanced with respect to patient characteristics (Table 1); there were no statistically significant differences in the distribution of these factors between groups.
Table 1.
Characteristics of Subjects (N=35) on COG ANBL1221
| I/T/TEM | I/T/DIN | Overall | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| 18 | 51 | 17 | 49 | 35 | 100 | |
| Age at Diagnosis (Years) | ||||||
| Median | 5·0 | 3·2 | 4·1 | |||
| Range | (2·1, 15·9) | (1·7, 11·9) | (1·7, 15·9) | |||
| Interquartile Range (IQR) | 3·6–7·8 | 2·5–4·6 | 2·8–6·9 | |||
| Age at Enrollment (Years) | ||||||
| Median | 7·0 | 4·7 | 5·7 | |||
| Range | (2·9, 16·2) | (2·1, 15·6) | (2·1, 16·2) | |||
| IQR | 5·1–9·4 | 3·6–6·5 | 4·5–9·1 | |||
| Time from Diagnosis of High-Risk Disease to Enrollment (Months) | ||||||
| Median | 9.74 | 9.79 | 9.79 | |||
| Range | (3·25, 60·39) | (4·27, 49·45) | (3·25, 60·39) | |||
| IQR | 7·00–32·13 | 6·08–29·96 | 6·08–32·13 | |||
| INRG Stage at Diagnosis of High-Risk Disease | ||||||
| Stage M | 17 | 94 | 17 | 100 | 34 | 97 |
| Not Stage M | 1 | 6 | 0 | 0 | 1 | 3 |
| MYCN Status at Diagnosis | ||||||
| Amplified | 5 | 29 | 3 | 20 | 8 | 25 |
| Non-amplified | 12 | 71 | 12 | 80 | 24 | 75 |
| Unknown | 1 | -- | 2 | -- | 3 | -- |
| Disease Status at Enrollment | ||||||
| Relapsed | 10 | 56 | 9 | 53 | 19 | 54 |
| Refractory | 8 | 44 | 8 | 47 | 16 | 46 |
| Site of Disease | ||||||
| Bone | 12 | 67 | 10 | 59 | 22 | 63 |
| Bone marrow | 6 | 33 | 13 | 76 | 19 | 54 |
| Soft tissue | 12 | 67 | 11 | 65 | 23 | 66 |
| Liver | 0 | 0 | 1 | 6 | 1 | 3 |
| Lung | 1 | 6 | 1 | 6 | 2 | 6 |
| Measureable Disease | ||||||
| Yes | 12 | 67 | 10 | 59 | 22 | 63 |
| Primary site | 2 | 11 | 4 | 23 | 6 | 17 |
| Metastatic sites | 10 | 56 | 8 | 47 | 18 | 51 |
| No | 6 | 33 | 7 | 41 | 13 | 37 |
| Prior treatment | ||||||
| Multi-modailty Induction Therapy (MMIT) Only | 7 | 39 | 5 | 29 | 12 | 34 |
| MMIT + MIBG Therapy | 2 | 11 | 1 | 6 | 3 | 9 |
| MMIT + ASCT | 9 | 50 | 10 | 59 | 19 | 54 |
| MMIT + ASCT + External Beam Radiation Therapy | 8 | 44 | 7 | 41 | 15 | 43 |
| MMIT + ASCT + Anti-GD2 Ab | 4 | 22 | 6 | 35 | 10 | 29 |
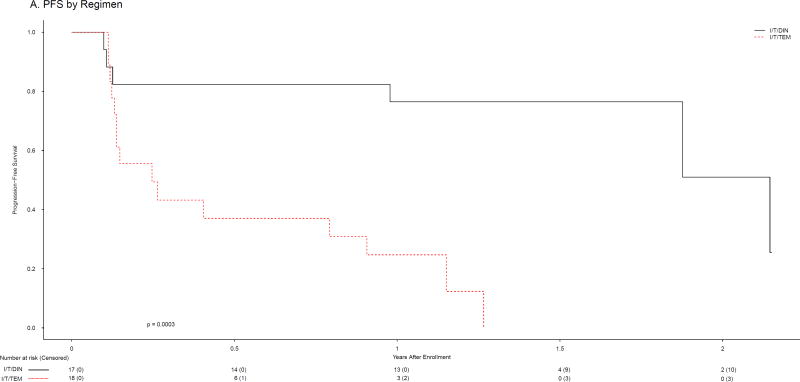
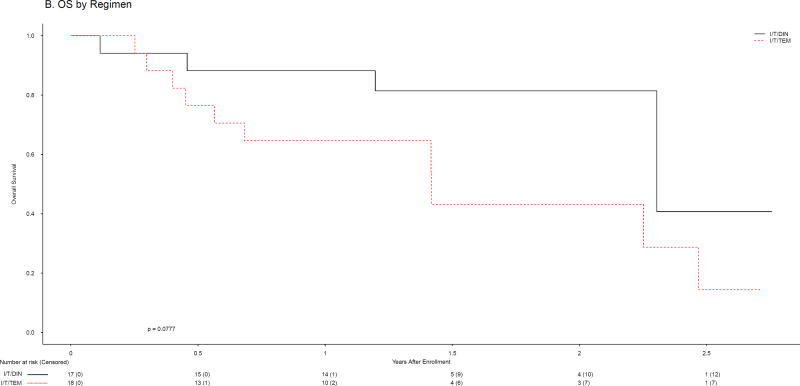
The 18 subjects randomised to I/T/TEM received 98 total courses (median 3, IQR 2–10); the 17 patients randomised to I/T/DIN received 148 courses (median 6, IQR 2·5–17). Objective responses (CR or PR; Table 2) were observed in one patient (5·6%, 95% CI: [0·0%, 16·1%]) randomised to I/T/TEM (1 PR) and 9 patients (53%, 95% CI: [29·2%, 76·7%]) randomised to I/T/DIN (5 CR, 4 PR). One I/T/DIN patient did not receive therapy due to withdrawal after randomisation but is included in this intent-to-treat analysis. In the Activity Design, I/T/TEM failed to meet the minimum level of activity while I/T/DIN exceeded the requirement (Table 2). Having eliminated I/T/TEM, application of the Selection Design was unnecessary. DIN met criteria to be designated the agent meriting further study. Because the stopping boundary at Stage 1 had been met for I/T/TEM, accrual to Stage 2 was not required for either arm. Among subjects assigned to I/T/DIN, 4/8 with refractory disease had objective responses, as did 5/9 with relapsed disease (Table 3, Web appendix pages 3–4). Objective responses were seen among patients with measurable (3/10) and evaluable (6/7) disease, and among those whose tumours were MYCN amplified (2/3) and non-amplified (6/12). Seven of 10 who had previously undergone SCT responded to I/T/DIN, as did 4/6 who had received prior anti-GD2 antibody therapy. Ten of 18 I/T/TEM and 4/17 I/T/DIN patients had SD as best response. A total of 11/18 (56%) of I/T/TEM and 13/17 (76%) of I/T/DIN patients therefore had SD or better. Among patients who experienced PD on therapy, none had PD in marrow only. PFS and OS were evaluated as a secondary objective of the trial. The 1-year PFS for I/T/TEM and I/T/DIN patients were 24·7±12·4% [PFS median 0.25, IQR 0·13–0·91, 95% CI: (0·13, 0·91) years] and 76·5±10·3% [PFS median 2.14, IQR 1·88-N/A, 95% CI: ≥0.98 years], respectively (p=0·0003; Figure 2A). There were 15 events among the 18 patients assigned to I/T/TEM and 6 events among the 17 patients assigned to I/T/DIN. The HR and 95% CI for the difference in PFS between treatment groups are 6·86 (2·15, 21·96). One-year OS rates were 64·7±12·2% [OS median 1.42, IQR 0·56–2·47, 95% CI: (0·45, 2·47) years] and 88·2±8·1% [OS median 2·30, IQR 2·30-N/A, 95% CI: ≥2.30 years] for I/T/TEM and I/T/DIN, respectively (p=0·0777; Figure 2B). There were 10 deaths among the 18 patients assigned to I/T/TEM and 4 deaths among the 17 patients assigned to I/T/DIN. The HR for the difference in OS between treatment groups is 2·74 [95% CI: (0·85, 8·82)].
Table 2.
Response by Treatment Regimen
| Overall Best Response |
N | % |
|---|---|---|
| I/T/TEM | ||
| CR | 0 | 0 |
| PR | 1 | 5 |
| SD | 10 | 56 |
| PD | 7 | 39 |
| I/T/DIN | ||
| CR | 5 | 29 |
| PR | 4 | 24 |
| SD | 4 | 24 |
| PD | 3 | 18 |
| Not evaluated* | 1 | 6 |
Not evaluated due to refusal of therapy after randomization
Table 3.
Characteristics of I/T/DIN Responders
| Responder | Disease Status |
Measurable Disease |
MYCN Amplified |
Prior Anti- GD2 Antibody |
Total Cycles Received |
|---|---|---|---|---|---|
| 1 | Relapsed | Yes | No | Yes | 6 |
| 2 | Relapsed | Yes | No | Yes | 17 |
| 3 | Relapsed | Yes | Yes | Yes | 17 |
| 4 | Relapsed | No | Yes | Yes | 10 |
| 5 | Relapsed | No | No | No | 14 |
| 6 | Refractory | No | No | No | 17 |
| 7 | Refractory | No | Unknown | No | 17 |
| 8 | Refractory | No | No | No | 3 |
| 9 | Refractory | No | No | No | 8 |
Figure 2.
Progression-free (A) and overall (B) survival by regimen
Grade ≥3 toxicities related to protocol therapy are shown (Table 4). There was no significant difference in Grade ≥3 diarrhea between groups [11% (2/18) vs. 6% (1/17) on I/T/TEM and I/T/DIN, respectively]. Rates of Grade ≥3 neutropenia [44% (8/18) vs. 25% (4/17)] and thrombocytopenia [(28% (5/18) vs. 25% (4/17)] did not differ. As expected, patients assigned to I/T/DIN experienced pain with treatment; 44% (7/17) had Grade ≥3 pain. Four patients had hypoxia during I/T/DIN therapy (three Grade 3 and one Grade 4; see web appendix page 5). One I/T/DIN patient experienced Grade 3 peripheral motor neuropathy beginning on day 6 of cycle 6. The patient had bilateral lower extremity weakness with inability to ambulate independently for 4 weeks. No additional I/T/DIN was administered and motor function returned to baseline within 6 weeks of onset of neuropathy. Ten I/T/DIN and 5 I/T/TEM patients required dose modifications. The TEM dose was modified in 4 patients; the dose reductions were due to hematologic toxicity (n=3; neutropenia and thrombocytopenia) and an infusion reaction (n=1). The temozolomide dose was reduced along with the TEM dose in 2 patients with hematologic toxicity. The irinotecan dose was also modified in one of those patients. Four I/T/DIN patients required temozolomide dose modifications; these were due to a formulation issue (n=1), emesis (n=1), and hematologic toxicity (n=2; neutropenia and thrombocytopenia). An additional 6 patients required dinutuximab dose modifications; these were due to hypoxia (n=1), bronchospasm (n=1), pain (n=2), infection (n=1) and hypotension (n=1). Among all patients requiring dose modifications, only those with the hypoxia and bronchospasm required discontinuation of protocol therapy due to toxicity.
Table 4.
Therapy-related Toxicities (≥Grade 3) by Treatment Regimen
| Toxicity | I/T/TEM | I/T/DIN | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | |
| Hematologic | ||||||
| Neutropenia | 6(33) | 2(11) | 0(0) | 4(25) | 0(0) | 0(0) |
| Thrombocytopenia | 2(11) | 3(17) | 0(0) | 4(25) | 0(0) | 0(0) |
| Anemia | 6(33) | 0(0) | 0(0) | 4(25) | 0(0) | 0(0) |
| Fever/Infection | 2^(11) | 0(0) | 0(0) | 4&(25) | 0(0) | 0(0) |
| Febrile Neutropenia | 3(17) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Capillary Leak Syndrome | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Hypotension | 0(0) | 0(0) | 0(0) | 2(13) | 0(0) | 0(0) |
| Hypoxia | 0(0) | 0(0) | 0(0) | 3(19) | 1(6) | 0(0) |
| Pain | 1(6) | 0(0) | 0(0) | 7(44) | 0(0) | 0(0) |
| Diarrhea | 2(11) | 0(0) | 0(0) | 1(6) | 0(0) | 0(0) |
| Vomiting | 2(11) | 0(0) | 0(0) | 3(19) | 0(0) | 0(0) |
| Mucositis | 2(11) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Dehydration | 3(17) | 0(0) | 0(0) | 3(19) | 0(0) | 0(0) |
| Elevated Creatinine | 0(0) | 0(0) | 0(0) | 0(0) | 1(6) | 0(0) |
| Increased Bilirubin | 1(6) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) |
| Hyponatremia | 0(0) | 0(0) | 0(0) | 3(19) | 0(0) | 0(0) |
| Hypokalemia | 3(17) | 1(6) | 0(0) | 6(38) | 0(0) | 0(0) |
| Increased ALT | 5(28) | 0(0) | 0(0) | 1(6) | 0(0) | 0(0) |
Both patients had bacteremia
Two of these patients had ≥Grade 3 fever during the time period in which the patient was receiving antibody. The other two had documented infections including bacteremia and urinary tract infection.
Deaths during protocol therapy included an I/T/DIN patient who had PD in the chest and died of respiratory failure during Cycle 2, and an I/T/DIN patient who achieved CR after cycle 6 and died unexpectedly after 14 cycles of treatment. The cause of death in this case was not determined despite a full autopsy, and the relationship of death to protocol therapy is unclear. There were no other deaths during protocol therapy and no deaths were directly attributed to treatment. One patient developed Grade 4 hypoxia possibly related to therapy and met protocol-defined criteria for unacceptable toxicity. No other events met this definition. The stopping rule for unacceptable toxicity was not met for either regimen.
Discussion
The chemoimmunotherapy combination I/T/DIN has significant activity in patients with relapsed/refractory neuroblastoma. Nine of 17 patients randomised to I/T/DIN had objective responses while only one patient randomised to I/T/TEM had a PR. Therefore the a priori benchmark for activity (≥4/17 responders) was met for I/T/DIN but not for I/T/TEM. It should be noted that the patient population in this study was not selected on the basis of PI3K/AKT/mTORC status. Further evaluation of I/T/TEM in patients whose tumours harbour alterations in PI3K/AKT/mTORC pathway molecules or in components of interacting pathways may yield different results.
The 53% response rate following I/T/DIN is striking in this disease setting. Reported 10-year OS rates for patients with relapsed metastatic neuroblastoma and those with progression on high-risk therapy are 2% and 1·5% respectively.(36) Reported objective response rates to I/T alone (2, 3) and the response rate to I/T/TEM during this trial are in marked contrast to the response rate observed in the I/T/DIN group.
Responses to I/T/DIN were not restricted based upon disease status (relapsed vs. refractory disease, measurable vs. evaluable disease), MYCN status (amplified vs. non-amplified), or prior therapy (prior DIN vs. no prior DIN), and most responses were detected early in treatment (Figure 2). In 7/9 responders, best response was seen after only two cycles. While 7/9 responders went on to other therapies based on family/physician preference, 3 did so after receiving the maximum number of cycles permitted, and none of the responders relapsed or progressed during I/T/DIN.
Response rates of the magnitude observed here have been reported previously, however these were studies of topotecan-containing regimens in topotecan-naïve patients.(37),(38, 39),(40),(41) A widely used induction regimen now includes topotecan;(42, 43) response rates to topotecan-based therapy are expected to be lower in previously-exposed patients. Similarly, while 9/17 patients in first relapse responded to high-dose ifosfamide/carboplatin/etoposide in one study,(44) a lower response rate could be expected in patients treated with the high-dose carboplatin and etoposide used in current-era North American consolidation regimens.(43, 45, 46) In a study of a GD2-directed antibody with GM-CSF without chemotherapy, a 38% response rate was observed in patients with non-progressing, primary refractory, evaluable (non-irradiated) osteomedullary disease.(47) The 53% response rate observed in this study requires confirmation, but suggests that addition of I/T to DIN and GM-CSF may result in activity in a broader group of patients. Subjects receiving I/T/DIN experienced toxicities known to accompany DIN therapy(19) (including pain, fever, and electrolyte abnormalities), however hematologic toxicity was relatively modest. Monitoring for peripheral motor neuropathy will be important going forward. No second malignancies among patients treated on this trial have been reported to date, however follow-up time is relatively short.
Because the primary objective of ANBL1221 was met after accrual to Stage 1 of the Activity design, the number of patients treated with I/T/DIN is small. This is an important limitation of the study. Treatment of additional patients is required to verify the encouraging response rate and better define the toxicity profile of I/T/DIN. Expansion of the patient population may also permit subgroup analyses. Another limitation is that this trial included only patients with a first episode of relapsed/refractory disease; the role of I/T/DIN in other settings is unknown. This study was not specifically powered to evaluate survival endpoints and survival data should be interpreted in light of the limited sample size and in light of the fact that EFS in children with neuroblastoma may vary considerably given the clinical heterogeneity of this disease. Results of OS analyses should also be interpreted with caution, as survival for patients on both arms may have been impacted by therapy received following study treatment.
The 5·6% response rate for I/T/TEM is not significantly lower than the response rate to I/T alone as reported in other studies (8 to 15%).(2, 3) In addition, 10 I/T/TEM patients had SD on this study. However, this study was not designed to compare clinical benefit following I/T/TEM to clinical benefit following I/T alone. To more rigorously compare these therapies, a much larger clinical trial would be required.
This study made use of the same modified version of the 1993 INRC criteria used in prior COG Phase 2 trials.(2, 14, 48, 49) Modification was necessary because the 1993 criteria do not include MIBG scans as an assessment modality and because the use of tumour volume for assessment of measurable disease (as in the 1993 criteria) does not permit comparison with modern-era studies that use RECIST-style approaches. A new version of the INRC has recently been developed that includes parameters for MIBG assessment and includes RECIST-style guidance for evaluation of soft tissue disease. However, these consensus criteria had not yet been agreed upon at the time of the development of this trial. The use of the COG modification of the 1993 INRC limits comparisons with studies conducted by other groups, however this limitation will be overcome in future trials when the new INRC are incorporated worldwide.
Potential mechanisms of response to this combination merit consideration. Chemotherapy-induced capillary modification could increase antibody dispersion and improve access to tumour cells.(50) I/T may also potentiate the effects of immunotherapy by altering the tumour microenvironment, producing response rates that exceed those observed following GD2-directed antibody therapy alone(51) or with cytokines but without I/T.(47, 48) Evaluation of cytokines in peripheral blood and assessment of tumour infiltrating leukocytes and macrophages may reveal the basis for responses.(52, 53) Assessment of expression of immune checkpoint proteins affecting activity of macrophages and NK cells(54–56) may aid in development of predictive biomarkers. NK-mediated cytotoxicity can be diminished due to inhibitory interactions between killer Ig-like receptors (KIR) and their ligands;(57) KIR/KIR ligand genotypes may correlate with response to anti-GD2 therapy.(58)These genotypes should also be evaluated as potential biomarkers. Fc receptor polymorphisms influence response to antibody therapy in adults with lymphoma;(59, 60) and may impact response to GD2-directed therapy in children;(61) further evaluation of Fc receptor genotypes may be warranted. Additional studies to elucidate the specific contributions of irinotecan, temozolomide and GM-CSF in augmenting DIN activity may also be helpful. During this study, GM-CSF was administered following completion of I/T/DIN. Alternate GM-CSF schedules could not be studied in this initial trial, but response following concurrent administration of GM-CSF and I/T/DIN could be evaluated in a future study. Response rates following I/T/DIN with GM-CSFcompared to response rates following DIN and GM-CSF alone could also be evaluated in future trials.
The combination of I/T/DIN is active in patients with relapsed and refractory neuroblastoma, and toxicities are manageable. ANBL1221 was designed to identify an agent meriting further study in combination with chemotherapy in the frontline setting. A GD2-directed antibody has been administered with a multi-agent induction regimen in a single institution study.(62) No unexpected toxicities were observed, however further study on DIN in combination with chemotherapy agents other than I/T is needed. A multi-institution pilot study to evaluate the safety of DIN in combination with induction chemotherapy is being developed.
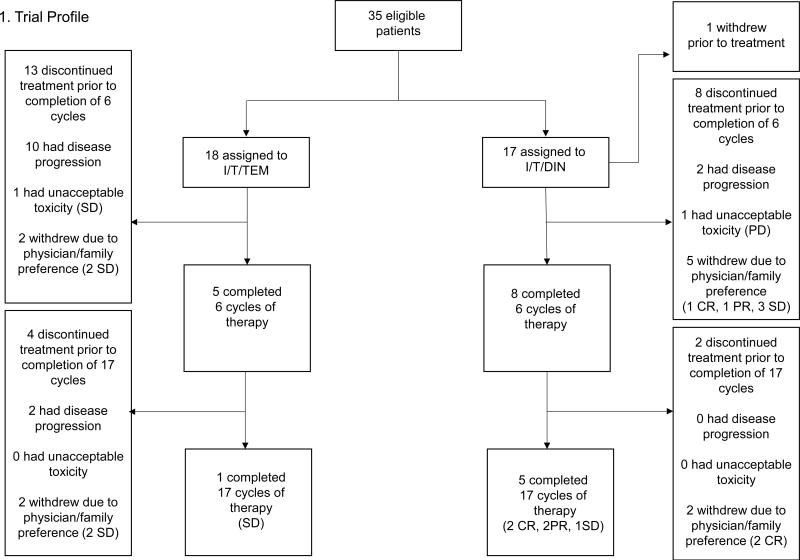
Figure 1. Trial profile.
Best response by Cycle 6 was the primary endpoint of the trial. Patients with SD or better could remain on study and receive a maximum of 17 cycles of treatment. Best response is indicated in parentheses.
Research in Context.
Evidence before this study
We searched MEDLINE on February 1, 2017, for publications until Dec 31st 2011, using the terms ((neuroblastoma) AND (irinotecan) NOT review [publication type]. We did not apply any language restrictions but used search terms in English only. Of the 11 publications, there were four addressing the use of this 2-drug combination in children with cancer. Among these were two trials of these agents in children with neuroblastoma. Both reported that this well-tolerated combination has activity (PMIDs: 17114661 and 21115869). We also searched using the terms ((neuroblastoma) AND (mTOR inhibitor)) NOT review [publication type]. Of the 25 publications, there were two clinical studies involving children, including one Phase 1 trial of temsirolimus in patients with refractory solid tumours. One patient with neuroblastoma had a complete response (PMID: 21690471). No randomised trials of temsirolimus in combination with chemotherapy for patients with neuroblastoma had been published before this study. We also searched using the terms ((neuroblastoma) AND (GD2 antibody) NOT review [publication type]. Among the 234 search results, there were 15 reports on Phase I through III clinical trials. Among these were single arm studies in patients with relapsed or refractory disease (including studies of an anti-GD2 antibody combined with GM-CSF) and single arm or pilot studies in patients receiving frontline therapy. There was one landmark randomised trial of an anti-GD2 antibody (dinutuximab) with cytokines in patients receiving frontline therapy (PMID: 20879881). Publications available prior to our study did not include reports describing randomised trials of anti-GD2 antibodies in patients with relapsed or refractory neuroblastoma. We also searched using the terms ((neuroblastoma) AND (GD2 antibody) AND (chemotherapy) NOT review [publication type]. Of the 86 publications, there were no publications reporting the results of a clinical trial of an anti-GD2 antibody in combination with chemotherapy in children with relapsed or refractory neuroblastoma.
Added value of this study
The primary objective of this randomised Phase II trial was achieved, as the targeted agent meriting further study in combination with chemotherapy was identified. The combination of irinotecan, temozolomide and temsirolimus was not active in a patient population that was not selected based on mTOR pathway mutation status, however the response rate of patients treated with the combination of irinotecan, temozolomide and dinutuximab was encouraging.
Implications of all the available evidence
Further study of chemo-immunotherapy for children with neuroblastoma is warranted.
Acknowledgments
Research support provided by NIH/NCI Grants U10 CA180899 and U10CA98413 (Children’s Oncology Group Statistics and Data Center), U10CA98543 (Children’s Oncology Group Chair’s Grant), U10CA180886 (NCTN Operations Center Grant) and the St. Baldrick’s Foundation
Footnotes
Presented in abstract form at the American Association for Clinical Oncology (ASCO) Annual Meeting 2016, Chicago, IL and Advances in Neuroblastoma Research (ANR) 2016, Cairns, Australia
Other information
This trial is registered on ClinicalTrials.gov (NCT01767194) with the title “Irinotecan Hydrochloride and Temozolomide With Temsirolimus or Dinutuximab in Treating Younger Patients With Refractory or Relapsed Neuroblastoma.” The full protocol can be accessed by COG members at www.childrensoncologygroup.org. The study was funded in part by the USA National Cancer Institute. Dinutuximab was provided through the NCI Cancer Therapy Evaluation Program.
Author Contributions
RM – literature search, figures, study design, data collection, data analysis, data interpretation, writing
AN – figures, study design, data collection, data analysis, data interpretation, writing
CVR - figures, study design, data collection, data analysis, data interpretation, writing
ALY – literature search, study design, data analysis, data interpretation, writing
WBL – literature search, figures, study design, data collection, data analysis, data interpretation, writing
BLS – figures, study design, data collection, data analysis, data interpretation, writing
MTP - figures, study design, data collection, data analysis, data interpretation, writing
SENS – study design, data collection, data analysis, data interpretation, writing
MBD - data collection, data analysis, data interpretation, writing
PMS - data analysis, data interpretation, writing
JMM – study design, data collection, data analysis, data interpretation, writing
JRP – study design, data collection, data analysis, data interpretation, writing
RB - literature search, figures, study design, data collection, data analysis, data interpretation, writing
Declaration of Interests
Dr. Shulkin is a member of the Data Safety Monitoring Board for a study of Lymphoseek (Nav 3–18).
The other authors have nothing to disclose
References
- 1.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33(27):3008–17. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(2):208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner BH, Kramer K, Modak S, Cheung NK. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24(33):5271–6. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 4.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnsen JI, Segerstrom L, Orrego A, Elfman L, Henriksson M, Kagedal B, et al. Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene. 2008;27(20):2910–22. doi: 10.1038/sj.onc.1210938. [DOI] [PubMed] [Google Scholar]
- 6.Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 7.Houghton PJ, Gorlick R, Kolb EA, Lock R, Carol H, Morton CL, et al. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;58(2):191–9. doi: 10.1002/pbc.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang MH, Reynolds CP, Maris JM, Gorlick R, Kolb EA, Lock R, et al. Initial testing (stage 1) of the investigational mTOR kinase inhibitor MLN0128 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61(8):1486–9. doi: 10.1002/pbc.24989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiessling MK, Curioni-Fontecedro A, Samaras P, Lang S, Scharl M, Aguzzi A, et al. Targeting the mTOR Complex by Everolimus in NRAS Mutant Neuroblastoma. PLoS One. 2016;11(1):e0147682. doi: 10.1371/journal.pone.0147682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10(20):7031–42. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 11.Piguet AC, Semela D, Keogh A, Wilkens L, Stroka D, Stoupis C, et al. Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol. 2008;49(1):78–87. doi: 10.1016/j.jhep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29(21):2933–40. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geoerger B, Kieran MW, Grupp S, Perek D, Clancy J, Krygowski M, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer. 2012;48(2):253–62. doi: 10.1016/j.ejca.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagatell R, Norris R, Ingle AM, Ahern C, Voss S, Fox E, et al. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children’s Oncology Group Study. Pediatr Blood Cancer. 2014;61(5):833–9. doi: 10.1002/pbc.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44(12 Pt 1):5914–20. [PubMed] [Google Scholar]
- 16.Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45(6):2642–9. [PubMed] [Google Scholar]
- 17.Svennerholm L, Bostrom K, Fredman P, Jungbjer B, Lekman A, Mansson JE, et al. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biophys Acta. 1994;1214(2):115–23. doi: 10.1016/0005-2760(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 18.Dobrenkov K, Cheung NK. GD2-targeted immunotherapy and radioimmunotherapy. Semin Oncol. 2014;41(5):589–612. doi: 10.1053/j.seminoncol.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 21.Forstpointner R, Dreyling M, Repp R, Hermann S, Hanel A, Metzner B, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–71. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 22.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20(5):545–57. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 25.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–6. [PubMed] [Google Scholar]
- 26.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 27.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69(12):1375–81. [PubMed] [Google Scholar]
- 29.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199–206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg SM, Venzon DJ. Early selection in a randomized phase II clinical trial. Stat Med. 2002;21(12):1711–26. doi: 10.1002/sim.1150. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Messina JA, Cheng SC, Franc BL, Charron M, Shulkin B, To B, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47(7):865–74. doi: 10.1002/pbc.20777. [DOI] [PubMed] [Google Scholar]
- 33.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan ER, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 35.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garaventa A, Parodi S, De Bernardi B, Dau D, Manzitti C, Conte M, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835–42. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Kushner BH, Kramer K, Modak S, Qin LX, Cheung NK. Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer. 2010;116(12):3054–60. doi: 10.1002/cncr.25232. [DOI] [PubMed] [Google Scholar]
- 38.Simon T, Langler A, Berthold F, Klingebiel T, Hero B. Topotecan and etoposide in the treatment of relapsed high-risk neuroblastoma: results of a phase 2 trial. J Pediatr Hematol Oncol. 2007;29(2):101–6. doi: 10.1097/MPH.0b013e3180320b48. [DOI] [PubMed] [Google Scholar]
- 39.Simon T, Langler A, Harnischmacher U, Fruhwald MC, Jorch N, Claviez A, et al. Topotecan cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma. Results of a phase-II trial. J Cancer Res Clin Oncol. 2007;133(9):653–61. doi: 10.1007/s00432-007-0216-y. [DOI] [PubMed] [Google Scholar]
- 40.Garaventa A, Luksch R, Biasotti S, Severi G, Pizzitola MR, Viscardi E, et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003;98(11):2488–94. doi: 10.1002/cncr.11797. [DOI] [PubMed] [Google Scholar]
- 41.London WB, Frantz CN, Campbell LA, Seeger RC, Brumback BA, Cohn SL, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2010;28(24):3808–15. doi: 10.1200/JCO.2009.27.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(33):4351–7. doi: 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al., editors. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study; American Society of Clinical Oncology Annual Meeting 2016; Chicago, IL. 2016. [Google Scholar]
- 44.Kushner BH, Modak S, Kramer K, Basu EM, Roberts SS, Cheung NK. Ifosfamide, carboplatin, and etoposide for neuroblastoma: a high-dose salvage regimen and review of the literature. Cancer. 2013;119(3):665–71. doi: 10.1002/cncr.27783. [DOI] [PubMed] [Google Scholar]
- 45.Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14(10):999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granger M, Naranjo A, McCune JS, Dubois SG, Bagatell R, Weiss BD, et al., editors. Myeloablative busulfan/melphalan (BuMel) consolidation following induction chemotherapy for patients with high-risk neuroblastoma: A Children’s Oncology Group (COG) study; American Society of Clinical Oncology Annual Meeting 2016; Chicago, IL. 2106. [Google Scholar]
- 47.Cheung NK, Cheung IY, Kramer K, Modak S, Kuk D, Pandit-Taskar N, et al. Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer. 2014;135(9):2199–205. doi: 10.1002/ijc.28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–75. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villablanca JG, London WB, Naranjo A, McGrady P, Ames MM, Reid JM, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children’s Oncology Group. Clin Cancer Res. 2011;17(21):6858–66. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holden SA, Lan Y, Pardo AM, Wesolowski JS, Gillies SD. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin Cancer Res. 2001;7(9):2862–9. [PubMed] [Google Scholar]
- 51.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–80. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 52.Pistoia V, Bianchi G, Borgonovo G, Raffaghello L. Cytokines in neuroblastoma: from pathogenesis to treatment. Immunotherapy. 2011;3(7):895–907. doi: 10.2217/imt.11.80. [DOI] [PubMed] [Google Scholar]
- 53.Borriello L, Seeger RC, Asgharzadeh S, DeClerck YA. More than the genes, the tumor microenvironment in neuroblastoma. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–14. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–5. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 57.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179(9):5977–89. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 58.Forlenza CJ, Boudreau JE, Zheng J, Le Luduec JB, Chamberlain E, Heller G, et al. KIR3DL1 Allelic Polymorphism and HLA-B Epitopes Modulate Response to Anti-GD2 Monoclonal Antibody in Patients With Neuroblastoma. J Clin Oncol. 2016;34(21):2443–51. doi: 10.1200/JCO.2015.64.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 60.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 61.Cheung NK, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24(18):2885–90. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 62.Furman WL, Federico SM, McCarville MB, Davidoff AM, Krasin MJ, Wu J, et al., editors. Improved clinical responses with the concomitant use of an anti-GD2 monoclonal antibody and chemotherapy in newly diagnosed children with high-risk (HR) neuroblastoma (NB): Preliminary results of a phase II study; American Society of Clinical Oncology Annual Meeting; Chicago, IL. 2016. [Google Scholar]