Abstract
Artesunate (ART) is a semisynthetic derivative of artemisinin. Artemisinin and its derivatives have shown profound cytotoxicity and antitumor activity in addition to antimalarial activity in various studies. As the in vivo chemopreventive efficacy of ART in colon carcinogenesis has not been investigated so far, the aim of the current study was to study the chemopreventive effect of ART in 1,2-dimethylhydrazine (DMH)-induced rat colon carcinogenesis. Animals were divided into four groups (n = 6): Group I - vehicle (1 mM ethylenediaminetetraacetic acid), Group II - DMH (20 mg/kg), Group III - DMH + 5-fluorouracil (81 mg/kg), Group IV - DMH + ART (6.7 mg/kg). After completion of 15 weeks of treatment, rats were sacrificed under ether anesthesia by cervical dislocation for assessment of lipid peroxidation (LPO), antioxidant status, average number of aberrant crypt foci (ACF), and cytokine levels. ART administration significantly decreased the average number of ACF/microscopic field. Similarly, LPO level was decreased and antioxidant activities were enhanced after ART treatment. ART decreased the levels of proinflammatory cytokines and induced apoptosis in the colons of DMH-treated rats. The results of this study suggest that ART has a beneficial effect against chemically induced colonic preneoplastic progression in rats.
Key words: Aberrant crypt foci, artemisinin, artesunate, apoptosis, cytokines, oxidative stress, tumor necrosis factor-α
INTRODUCTION
Artesunate (ART), a hemisuccinate ester prodrug, is a semisynthetic derivative of artemisinin. Artemisinin and its derivatives have secured themselves a place in the new generation antimalarial drug armamentarium because of their promising potential in the treatment of multidrug-resistant Plasmodium falciparum and Plasmodium vivax infections.[1] Various studies have reported that in addition to the antimalarial activity, artemisinins have shown profound cytotoxicity and antitumor activity in vitro and in vivo.[2,3,4] However, a previous study has reported that ART exhibits the greatest cytotoxicity among all the artemisinins which are clinically used for malaria treatment.[5] Studies involving the nude mice bearing human xenograft tumors have demonstrated that ART strongly retards the growth of Kaposi's sarcoma and colorectal carcinoma.[6,7] Results of a study analyzing the anticancer activity of ART against 55 cell lines demonstrated that ART was most active against leukemia and colon cancer cell lines.[8] ART significantly slows the growth of colorectal tumor xenografts.[7] Based on the significant results and low toxicity observed in the experimental studies, ART is being considered as a promising drug candidate for the treatment of colorectal carcinoma.
In addition to the cytotoxic effects, ART has shown antiangiogenic activity in different in vivo and in vitro studies.[9,10] ART downregulates vascular endothelial growth factor (VEGF) expression in tumor cells.[10] As many chemopreventive drugs exert antiangiogenic features, it is suggested that ART might also be chemopreventive. Due to the lack of investigative studies regarding in vivo chemopreventive efficacy of ART in colon cancer, the present study was designed to investigate the chemopreventive efficacy of ART in 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis in rat model.
MATERIALS AND METHODS
Animals and diet
Adult Wistar rats of either sex weighing between 150 and 250 g were procured from the Central Animal House, PGIMER, Chandigarh. The entire study was conducted after approval from the Institutional Animal Ethics Committee (PGIMER, Chandigarh, IAEC/165). The animals were housed in polypropylene cages under standard laboratory conditions at 25°C and 12-h light/dark cycles until the end of the experimental period. Animals were given free access to rat chow diet and water. After a 1-week acclimatization period, rats were stratified and assigned to the experimental groups.
Drugs and chemicals
ART and 5-fluorouracil (5-FU) were generous gifts from IPCA Laboratories Ltd., (Maharashtra, India) and Cadila Pharmaceuticals Ltd., (Gujarat, India), respectively. DMH was purchased from Sigma-Aldrich Chemicals Pvt. Ltd., (Bengaluru, India). ELISA kits for rat tumor necrosis factor (TNF)-α, and other cytokines' estimation were purchased from Diaclone and RayBio. DNA isolation kit was obtained from Real Genomics. All other chemicals and reagents used were of analytical grade and purchased from the reputed Indian manufacturers.
Experimental design
Rats were assorted into four groups with six animals per group:
Control group (Group I): Animals were administered the vehicle (1 mM ethylenediaminetetraacetic acid) subcutaneously in weekly injection for 15 weeks
DMH group (Group II): Weekly subcutaneous injection of 20 mg/kg DMH was given to the rats for 15 weeks
DMH + 5-FU group (Group III): DMH was administered as per Group II. Along with this, weekly dose of 81 mg/kg of 5-FU was given intraperitoneally for 15 weeks
DMH + ART group (Group IV): DMH was administered as per Group II. In addition, ART was given orally in a dose of 6.7 mg/kg daily for 15 weeks.
Animal sacrifice and tissue preparation
At the end of 15 weeks, after anesthetizing animals, about 2 ml blood was collected. Colon was quickly excised after sacrificing the animals. Then, sections of colon were prepared for histomorphological evaluation and DNA isolation. The remaining colonic sections were gently scraped with a microscopic slide, and the mucosa was used for various biochemical assessments after homogenization with the appropriate buffer.
Observation for colon carcinogenesis
Throughout the experimental period, rats were weighed weekly. Any changes in the body weight were noted.
Gross morphological assessment
Any gross changes in the colon were noted. The tumors, if present, were counted on gross examination and their sizes were measured. Tumor incidence was calculated as the number of tumor-bearing rats divided by the total number of rats, and tumor multiplicity was calculated as the number of tumors divided by the number of tumor-bearing rats.
Histomorphologic evaluation
All the groups were subjected to histological examination by a qualified pathologist in a blinded fashion. Aberrant crypt foci (ACF) were identified by their increased size, thicker epithelial lining, and increased pericryptal zone.[11] Average number of ACF present per microscopic field was determined by counting the ACF in ten fields randomly chosen for each rat. These ACF were evaluated for the following characteristics: pattern of luminal outline of the ACF, i.e., luminal alteration, nuclear alteration, crypt orientation, and reduction in the number of goblet cells. Moreover, the overall distribution of different grades of morphological alteration observed in ACF was calculated as the percentage of ACF over total ACF in each grade of morphological alteration.[12]
Biochemical estimations
Lipid peroxidation (LPO) was estimated in the colonic mucosa by measuring the levels of thiobarbituric acid reactive substances (TBARS) according to the method of Ohkawa et al.[13] The values were expressed as nmoles of malondialdehyde (MDA)/mg protein. Superoxide dismutase (SOD, EC.1.15.1.1) was estimated by the method of Kono.[14] The values were expressed as units/mg protein/min, and one unit of SOD activity was defined as the amount of enzyme required for 50% inhibition of nitroblue tetrazolium reduction/min/mg protein. The activity of catalase (CAT, EC.1.11.16) was measured by the method of Luck.[15] The values were expressed as μmoles of H2O2 decomposed/min/mg protein. Glutathione peroxidase (GSH-Px, EC.1.11.1.9) activity was measured by the method of Paglia and Valentine.[16] GSH-Px enzyme activity was expressed as μmoles of NADPH consumed/min/mg protein.
Cytokine estimation
The levels of TNF-α, interleukin (IL)-1β, and VEGF in plasma were estimated using ELISA kits as per the directions of the manufacturer.
DNA fragmentation
DNA fragmentation is a characteristic feature of apoptosis at the biochemical level. To determine apoptosis induction, DNA fragmentation gel electrophoresis was performed.[17] Gel photographs were evaluated for typical ladder patterns of low molecular weight DNA fragments in multiples of 180–200 base pairs, a hallmark of apoptosis.
Statistical analysis
All the data were evaluated with SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. (Chicago, SPSS Inc.) version 16 software. Mann–Whitney U-test was used for analysis. P < 0.05 was considered to indicate statistical significance. All these results were expressed as mean ± standard deviation for six animals in each group.
RESULTS
General observations
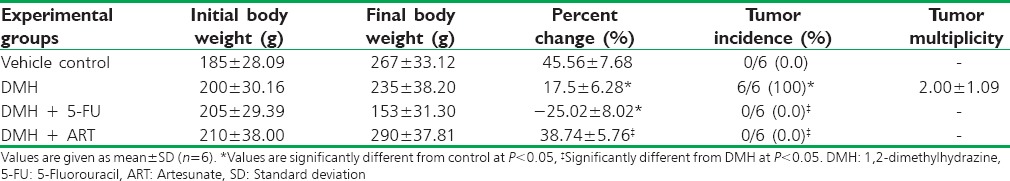
The mean body weights in all groups are shown in Table 1. Control, DMH + ART, and DMH + 5-FU treated animals showed no gross morphological changes, and the colonic mucosa was also normal, devoid of any tumor/lesion growth. However, in the DMH-treated group, the tumor incidence in the colon was 100% and the average size of the tumor was approximately 0.5 cm [Table 1].
Table 1.
Effect of artesunate on body, liver, and kidney weights and incidence of colonic neoplasms
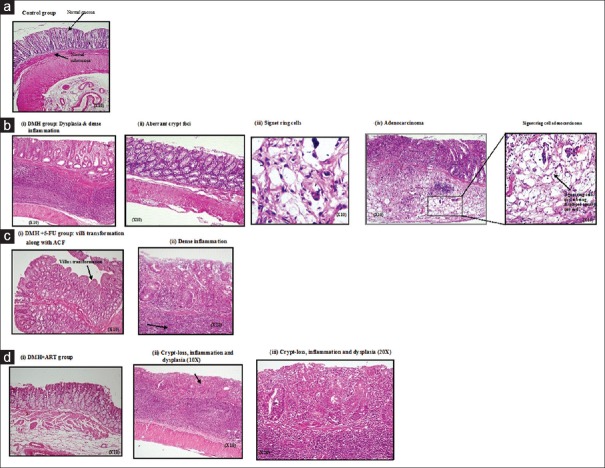
Histomorphologic evaluation
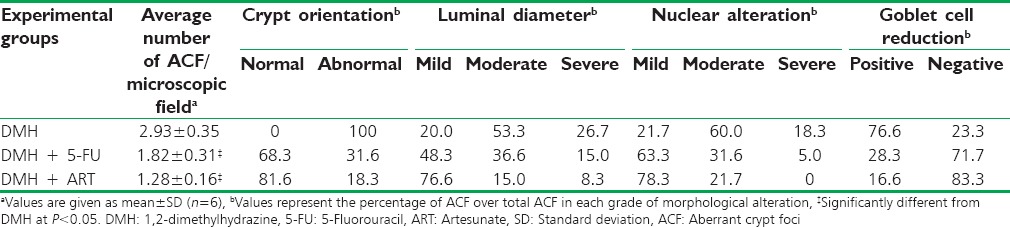
The histomorphologic observations are represented by Figure 1. DMH + ART-treated animals exhibited normal mucosal and submucosal layers [Figure 1di], while occasional early changes of ACF or crypt abscess were noted too. Likewise, occasional loss of crypts, inflammation, and dysplasia were observed too [Figure 1dii and iii]. Results of histologic morphometry showed that the average number of ACF/microscopic field decreased significantly in the DMH + ART (1.28 ± 0.16) and DMH + 5-FU (1.82 ± 0.31) groups as compared with the DMH group (2.93 ± 0.35). However, no ACF were found in the colon of vehicle-treated rats [Table 2]. The overall distribution of different grades of morphological alterations observed in ACF is also shown in Table 2.
Figure 1.
Photomicrographs of histologic (H and E) cross sections of the colons from the control, DMH, DMH + 5-FU, and DMH + ART groups examined after 15 weeks of treatment schedule. (a) Control sections showing the normal mucosal and submucosal architecture (×10). (b) (i) Dysplasia and dense inflammation. (ii) Aberrant crypt foci (×10). (iii) A typical illustration of Signet ring cells with nuclei displayed toward one end (×10). (iv) Mucosal ulceration with tumor infiltrating into the mucosal and muscular layers (×10), inset shows Signet ring cell adenocarcinoma (×10). (c) (i) Section from DMH + 5-FU group showing villi transformation along with ACF (×10) and (ii) showing dense inflammation (×20). (d) (i) Section from DMH + ART-treated group showing the normal histology of mucosal and submucosal layers (×10) (ii) shows dysplasia, loss of crypts, and heavy inflammation (×10). (iii) Same microphotograph at higher magnification (×20). DMH: 1,2-dimethylhydrazine, ART: Artesunate, 5-FU: 5-Fluorouracil, ACF: Aberrant crypt foci
Table 2.
Effect of artesunate on the distribution of different grades of morphological alteration observed in aberrant crypt foci
Biochemical estimations
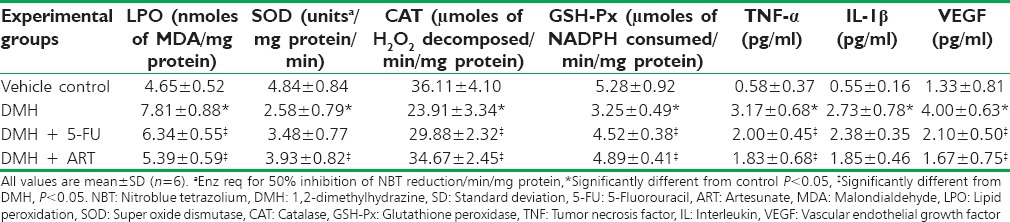
Table 3 shows the levels of TBARS in the colonic mucosa of all the experimental groups of rats and summarizes the activities of enzymic antioxidants in colonic mucosa of different experimental group of animals. A significant reduction in the activities of SOD, CAT, and GSH-Px was evident in DMH-treated rats (Group II) when compared with the control (Group I). ART administration significantly increased the activities of these enzymes as compared with DMH-induced group [Table 3].
Table 3.
Effect of artesunate on different biochemical parameters and different cytokines
Cytokine estimation
Results of the present study indicated that TNF-α levels were significantly increased in DMH group as compared to control group [Table 3]. ART treatment significantly reduced the TNF-α level as compared to DMH group (P < 0.05) [Table 3]. Similarly, both 5-FU and ART decreased the levels of IL-1β as compared to control group; however, the decrease was not significant in both cases. Results of VEGF estimation indicated that after DMH treatment, VEGF levels increased significantly, which were significantly reduced by both 5-FU and ART.
DNA fragmentation
DMH + ART group exhibited the distinct ladder pattern similar to that of 5-FU thereby indicating that ART-induced apoptosis in colons of rats [Figure 2].
Figure 2.
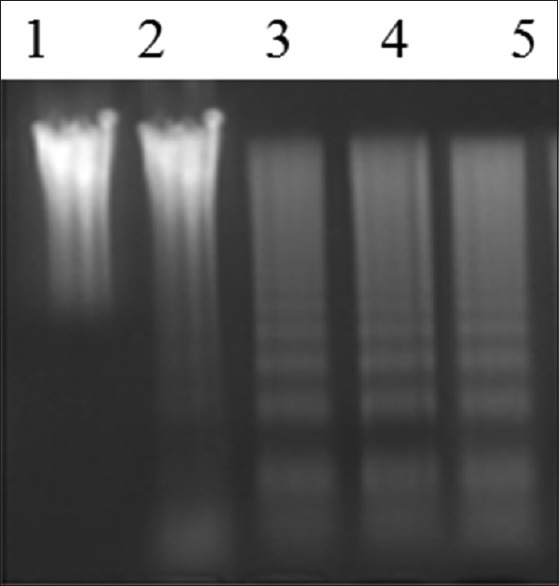
Representative picture showing the assessment of apoptosis by DNA fragmentation analysis in the colon of control and experimental groups of rats. Lane 1 - DMH, Lane 2 - Vehicle control, Lane 3 - DMH + 5-FU, and Lane 4, 5 - DMH + ART. DMH: 1,2-dimethylhydrazine, ART: Artesunate, 5-FU: 5-Fluorouracil
DISCUSSION
Since ACF are regarded as the earliest identified putative premalignant precursors of both human and experimental colon carcinogenesis, the ACF-inhibiting agents are considered protective against colon cancer.[18] Results of the present study indicated that ART given along with DMH significantly inhibited the formation of ACF as well as tumors. A series of morphological alterations in ACF were also identified and quantified in the sectioned colons. It was observed that ART treatment decreased the morphological alterations (such as nuclear/luminal alterations and goblet cell reduction) as compared to DMH group. Moreover, ART restored the crypt orientation to near normal pattern which was 100% lost in DMH group. These findings suggest that ART commences its cancer preventive activity much before the onset of the occurrence of the carcinogenic events as exhibited in the present model of colon carcinogenesis.
Oxidative stress plays an important role in all stages of chemical carcinogenesis and tumorigenesis. It is characterized as increase in LPO and a decrease in antioxidant enzymes.[19] DMH, a potent colon-specific carcinogen, used in the present study is metabolized in the liver to azoxymethane (a known colon carcinogen) and ultimately to a methyl-free radical that in turn generates hydroxyl radical in the presence of metal ions that may contribute to the initiation of LPO.[20] MDA, a secondary product of LPO, itself is a known mutagen. Previous studies have reported increased levels of MDA in colon cancer tissues as compared to the surrounding normal mucosa.[21] Similarly, in the present study, significantly elevated levels of LPO were observed in DMH-treated rats as compared with the control [Table 3]. Treatment with ART significantly (P < 0.05) lowered the levels of LPO, and the effect was more pronounced than that observed in 5-FU-treated group. Antioxidant enzymes such as SOD, CAT, and GSH-Px play a protective role against a variety of endogenous and exogenous toxic compounds such as ROS and chemical carcinogens.[22] Our results showed decreased levels of SOD and CAT activities in DMH-induced rats [Table 3] as compared to control rats which were significantly elevated after ART treatment. As low levels of SOD and CAT activity promote cancerous growth, which may lead to invasion and metastasis,[23] above results suggest that ART could be effective in inhibiting the DMH-induced carcinogenesis. Furthermore, it was noted that ART significantly enhanced the GSH-Px levels which were decreased significantly in DMH group as compared to the control group [Table 3]. All these findings suggest about the selective antioxidant nature of ART in normal colon mucosa. This fact is confirmed by earlier studies which have reported that artemisinin, a sesquiterpene trioxane lactone, kills the cells by oxidative stress when its endoperoxide bridge reacts with a ferrous iron atom to form activated oxygen species, such as oxygen radicals, or of carbon-centered radicals which are toxic to cells.[24,25] In other words, artemisinin and its derivatives require iron to form free radicals which can kill cells. Since cancer cells require and uptake a large amount of iron to proliferate, they are more susceptible to the cytotoxic effect of artemisinin than normal cells. Therefore, in the present study, enhancement of antioxidant activities by ART indicates the lack of ferrous iron atoms which depicts the antiproliferative activity of ART despite the concomitant DMH administration. This again suggests that ART commences its cancer preventive activity much before the onset of the occurrence of the carcinogenic events.
TNF-α, an important inflammatory cytokine, is quite crucial for the development of inflammation-associated cancers. Moreover, it is also produced by tumors and can act as an endogenous tumor promoter.[26] The present study showed that TNF-α level was significantly increased in DMH group as compared to control group which was significantly reduced by ART treatment [Table 3]. Furthermore, ART decreased the levels of IL-1β as well as VEGF, but the decrease was statistically significant only in the latter case. In addition to this, DNA fragmentation analysis evidently indicated that ART induces apoptosis in colons of rats.
CONCLUSION
The results of the present study have shown that ART inhibits ACF as well as tumor formation, modulates LPO, restores the antioxidant defenses, decreases the levels of proinflammatory cytokines, and induces apoptosis in the concomitantly DMH-administered animals. All these findings indicate that ART may constitute an effective drug regimen for chemoprevention. Further studies are required to elucidate its molecular mechanistic pathways of chemoprevention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Indian Council of Medical Research, New Delhi, for supporting the work by providing the research grant (ICMR-JRF fellowship). The authors would also like to thank IPCA Laboratories Ltd., and Cadila Pharmaceuticals Ltd., for supplying artesunate and 5-FU.
REFERENCES
- 1.Medhi B, Patyar S, Rao RS, Byrav DS, Prakash A. Pharmacokinetic and toxicological profile of artemisinin compounds: An update. Pharmacology. 2009;84:323–32. doi: 10.1159/000252658. [DOI] [PubMed] [Google Scholar]
- 2.Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets. 2006;7:407–21. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- 3.Lai H, Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–8. doi: 10.1016/j.canlet.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC, et al. Second generation, orally active, antimalarial, artemisinin-derived trioxane dimers with high stability, efficacy, and anticancer activity. J Med Chem. 2006;49:2731–4. doi: 10.1021/jm058288w. [DOI] [PubMed] [Google Scholar]
- 5.Efferth T, Oesch F. Oxidative stress response of tumor cells: Microarray-based comparison between artemisinins and anthracyclines. Biochem Pharmacol. 2004;68:3–10. doi: 10.1016/j.bcp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Dell'Eva R, Pfeffer U, Vené R, Anfosso L, Forlani A, Albini A, et al. Inhibition of angiogenesis in vivo and growth of Kaposi's sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol. 2004;68:2359–66. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int J Cancer. 2007;121:1360–5. doi: 10.1002/ijc.22804. [DOI] [PubMed] [Google Scholar]
- 8.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767–73. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Shi L, Yang X, Li S, Guo X, Pan L. Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol. 2010;92:587–97. doi: 10.1007/s12185-010-0697-3. [DOI] [PubMed] [Google Scholar]
- 10.Lai JP, Huang J. Suppressive effect of artesunate on K562 cell growth and its influence on VEGF expression. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:777–80. [PubMed] [Google Scholar]
- 11.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987;37:147–51. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 12.Caderni G, Giannini A, Lancioni L, Luceri C, Biggeri A, Dolara P. Characterisation of aberrant crypt foci in carcinogen-treated rats: Association with intestinal carcinogenesis. Br J Cancer. 1995;71:763–9. doi: 10.1038/bjc.1995.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 15.Luck H. Catalase. In: Bergmeyer HW, editor. Methods of Enzymatic Analysis. Sec. 3. New York: Academic Press; 1963. pp. 855–94. [Google Scholar]
- 16.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;701:158–69. [PubMed] [Google Scholar]
- 17.Daniel PT, Sturm I, Ritschel S, Friedrich K, Dörken B, Bendzko P, et al. Detection of genomic DNA fragmentation during apoptosis (DNA ladder) and the simultaneous isolation of RNA from low cell numbers. Anal Biochem. 1999;266:110–5. doi: 10.1006/abio.1998.2929. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal P. Potential biomarkers associated with oxidative stress for risk assessment of colorectal cancer. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:557–65. doi: 10.1007/s00210-017-1352-9. [DOI] [PubMed] [Google Scholar]
- 20.Bobek P, Galbavý S, Mariássyová M. The effect of red beet (Beta vulgaris var.rubra) fiber on alimentary hypercholesterolemia and chemically induced colon carcinogenesis in rats. Nahrung. 2000;44:184–7. doi: 10.1002/1521-3803(20000501)44:3<184::AID-FOOD184>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickse CW, Kelly RW, Radley S, Donovan IA, Keighley MR, Neoptolemos JP. Lipid peroxidation and prostaglandins in colorectal cancer. Br J Surg. 1994;81:1219–23. doi: 10.1002/bjs.1800810849. [DOI] [PubMed] [Google Scholar]
- 22.Nimse SB, Pal DK. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–8006. [Google Scholar]
- 23.Janssen AM, Bosman CB, Kruidenier L, Griffioen G, Lamers CB, van Krieken JH, et al. Superoxide dismutases in the human colorectal cancer sequence. J Cancer Res Clin Oncol. 1999;125:327–35. doi: 10.1007/s004320050282. [DOI] [PubMed] [Google Scholar]
- 24.Lai H, Sasaki T, Singh NP. Targeted treatment of cancer with artemisinin and artemisinin-tagged iron-carrying compounds. Expert Opin Ther Targets. 2005;9:995–1007. doi: 10.1517/14728222.9.5.995. [DOI] [PubMed] [Google Scholar]
- 25.Cai HH, Cai J, Yang PH. Electrochemical activity of holotransferrin and its electrocatalysis-mediated process of artemisinin. Bioorg Med Chem Lett. 2009;19:863–6. doi: 10.1016/j.bmcl.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–41. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]