Abstract
To replace and avoid synthetic chemicals toxicity, there is a growing interest in the investigation of natural products from plant origin for the discovery of active compounds with antimicrobial properties. This work was devoted to determine chemical composition and antimicrobial properties of the EO of M. piperita harvested in the garden of the National Institute of Medicinal and Aromatic Plants of Morocco. Experiments have been conducted at the Microbial Biotechnology Laboratory at the Sciences and Technology Faculty, Sidi Mohamed Ben Abdellah University, Fez, Morocco. M. piperita oil was screened for its antimicrobial activity against seven bacteria and two fungi using broth microdilution method. Chemical EO analysis was performed using CPG/MS. The EO revealed menthol (46.32%), menthofuran (13.18%), menthyl acetate (12.10%), menthone (7.42%), and 1,8-cineole (6.06%) as the main constituents. The tested EO exhibited strong inhibitory effect against all tested microorganisms with minimum inhibitory concentrations ranging from 0.062% to 0.5% (v/v), except for Pseudomonas aeruginosa that was the least sensitive and was only inhibited by concentrations as high as 0.5% (v/v). The studied EO showed an antimicrobial potential. This reinforces its use as an alternative to chemical additives that can be applied to the food and drug industry.
Key words: Antimicrobial activity, chemical composition, essential oil, Mentha piperita, Morocco
INTRODUCTION
The extensive use of chemical antimicrobial and antiseptic agents in human mediation as well as in animal breeding led to the selection of resistant strains permitting the development of resistance, which is a biological phenomenon very hard to remove. There are a few decades; several diseases appeared under control using antibiotics. Scientific and technological progress believed in a possible eradication of many diseases;[1] however, the resistance developed increasingly by microorganisms and the regular emergence of new infectious agents have denied this optimistic prognosis.
Faced with this phenomenon, the discovery of new antibacterial molecules that could provide an alternative to the use of conventional antibiotics which became ineffective seems an absolute necessity. The research tracks are numerous, but the exploration of natural resources appears to be more promising because they constitute, by their biodiversity, the largest reserve of active substances and especially the medicinal and aromatic plants, which are the source of high-value products, such as essential oils (EOs).
The genus Mentha, known as peppermint, is a cultivated natural hybrid of Mentha aquatic L. (water mint) and Mentha spicata L. (spearmint). Although being a native genus of the Mediterranean region, it is cultivated all over the world and the EO of this plant has been reported by other works for its insecticidal,[2] antimicrobial,[3] antioxidant effects.[4]
The present study aims to investigate the chemical composition of Moroccan M. piperita EO, to assess its antimicrobial activity against seven bacteria and two fungi causing spoiling and pathogenicity, in an attempt to contribute to the use of these as alternative products for microbial control and food preservation.
MATERIALS AND METHODS
Plant material
Fresh aerial part of Mentha piperita was harvested from the National Institute of the Medicinal and Aromatic Plants garden in Taounate city (34°32′11″ N, 4°38′24″W, altitude: 600 m) (Morocco).
Essential oils extraction
The fresh aerial parts of M. piperita (leaves and stems) were subjected to hydrodistillation for 3 h using a Clevenger-type apparatus. The recovered EO was kept in the dark at 4°C until further use.
Chemical analysis of essential oil
The EO was analyzed using gas chromatography (GC) coupled to mass spectrometry (MS) (GC/MS) (PolarisQ ion trap MS). Hence, analyses were performed on a Hewlett-Packard (HP 6890) gas chromatograph (flame ionization detector [FID]). The temperature was programmed from 50°C after 5 min initial hold to 200°C at 4°C/min. Chromatography carrier gas was N2 (1.8 ml/min), split mode was used (flow: 72.1 ml/min. ratio: 1/50), temperature of injector and detector was 250°C, and final hold time was 48 min. The machine was led by a computer system type “HP ChemStation,” managing its functioning and allowing to follow the evolution of chromatographic analyses. Diluted samples (1/20 in methanol) of 1 μl were injected manually.[3]
Bacterial strain
Tested bacteria include seven isolates of Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Micrococcus luteus ATCC 14452, Staphylococcus aureus ATCC 29213, Bacillus subtilis ATCC 6633, Salmonella typhimurium, and Bacillus cereus. Before use, strains were revivified by subcultures in Luria-Bertani (LB) plates at 37°C for 24 h.
The tested yeast includes Candida albicans and Candida tropicalis. Their revivification was made by subculturing each strain in yeast extract-peptone-glucose (YPG) agar plates at 30°C for 48 h.
These strains belong to the microbial biotechnology laboratory.
Determination of minimum inhibitory concentration
The minimum inhibitory concentrations (MICs) were determined in 96-well microplate using the microdilution assay according to Balouiri et al.[5] with slight modifications. Bacteriological agar at 0.15% (w/v) was used as an emulsifier of the EO in the culture medium. For bacteria, the EO was serially diluted in Muller-Hinton broth supplemented with agar to obtain final concentrations ranging between 8% and 0.007% (v/v). The 12th well was considered as growth control (free-EO control). Then, 50 μl of bacterial inoculum, previously prepared and adjusted to 0.5 McFarland, were added to each well to reach the final concentration of 106 CFU/ml. After incubation at 37°C for 24 h, 10 μl of resazurin was added to each well as bacterial growth indicator. After a further incubation at 37°C for 2 h, the bacterial growth was revealed by coloration change from purple to pink.
For yeasts, the microdilution assay was conducted according to the protocol previously described by Clinical and Laboratory Standards Institute[6] with slight modifications. First, the EO was serially diluted in YPG broth, supplemented with agar at 0.15% (w/v), to achieve final concentrations ranging between 4 and 0.003% (v/v). The 12th well was also considered as growth control. Then, 50 μl of inoculum was added to each well at final concentration of 103 CFU/ml. Finally, the microplate was incubated at 30°C for 48 h.
Determination of minimal (bactericidal/fungicidal) concentration
The minimal bactericidal/fungicidal concentration (MBC/MFC) was determined by spotting 3 μL from each negative well on LB agar plates for bacteria and YPG for yeasts and incubating at 37°C for 24 h and 30°C for 48 h, respectively.[7]
RESULTS
Chemical composition
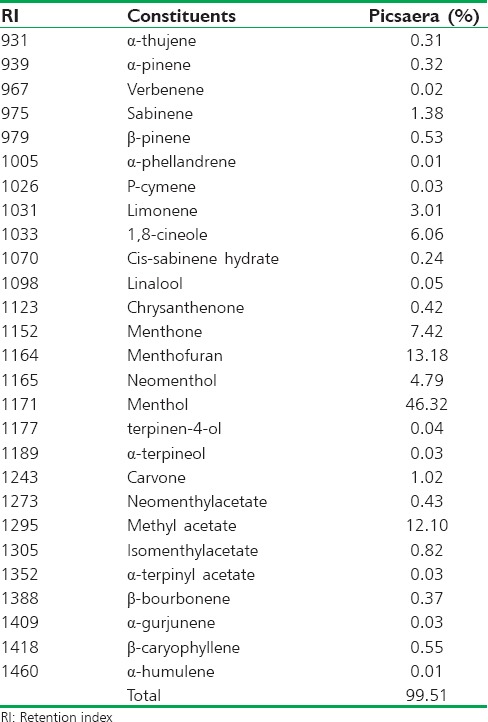
The studied EO has been previously subjected to the GC-MS analysis. Twenty-seven constituents, representing 99.51% of the total EO of M. piperita L., were identified. Its major constituents were menthol (46.32%), menthofuran (13.18%), menthyl acetate (12.10%), menthone (7.42%), and 1.8-cineole (6.06%) [Table 1].
Table 1.
Chemical composition of Mentha piperita essential oil
Antimicrobial effect of essential oil from Mentha piperita
As shown in Table 2, the EO of M. piperita exercised an important inhibitory activity against all studied bacteria, especially M. luteus and B. subtilis which showed a high sensitivity to this oil and were inhibited from very low concentrations of 0.062% (v/v) and 0.125% (v/v), respectively. Furthermore, the concentration of 0.25% (v/v) was sufficient to stop the growth of S. aureus and B. cereus.
Table 2.
The minimum inhibitory concentrations of Mentha piperita essential oil against bacterial strains tested
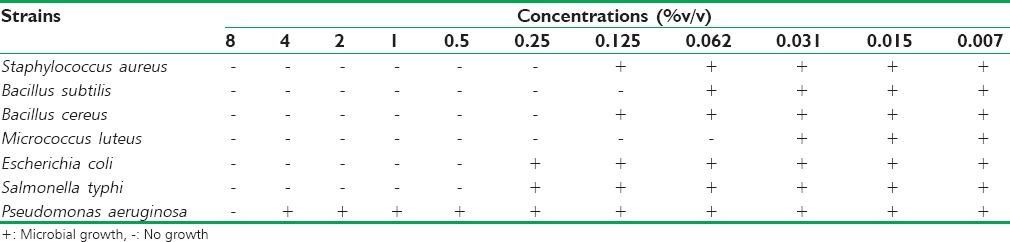
For Gram-negative bacteria, M. piperita EO was more effective against EO and S. typhimurium with a MIC of 0.5% (v/v). In contrast, P. aeruginosa was the least sensitive and was only inhibited by concentrations as high as 0.5% (v/v).
With regard to fungal strains, M. piperita EO exhibited a remarkable antifungal effect against both yeast strains tested with an MIC value of 0.062% (v/v) [Table 3].
Table 3.
The minimum inhibitory concentrations of Mentha piperita essential oil against fungal strains tested

Regarding the MBC values of M. piperita EO tested [Table 4], we found that MBC values could well be similar to their MIC values against S. aureus, B. cereus, and M. luteus and 2-fold higher toward B. subtilis, S. typhi, and EO and C. albicans. In addition, the MFC of C. tropicalis was 4-fold higher than its MIC value [Table 5].
Table 4.
The minimum bactericidal concentrations of Mentha piperita essential oil against bacterial strains tested
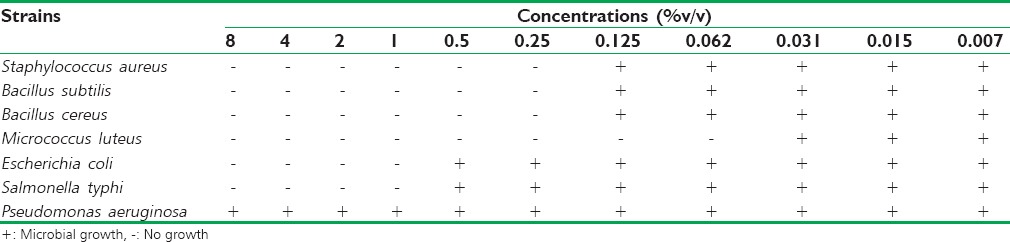
Table 5.
The minimum fungicidal concentrations of Mentha piperita essential oil against fungal strains tested

DISCUSSION
The present study has demonstrated the potential of M. piperita EO as an antimicrobial agent in the liquid phase.
The analysis results of M. piperita EO showed that its chemical composition is broadly similar to EO of M. piperita from Turkey regarding menthol, menthone, menthyl acetate, and menthofuron as main compounds.[8]
Hence, it is clear that menthol is the principal major component of the studied EO, which is in agreement with several other works.[9,10] Regarding other components, our results diverge from those published by other authors; in fact, the team research of Laghouiter et al.[11] has found that M. piperita from South Algeria is composed by trans-carveol (58.98%), D-limonene (19.94%), carvone (2.07%), and 4-terpineol (3.01%); hence, Yadegarinia et al.[12] showed that EO of M. piperita from Iran presented a significantly different chemical composition, consisting of a-terpinene (19.7%), isomenthone (10.3%), trans-carveol (14.5%), piperitenone oxide (19.3%) and β-caryophyllene (7.6%).
In fact, the compositions of EO vary significantly because of different species and chemotypes, geographical origin, plant's age and maturity season, and extraction procedure.[13,14,15]
M. piperita EO is known for its antimicrobial properties which have been reported in several studies.[16,4,8] These antibacterial activities could be mainly attributed to its chemical composition, which is very rich with oxygenated monoterpenes (92.95%) known for their higher efficiency and broader spectrum of antimicrobial activity.
In addition, Bakkali et al.[17] stipulate that the major compounds determine the biological EO activity. However, the antimicrobial power might also be attributed to the synergy between the various components of this oil.[18] Furthermore, the influence of minor compounds on antimicrobial activity should be also taken into consideration.
Moreover, among the identified compounds, some were previously reported to have antimicrobial activity including menthol and menthone that have been proven effective against human pathogenic[16,19] and also 1,8-cineole[20] and menthofuran.[21]
In addition, the antimicrobial effects of an EO can be attributed to its action mechanisms at cytoplasmic membrane level. In fact, EOs are selectively absorbed and interfered with the functions of biological cell membrane which involve lysis and loss of membrane integrity, causing damage to essential processes for cell survival.[22]
Hence, the antibacterial activity of EO tested in this study was more marked against Gram-positive than Gram-negative bacteria, which can be explained by the structure of the cell envelope. In fact, Gram-negative bacteria possess an additional membrane which gives them a double protection against toxic agents and hydrophobic compounds. However, the absence of this structure in Gram-positive bacteria makes them sensitive to attacks from external agents.[23]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.El Amri J, Elbadaoui K, Zair T, Bouharb H, Chakir S, Alaoui TI. Study of the antibacterial activity of Teucrium capitatum L essential oils and of Silene vulgaris extract on different tested strains. J Appl Biosci. 2014;82:7481–92. [Google Scholar]
- 2.Kumar P, Mishra S, Malik A, Satya S. Insecticidal properties of Mentha species. Ind Crops Prod. 2011;34:802–17. [Google Scholar]
- 3.Chraibi M, Fikri-Benbrahim K, Ou-yahyia D, Balouiri M, Farah A. Radical scavenging and disinfectant effect of essential oil from Moroccan Mentha pulegium. Int J Pharm Pharm Res. 2016;8:116–9. [Google Scholar]
- 4.Singh R, Shushni MA, Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab J Chem. 2015;8:322–8. [Google Scholar]
- 5.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity. JPharm Anal. 2016;6:71–9. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard - Second Edition Serving the World's Medical Science Community through Voluntary Consensus. The National Committee for Clinical Laboratory Standards. 2002 [Google Scholar]
- 7.Mattazi N, Farah A, Fadil M, Chraibi M, Fikri-Benbrahim K. Essential oils analysis and antibacterial activity of the leaves of Rosmarinus officinalis, Salvia officinalis and Mentha piperita cultivated in agadir (Morocco) Int J Pharm Pharm Sci. 2015;7:73–9. [Google Scholar]
- 8.Aridogan BC, Baydar H, Kaya S, Demirci M, Ozbasar D, Mumcu E. Antimicrobial activity and chemical composition of some essential oils. Arch Pharm Res. 2002;25:860–4. doi: 10.1007/BF02977005. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto GS, Neto FM, Ruiz ML, Acchile M, Chagas EC, Chaves FC, et al. Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture. 2016;45:182–6. [Google Scholar]
- 10.Malheiros DF, Maciel PO, Videira MN. Toxicity of the essential oil of Mentha piperita in Arapaima gigas (pirarucu) and antiparasitic effects on Dawestrema spp. (Monogenea) Aquaculture. 2016;455:81–6. [Google Scholar]
- 11.Laghouiter OK, Gherib A, Laghouiter H. Study of the antioxidant activity of essential oils from some cultivated mints in the region of Ghardaïa. El Wahat Review for Research and Studies. 2015;1:84–93. [Google Scholar]
- 12.Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry. 2006;67:1249–55. doi: 10.1016/j.phytochem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Palá-Paúl J, Pérez-Alonso MJ, Velasco-Negueruela A, Palá-Paúl R, Sanz J, Conejero F. Seasonal variation in chemical constituents of Santolina rosmarinifolia L. ssp. rosmarinifolia. Biochem Syst Ecol. 2001;29:663–72. doi: 10.1016/s0305-1978(01)00032-1. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Gomes PC, Fernandes-Ferreira M. Organ-and season-dependent variation in the essential oil composition of Salvia officinalis L. cultivated at two different sites. J Agric Food Chem. 2001;49:2908–16. doi: 10.1021/jf001102b. [DOI] [PubMed] [Google Scholar]
- 15.Lalli JY, Viljoen AM, Van Vuuren SF. Potential interaction between the volatile and non-volatile fractions on the in vitro antimicrobial activity of three South African Pelargonium (Geraniaceae) species. Nat Prod Commun. 2010;5:1395–400. [PubMed] [Google Scholar]
- 16.Iscan G, Kirimer N, Kürkcüoglu M, Baser KH, Demirci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem. 2002;50:3943–6. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- 17.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – A review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 18.Vinda-Martos M, Ruiz-Navaja Y, Fernanadez-Lopez J, Perez-Alvarez AJ. Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet. Int J Food Sci Technol. 2008;43:526–31. [Google Scholar]
- 19.Ben Arfa A, Combes S, Preziosi-Belloy L, Gontard N, Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol. 2006;43:149–54. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 20.Kifer D, Mužinic V, Klaric MŠ. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J Antibiot (Tokyo) 2016;69:689–96. doi: 10.1038/ja.2016.10. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoudi R, Katiraee F, Tajik H, Abbas A. Inhibitory effect of Mentha Longifolia L. essential oil against Listeria Monocytogenes using transmission electron microscopy. Int J Vet Sci Res. 2016;2:14–7. [Google Scholar]
- 22.Cleff MB, Meinerz AR, Xavier M, Schuch LF, Schuch LF, Araújo Meireles MC, et al. In vitro activity of origanum vulgare essential oil against Candida species. Braz J Microbiol. 2010;41:116–23. doi: 10.1590/S1517-838220100001000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian J, Yang G, Zhang Y, Chen Y, Luo Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2008;11:373–9. [Google Scholar]


