Abstract
Mycetoma is a localized chronic, suppurative, and deforming granulomatous infection seen in tropical and subtropical areas. It is a disorder of subcutaneous tissue, skin and bones, mainly of feet, characterized by a triad of localized swelling, underlying sinus tracts, and production of grains or granules. Etiological classification divides it into eumycetoma caused by fungus, and actinomycetoma caused by bacteria. Since the treatment of these two etiologies is entirely different, a definite diagnosis after histopathological and microbiological examination is mandatory, though difficult. Serological test exists but is not so reliable; however, molecular techniques to identify relevant antigens have shown promise. The disease is notoriously difficult to treat. Eumycetoma may be unresponsive to standard antifungal therapy. Actinomycetoma responds to antibiotic therapy, but prolonged treatment is necessary. This review focuses on the etiopathogenesis, clinical features, laboratory diagnosis, and treatment of mycetoma.
KEY WORDS: Actinomycetoma, eumycetoma, Madura foot
What was known?
Diagnosis of mycetoma can be made by the classic triad of painless soft tissue swelling, draining sinus tracts, and extrusion of grains
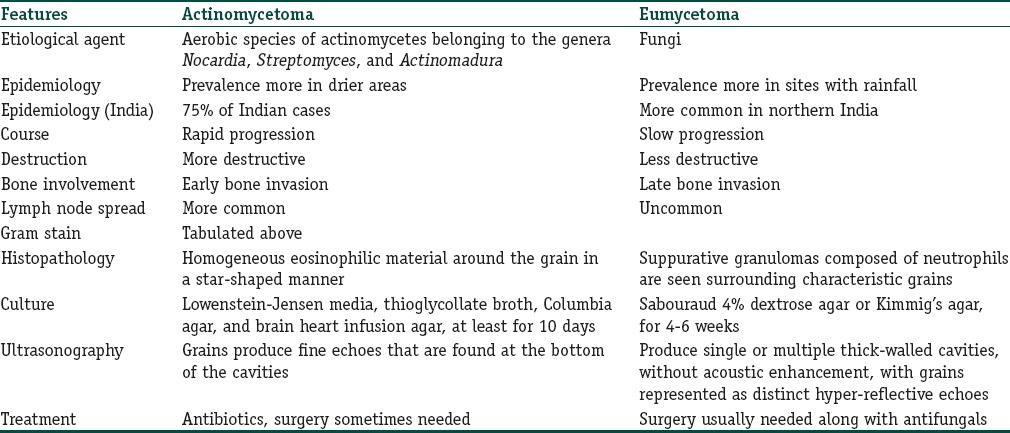
A diagnosis of actinomycetoma or eumycetoma can be made on outpatient basis based on morphology of grains
Treatment of actinomycetoma involves long-term use of antibiotics whereas that of eumycetoma is surgery followed by antifungals.
Introduction and Historical Aspects
which is amenable to prolonged treatment.[1,2] Mycetoma is a chronic suppurative infection affecting skin, subcutaneous tissue, and bones prevalent in tropical and subtropical regions. The oldest description of this disease dates back to the ancient Indian Sanskrit text Atharva Veda in which reference is made to padavalmikam, meaning “anthill foot.”[3] In more modern times, Gill first recognized mycetoma as a disease entity in 1842 in the southern province of Madura[4] from where the commonly used name “Madura foot” got prevalent. Godfrey first documented a case of mycetoma in Madras, India. However, the term “Mycetoma” (meaning fungal tumor) was proposed by Carter, who established the fungal etiology of this disorder.[4] He classified his cases by the color of the grains. Later, Pinoy recognized the possibility of classifying the cases of mycetoma by grouping the causative organisms, and the formal classification was put into place by Chalmers and Archibald who divided them into two groups.[5,6]
Group 1: Maduramycosis, caused by true fungi, and
Group 2: Actinomycosis, caused by actinomyces which belongs to higher bacteria.
Etiology
Mycetomas are caused by various species of fungi and bacteria, which occur as saprophytes in soil or on the plants. Actinomycotic mycetoma is caused by aerobic species of actinomycetes belonging to the genera Nocardia, Streptomyces and Actinomadura with Nocardia brasiliensis, Actinomadura madurae, Actinomadura pelletieri, and Streptomyces somaliensis being most common.
Eumycotic mycetoma is associated with a variety of fungi, the most common being Madurella mycetomatis.
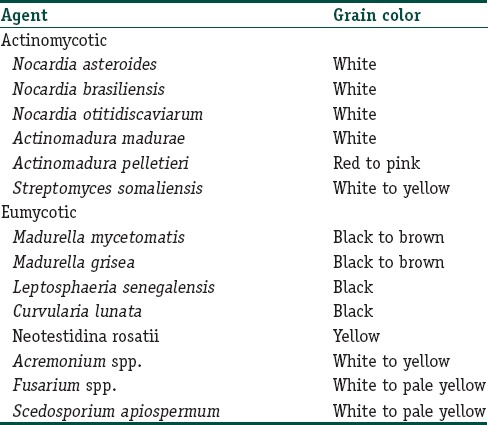
Clinically, the different species produce grains of different colors[7,8] in Table 1.
Table 1.
Mycetoma causing organisms and the color of grains they produce
Epidemiology
Mycetoma is reported to occur worldwide. It is endemic in tropical and subtropical regions, particularly between latitudes 15° S and 30° N, also known as the “Mycetoma belt” (Sudan, Somalia, Senegal, India, Yemen, Mexico, Venezuela, Colombia, and Argentina); however, the actual endemic area extends beyond this belt. Most cases are reported from Sudan and Mexico with Sudan being the most endemic country.[9] The species causing mycetoma vary from country to country, and agents that are more common in one region are rarely seen in other areas. Worldwide, M. Mycetomatis is the most common cause of this affliction. A. madurae, M. mycetomatis, and S. somaliensis are more commonly reported from drier regions, whereas Pseudallescheria boydii, Nocardia species, and A. pelletieri are more common in those areas with higher annual rainfall. In India, Nocardia species and Madurella grisea are the most common causes of mycetoma.[10]
Overall, most cases occur in arid and hot climates, which have a short period of heavy rainfall with milder temperatures. Actinomycetoma is more prevalent in drier areas, whereas eumycetoma is more common in sites with more rainfall.
Around 75% of mycetomas are actinomycotic in certain parts of India.[11] However, eumycotic mycetoma accounts for the majority of cases reported from the Northern region.[12] Mycetoma is more commonly reported in males than females (3:1), probably attributable to men being more commonly involved in agricultural work.[13,14] The condition is most common in young adults (16–40 years old)[15] and is uncommon in children.
Pathogenesis
Although antibodies against the causative organism are found in number of individuals, only few develop the disease and this may be attributable to a complex interplay of factors between the host and pathogen.
Host factors
The organism is usually implanted after a penetrating injury while performing agricultural work barefoot or through preexisting abrasions. Increased number in tropical regions may be due to decreased use of protective clothing, chiefly shoes, and due to the warmer, poorer conditions. Usually, some predisposing condition may be found such as poor general health, diabetes, and malnutrition, and this may lead to a more invasive and widespread infection. Complement-dependent chemotaxis of polymorphonuclear leukocytes has been shown to be induced by both fungal and actinomycotic antigens in vitro.[16] Cells of the innate immune system attempt to engulf and inactivate these organisms but in disease ultimately fail to accomplish this goal. Three types of immune responses have been described in response to the grains of mycetoma.[17]
Type a: Neutrophil degranulation and adherence to the grain surface, leading to gradual disintegration of the grain. Outside the zone of neutrophils is a zone of granulation tissue containing macrophages, lymphocytes, and plasma cells
Type b: Disappearance of neutrophils and arrival of macrophages to clear the grains and neutrophil debris
Type c: Marked by the formation of epithelioid cell granulomas.
T-cell responses also seem to play an important part in the development of mycetoma. Th2-like responses (interleukin (IL)-10 and IL-4) were found in primary lesions and in draining lymph nodes in S. somaliensis infection and after stimulation of peripheral blood mononuclear cells by M. mycetomatis antigens. Th1 responses are found in the acute phase of infection and in healthy endemic controls.[18]
Humoral antibodies also have a role in pathogenesis; in immunocompetent BALB/c mice, IgM antibodies induced specific protection in experimental N. brasiliensis infection. The disappearance of IgM antibodies and the appearance of IgG are postulated to account for the slow onset and the delay in development in experimental actinomycetoma.[19]
Recently, it has also been suggested that the greater frequency of disease in men may be due to progesterone inhibiting the growth of organisms.[20,21] In another study, estradiol was seen to limit the disease produced in animals.[20]
Factors related to pathogen
It has been found that certain species are more commonly found in the immunocompetent individuals such as N. brasiliensis, whereas others such as Nocardia farcinica, Nocardia nova, and Nocardia cyriacigeorgica mostly affect immunosuppressed individuals, and this may be due to ability of N. brasiliensis to survive the first-line innate immune response by phagocytes. The persistence of the organism after an initial inoculation appears to be related to its ability to evade host defenses through a variety of adaptations such as cell wall thickening and melanin production which protect microorganisms against ultraviolet radiation and destruction by alveolar macrophages, enzymatic lysis, and oxidants and might protect against antifungal drugs.
Ekizlerian et al.[22] studied the pathogenesis of mycetoma in animals injected with N. brasiliensis. It was suggested that the fractions of organisms are chemotactic for granulocytes, and the resultant influx of leukocytes to the site of inoculation is attributed to chemotactic activity induced by products of complement activation, formyl-methionyl peptides, leukotriene B4, and a soluble low molecular weight factor produced by macrophages. The lipid and polysaccharide constituents of bacteria probably participate in this inflammatory response inducing the liberation of products of complement activation or by inducing macrophage to secrete potent mediators of the acute inflammatory response. This host response does not appear to be able to control infection but likely accounts for the partial spontaneous healing that is seen in the disease.
Clinical Presentation of the Disease
Over 75% of patients have a lesion of lower extremity, most commonly in the foot (70%) followed by hand involvement. Other sites include the head, neck, chest, shoulder, and arms. The incubation period is variable, from 3 months to 9 years in natural infections. Since the mean duration before the first medical evaluation is long, patients often do not remember the preceding trauma.[23,24] The clinical features are fairly uniform, regardless of the organism involved.[25,26] The pathognomonic feature is a triad of painless firm subcutaneous mass, multiple sinus formation, and a purulent or seropurulent discharge containing grains.
Most cases start as small, painless, subcutaneous nodule at the site of injury which over time softens and ulcerates to discharge a viscous, purulent, or serosanguinous fluid [Figure 1a and b] containing characteristic granules. The granules, composed of colonies of causative organism, are hallmark of mycetoma and vary in size, color, and consistency depending on the etiological species. With time papules, pustules and nodules appear which also break down to form draining sinuses developing on the skin surface [Figure 2a and b]. Overlying skin appears smooth and shiny and is commonly fixed to the underlying tissue. Skin may be hypo- or hyper-pigmented, with signs of both old healed and active sinuses, displaying the cycle of spontaneous healing of older sinus tracts and simultaneous spread of infection to new areas typical of this disease. Sometimes, the overlying skin may display an increase in sweating secondary to sweat gland hyperplasia and increased local temperature due to increased blood flow secondary to inflammatory process.
Figure 1.
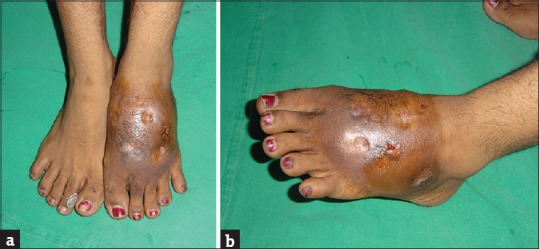
(a and b) A female patient with gross swelling of the left foot and ulcers in various stages of healing, with serosanguinous discharge from the active ulcer. Furthermore, evident is the pigmentary changes on the overlying skin
Figure 2.
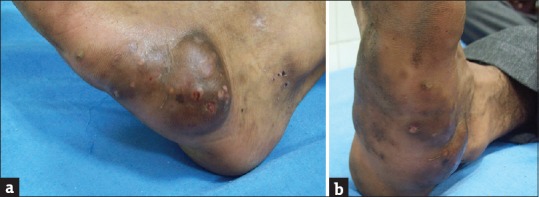
(a and b) A male patient with mycetoma with multiple papules, pustules, and nodules breaking down to form draining sinuses on the skin surface. Dorsum of foot of the same patient showing hyperpigmentation of skin with signs of both old healed and active sinuses
The disease progresses to involve the surrounding tissues which become swollen, indurated, and deformed by fibrous tissue reaction and multiple sinus formation. The condition is usually painless but may become very painful with the involvement of bones or as a result of secondary bacterial infection. Nerves and tendons are rarely affected till late in the disease.
Mycetoma is usually localized but may extend slowly by direct contiguity along the fascial planes, invading the subcutaneous tissue, fat, ligaments, muscles, and bones. In eumycotic mycetoma, there may be multiple punched out lytic lesions in bones whereas actinomycotic mycetoma is characterized by both osteolytic and osteosclerotic lesions. The result is gross swelling of the affected part with deformity. Actinomycetoma tends to progress more rapidly, with greater inflammation and tissue destruction and earlier invasion of bone than implantation mycosis.
Spread of the infection may also occur through the lymphatics, resembling sporotrichosis.[27] Metastatic lesions can also occur at various distant lymph nodes, which might become suppurative. These lymph node lesions are more common in actinomycetoma than in eumycetoma. Hematological spread has also been described.
Complications
The disease causes disfigurement but is rarely fatal. When left untreated, disease continues to progress, and bacterial superinfection leads to increased morbidity from local abscess formation, cellulitis, and bacterial osteomyelitis. In advanced cases, deformities or ankylosis may occur.
Differential Diagnosis
Mycetoma has to be differentiated from various infectious and noninfectious pathologies. Infectious pathology would usually include chronic infections such as cutaneous tuberculosis, nontuberculous mycobacterial infections of the skin, osteomyelitis (bacterial or tubercular), actinomycosis, chromomycosis, sporotrichosis, blastomycosis of the skin, dermatophyte pseudomycetomas, and botryomycosis.
Noninfectious differentials would include mossy foot or podoconiosis, malignant tumors, such as sarcoma of the skin and soft tissue or bones, and Kaposi sarcoma.
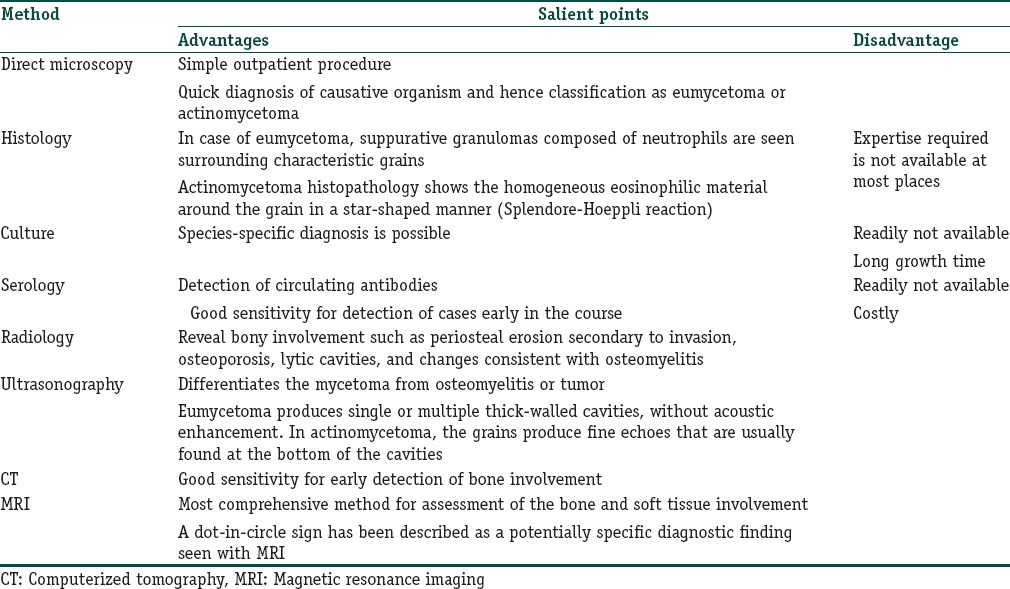
Laboratory Diagnosis
A diagnosis of mycetoma can be made by the classic triad. Diagnosis of the causative organism can be made by microscopic observation of a grain only. Histopathology and culture is usually not necessary. Finally, various serological and molecular tests have also found place in diagnosis of mycetoma.
Specimen collection
Ideally, the discharging fluid, scrapings of sinus walls, and tissue biopsy should be examined for the presence of grains. Saline dressings applied overnight over the swelling or aspiration of grains directly from an unopened sinus tract can also be used. Evaluation of spontaneously extruded grains may not allow diagnosis because these grains may be composed of dead organisms.
The grains extracted are evaluated in three ways to confirm the diagnosis: direct clinical examination, microscopy, and culture. Direct clinical examination involves evaluating the variation in size, color, and consistency of the grains, which can be helpful in rapid but provisional identification of the etiological agent.[7,8]
Direct microscopy
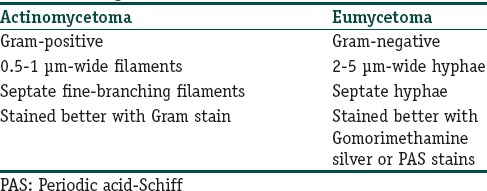
A Gram's stained preparation is of considerable value in distinguishing between actinomycetoma and eumycetoma in Table 2.[26,28] The study of discharged granules crushed on the slide and stained with special stains, most notably lactophenol blue, particularly allows differentiation between the thin filaments of actinomycetoma and the thicker hyphae of eumycetoma.
Table 2.
Difference between mycetoma causing organisms on basis of Gram stain
Histology
It is usually needed when the drainage material cannot be obtained, and in these cases, ideally a deep punch biopsy should be taken to include the subcutaneous tissue.
Hematoxylin and eosin (H and E) stain shows [Figure 3] suppurative granulomas (composed of neutrophils), surrounding characteristic grains which are present in the subcutaneous tissue. Grains or druses are aggregates of septated and branched, radially arranged broad hyphae, sometimes with vacuole formation. They are seen as broad, pink-stained hyphae surrounded by a sharp basophilic strand. The neutrophilic infiltrate is, in turn, surrounded by palisading histiocytes, beyond which a mixed inflammatory infiltrate comprising lymphocytes, plasma cells, eosinophils, and macrophages is seen. In long-standing cases, fibrosis may also be appreciated in the outermost layer.
Figure 3.
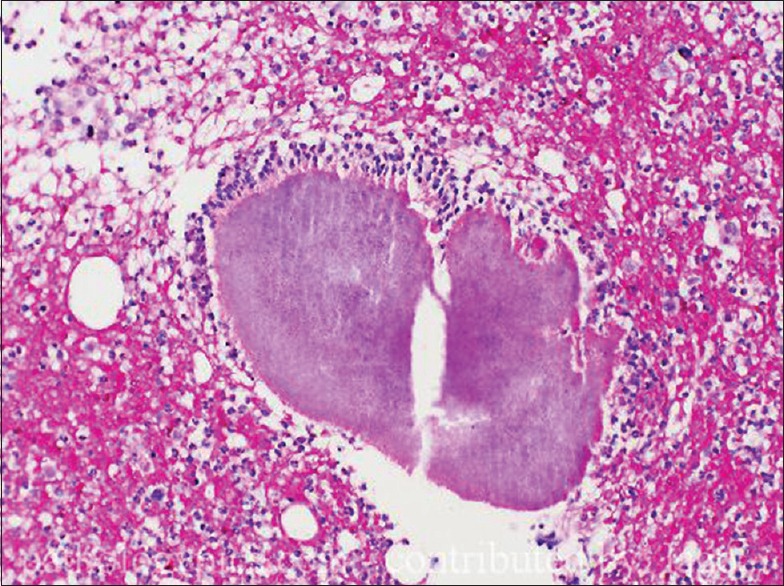
Skin biopsy, stained with H and E, ×40 view showing granulomas surrounded by a mixed inflammatory infiltrate comprising lymphocytes, plasma cells, eosinophils, macrophages are seen. Some amount of fibrosis can be seen in the periphery
In eumycetomas apart from H and E, periodic acid–Schiff and Grocott–Gomori staining may be performed for finer details. When an actinomycetoma is suspected, an additional Gram staining should be performed.
Culture
Grains of many species have overlapping morphological features, and therefore, culture in special media such as Sabouraud 4%, dextrose agar, or Kimmig's agar is capable of providing an accurate identification of the causative agent. Culture can be performed on grains or from biopsy sample, and incubation at 37°C is necessary because the isolates are human pathogens and probably will grow at 37°C. Fungal cultures should be kept for a longer time, approximately 4–6 weeks, in order not to overlook slowly growing fungi. M. mycetomatis, an important causative agent of eumycetomas, grows very slowly. First, growth of colonies is usually not seen before 10–15 days of cultivating.
Cultivation of actinomycetes requires special media and a longer duration than other bacterial cultures (10 days). Recommended culture media for actinomycetes are Lowenstein–Jensen media, thioglycollate broth, Columbia agar, and brain heart infusion agar. Incubation time should comprise approximately 48–72 h and longer at 35°C–37°C.
Serology
Serological tests are not usually required; however, they can prove useful in the early stages of the disease, even before granule formation. At present, no useful serological test exists that can reliably diagnose mycetoma. However, several serological assays have been used, including immunoblots, indirect hemagglutination assays, immunodiffusion, counterimmunoelectrophoresis, and ELISA. ELISA appears to be a sensitive test for the detection of circulating antibodies and has been especially used in epidemiological studies.[29] Serological diagnosis has been used in few studies for N. brasiliensis, M. mycetomatis, and P. boydii; however, their sensitivity and specificity is low and may be positive in healthy endemic controls.[30]
Recent advances in diagnostic techniques
The development of rapid and inexpensive species-specific polymerase chain reaction (PCR) analyses permits identification of new species and phylogenetic relationships.[31,32,33] PCR is done directly on the biopsy specimen, and sequencing of gene regions, for example, internal transcribed spacer 1 (ITS1), ITS2 is usually sufficient in most isolated fungi. In distinct cases, to identify the mold species, however, it will be necessary to use other gene regions as a multilocus sequence analysis, for example, of the large subunit, small subunit 18S nrDNA, β-tubulin, and chitin synthase 1 regions.[34]
Imaging techniques
Radiology and ultrasonography enable assessment of disease extent and bony involvement if any.[35] Standard X-ray studies can reveal bony involvement such as periosteal erosion secondary to invasion, osteoporosis, and changes consistent with osteomyelitis, osteolysis, and osteosclerosis [Figure 4]. However, it can pick up only fairly advanced disease.
Figure 4.
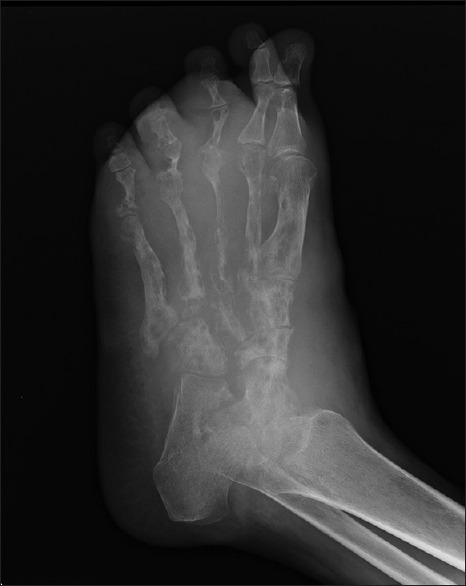
X-ray left foot anteroposterior view showing periosteal reaction, osteoporosis, and osteolysis
Ultrasonography successfully differentiates the mycetoma from osteomyelitis or tumor. Subtle differences between actinomycetoma and eumycetoma can also be picked up. Eumycetoma produces single or multiple thick-walled cavities, without acoustic enhancement, with grains represented as distinct hyper-reflective echoes. Actinomycetoma produced similar results except grains produced fine echoes that were found at the bottom of the cavities.
The use of helical computerized tomography has recently been shown to provide detailed assessments of soft tissue and visceral involvement[36] and appears to be more sensitive for detecting early changes. However, magnetic resonance imaging (MRI) provides the most comprehensive method for assessment of the bone and soft tissue involvement and may also be useful in evaluating the differential diagnosis of the swelling.[37,38] A “dot-in-circle sign” has been described as a potentially specific diagnostic finding seen with MRI.[37] The dots are tiny hypointense foci (believed to be grains) within spherical, high-intensity lesions (the circle) surrounded by low-intensity matrix on T2-weighted imaging, which represent granulomas scattered in areas of fibrosis. T1-weighted, fat-saturated, postgadolinium images may also produce this appearance.
Table 3 briefly summarizes the various diagnostic tests.
Table 3.
Summary of diagnostic investigations
Treatment
Treatment of mycetoma has proven to be difficult and typically includes antimicrobial agents and surgery. Surgery alone is rarely successful, but the removal of smaller lesions or debulking of larger ones does play an important role, especially in the management of fungal disease. As chemotherapeutic options for actinomycetoma and eumycetoma vary, the clinician must confirm the diagnosis before starting the treatment.
Treatment of actinomycetoma
Actinomycetomas are usually amenable to antibiotic treatment. Several antibiotics, among these cotrimoxazole, dapsone, streptomycin, trimethoprim (TMP), rifampicin, and amoxicillin-clavulanic acid combination, have been used and found to be effective.[2,39,40,41,42] In addition, combinations such as netilmicin with cotrimoxazole, amikacin with cotrimoxazole and rifampicin, and meropenem have also been used. In vitro sensitivity of actinomycetes to ciprofloxacin[11] and linezolid[42] has also been demonstrated, but these are currently not used as a first-line therapy. Today, the common consensus is that cotrimoxazole should be administered as a gold standard therapy in all actinomycetoma patients. Combination antibiotic therapy is preferable to monotherapy to avoid development of drug resistance and to eradicate residual infection.[43] Surgery may be required for some patients unresponsive to medical therapy alone.
The most commonly described regimens for actinomycetoma include streptomycin plus either TMP-sulfamethoxazole (TMP-SMX)[44] or dapsone. In this regimen, streptomycin (14 mg/kg/day intramuscularly) is given for the 1st month (and sometimes three times weekly thereafter for several months) in addition to a long course of TMP-SMX (usually one double-strength [DS] tablet [160 mg TMP and 800 mg SMX] twice daily), or dapsone (1.5 mg/kg/day twice daily).[45]
In 1987, Welsh demonstrated excellent therapeutic response with amikacin alone and in combination with TMP-SMX (Welsh regimen) in the treatment of 15 patients with poorly responsive actinomycotic mycetoma and those with systemic involvement. The regimen included cyclical dosing of amikacin 15 mg/kg/day, in two divided doses in cycles of 21 days for 1–3 cycles with intervals of 15 days between cycles while cotrimoxazole (one DS tablet BD) was administered continuously for 35–105 days. The 2-week interval of amikacin in the 5-week cycle is used for renal and audiometric monitoring. All patients achieved remission with this regimen with most patients requiring two cycles (42 days) of amikacin and 70 days of cotrimoxazole therapy. Damle et al. in 2008 introduced the modified Welsh regimen in unresponsive patients by adding rifampicin as the third drug.[46]
Ramam et al. initially described a two-step regimen consisting of an intensive phase with penicillin, gentamicin, and cotrimoxazole for 5–7 weeks, followed by maintenance therapy with amoxicillin and cotrimoxazole continued 5–6 months after clinical remission;[47] however, they later modified this to gentamicin (1.5 mg/kg IV) plus TMP-SMX (two DS tablets) given twice daily for 4 weeks followed by continuation of TMP-SMX plus doxycycline (100 mg twice daily). This modified approach had the advantage of reducing the number of injections and duration of intensive phase and reducing the cost of therapy but still maintaining the efficacy.[48]
Some other combinations that have been found useful are cotrimoxazole with penicillin,[49] dapsone with ampicillin and amikacin,[50] and tetracycline or chloramphenicol.[41]
As a general rule, actinomycetomas should be treated with a combination of cotrimoxazole and dapsone; however, in case of widespread infections, amikacin can be added for 3–5 pulses in the intensive phase.
In case of resistance or allergy to co-trimoxazole or amikacin, co-amoxiclav can be used as an alternative to co-trimoxazole and netilmicin to amikacin. Co-amoxiclav can also be used alone during pregnancy; however, chances of resistance are there. Amikacin combined with a carbapenem, such as imipenem or meropenem, could also be used in refractory cases.[30]
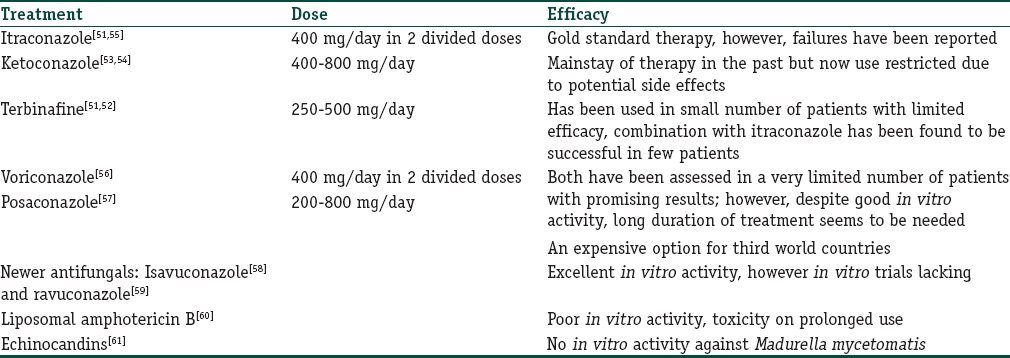
Treatment of eumycetoma
Eumycotic mycetomas usually respond less well to drug therapy, and therefore, a combined approach of both medical and surgical therapy is usually undertaken. Complete surgical excision of the lesion followed by long courses of antifungals should form the first line of management in eumycotic mycetoma. Triazole antifungals (itraconazole) are the treatment of choice and usually a prolonged treatment of 1–2 years is required, however, due considerations should be made at appropriate intervals about the response and treatment side effects. Various treatments available for eumycotic mycetomas are in Table 4.
Table 4.
Treatment options for eumycotic mycetoma
End point of treatment
Since the treatment is prolonged, few indicators may be used to define the end point of treatment including skin becoming normal, disappearance of mass, healing of sinuses, and elimination of organisms from the tissue evidenced by the absence of grains in fine-needle aspiration cytology with a type 3 tissue reaction and the disappearance of the grains and cavities on ultrasonography.[17] Radiological examination is essential for follow-up of patients on medical treatment and to assure cure. It usually shows reappearance of normal bone pattern and the disappearance of the soft tissue mass.
Conclusion
Mycetoma, being a relatively painless condition, is often diagnosed at an advanced stage where permanent deformity of affected part has already occurred. The chronic nature of the disease leads to a high possibility of superimposed bacterial infection which can worsen the disease. This can cause increased pain and disability as well as septicemia which may be fatal if untreated. Thus, there is a need for correct diagnosis of mycetoma after meticulous clinical examination, assisted by histological and microbiological studies along with the use of special stains and a proper treatment.
Table 5 summarizes the important differences between actinomycetoma and eumycetoma.
Table 5.
Summary of differences between actinomycetoma and eumycetoma
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Greater frequency of disease in men may not be just attributable to environmental factors but also to hormonal factors
Various serological and molecular tests have also found place in diagnosis of mycetoma allowing early diagnosis and identification of new species and phylogenetic relationships
Magnetic resonance imaging provides the most comprehensive method for assessment of the bone and soft tissue involvement and may also be useful in evaluating the differential diagnosis of the swelling
Combination antibiotic therapy is a must in case of actinomycetomas
Several newer antifungals have been tried for eumycetomas though in vivo studies are lacking.
References
- 1.McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97–104. doi: 10.1016/s0733-8635(05)70329-6. [DOI] [PubMed] [Google Scholar]
- 2.Mahgoub ES. Medical management of mycetoma. Bull World Health Organ. 1976;54:303–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon-Chung KJ, Bennett JE. Medical Mycology. Philadelphia: Lea & Febiger; 1992. Mycetoma; pp. 560–93. [Google Scholar]
- 4.Carter HV. On a New and Striking form of Fungus Disease Principally Affecting the Foot and Prevailing Endemically in Many Parts of India. Transactions of the Medical and Physical Society of Bombay. 1860;6:104–42. [Google Scholar]
- 5.Pinoy E. Actinomycoses and mycetomas. Bull Inst Pasteur. 1913;11:929. [Google Scholar]
- 6.Chalmers AJ, Archibald RG. A Sudanese maduromycoses. Ann Trop Med. 1916;10:169. [Google Scholar]
- 7.Palestine RF, Rogers RS., 3rd Diagnosis and treatment of mycetoma. J Am Acad Dermatol. 1982;6:107–11. doi: 10.1016/s0190-9622(82)70009-x. [DOI] [PubMed] [Google Scholar]
- 8.Magana M. Mycetoma. Int J Dermatol. 1984;23:221–36. doi: 10.1111/j.1365-4362.1984.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 9.Hay RJ, Mackenzie DW. Mycetoma (madura foot) in the United Kingdom – A survey of forty-four cases. Clin Exp Dermatol. 1983;8:553–62. doi: 10.1111/j.1365-2230.1983.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 10.Green WO, 3rd, Adams TE. Mycetoma in the United States; a review and report of seven additional cases. Am J Clin Pathol. 1964;42:75–91. doi: 10.1093/ajcp/42.1.75. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri BN, Maiti PK, Sil J. Antibiotic sensitivity patterns of actinomycetes isolated from patients of actinomycetoma. Indian J Med Res. 1997;105:162–6. [PubMed] [Google Scholar]
- 12.Taralakshmi VV, Pankajalakshmi VV, Arumugam S, Subramanian S. Mycetoma caused by Madurella mycetomii in Madras. Australas J Dermatol. 1978;19:125–9. doi: 10.1111/j.1440-0960.1978.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Fahal AH, Sabaa AH. Mycetoma in children in Sudan. Trans R Soc Trop Med Hyg. 2010;104:117–21. doi: 10.1016/j.trstmh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Zarei Mahmoudabadi A, Zarrin M. Mycetomas in Iran:a review article. Mycopathologia. 2008;165:135–41. doi: 10.1007/s11046-007-9066-z. [DOI] [PubMed] [Google Scholar]
- 15.López Martínez R, Méndez Tovar LJ, Lavalle P, Welsh O, Saúl A, Macotela Ruíz E. Epidemiology of mycetoma in Mexico: Study of 2105 cases. Gac Med Mex. 1992;128:477–81. [PubMed] [Google Scholar]
- 16.Yousif MA, Hay RJ. Leucocyte chemotaxis to mycetoma agents – The effect of the antifungal drugs griseofulvin and ketoconazole. Trans R Soc Trop Med Hyg. 1987;81:319–21. doi: 10.1016/0035-9203(87)90252-5. [DOI] [PubMed] [Google Scholar]
- 17.Fahal AH, el Toum EA, el Hassan AM, Mahgoub ES, Gumaa SA. The host tissue reaction to Madurella mycetomatis: New classification. J Med Vet Mycol. 1995;33:15–7. [PubMed] [Google Scholar]
- 18.el Hassan AM, Fahal AH, Ahmed AO, Ismail A, Veress B. The immunopathology of actinomycetoma lesions caused by Streptomyces somaliensis. Trans R Soc Trop Med Hyg. 2001;95:89–92. doi: 10.1016/s0035-9203(01)90346-3. [DOI] [PubMed] [Google Scholar]
- 19.Salinas-Carmona MC, Pérez-Rivera I. Humoral immunity through immunoglobulin M protects mice from an experimental actinomycetoma infection by Nocardia brasiliensis. Infect Immun. 2004;72:5597–604. doi: 10.1128/IAI.72.10.5597-5604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez-Hernandez F, Lopez-Martinez R, Mendez-Tovar LJ, Manzano-Gayosso P. Nocardia brasiliensis: In vitro and in vivo growth response to steroid sex hormones. Mycopathologia. 1995;132:79–85. doi: 10.1007/BF01103779. [DOI] [PubMed] [Google Scholar]
- 21.Méndez-Tovar LJ, de Biève C, López-Martínez R. Effects of human sex hormones on in vitro development of agents of eumycétomes. J Mycol Méd. 1991;1:141–3. [Google Scholar]
- 22.Ekizlerian SM, Brandão Filho SL, Tincani I, Alves LM, Silva CL. Studies on the pathogenesis of actinomycotic mycetoma in animals injected with fractions isolated from Nocardia brasiliensis. Br J Exp Pathol. 1987;68:115–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Maiti PK, Ray A, Bandyopadhyay S. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop Med Int Health. 2002;7:788–92. doi: 10.1046/j.1365-3156.2002.00915.x. [DOI] [PubMed] [Google Scholar]
- 24.Dieng MT, Sy MH, Diop BM, Niang SO, Ndiaye B. Mycetoma: 130 cases. Ann Dermatol Venereol. 2003;130(1 Pt 1):16–9. [PubMed] [Google Scholar]
- 25.Mahgoub ES, Murray IG. Mycetoma. London: Heinemann Medical; 1973. [Google Scholar]
- 26.Zaias N, Taplin D, Rebell G. Mycetoma. Arch Dermatol. 1969;99:215–25. [PubMed] [Google Scholar]
- 27.el Hassan AM, Mahgoub ES. Lymph node involvement in mycetoma. Trans R Soc Trop Med Hyg. 1972;66:165–9. doi: 10.1016/0035-9203(72)90065-x. [DOI] [PubMed] [Google Scholar]
- 28.Pilsczek FH, Augenbraun M. Mycetoma fungal infection: Multiple organisms as colonizers or pathogens? Rev Soc Bras Med Trop. 2007;40:463–5. doi: 10.1590/s0037-86822007000400017. [DOI] [PubMed] [Google Scholar]
- 29.Salinas-Carmona MC, Welsh O, Casillas SM. Enzyme-linked immunosorbent assay for serological diagnosis of Nocardia brasiliensis and clinical correlation with mycetoma infections. J Clin Microbiol. 1993;31:2901–6. doi: 10.1128/jcm.31.11.2901-2906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zijlstra EE, van de Sande WW, Welsh O, Mahgoub el S, Goodfellow M, Fahal AH. Mycetoma: A unique neglected tropical disease. Lancet Infect Dis. 2016;16:100–12. doi: 10.1016/S1473-3099(15)00359-X. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed AO, Mukhtar MM, Kools-Sijmons M, Fahal AH, de Hoog S, van den Ende BG, et al. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol. 1999;37:3175–8. doi: 10.1128/jcm.37.10.3175-3178.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desnos-Ollivier M, Bretagne S, Dromer F, Lortholary O, Dannaoui E. Molecular identification of black-grain mycetoma agents. J Clin Microbiol. 2006;44:3517–23. doi: 10.1128/JCM.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borman AM, Linton CJ, Miles SJ, Johnson EM. Molecular identification of pathogenic fungi. J Antimicrob Chemother. 2008;61(Suppl 1):i7–12. doi: 10.1093/jac/dkm425. [DOI] [PubMed] [Google Scholar]
- 34.Nenoff P, van de Sande WW, Fahal AH, Reinel D, Schöfer H. Eumycetoma and actinomycetoma – An update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873–83. doi: 10.1111/jdv.13008. [DOI] [PubMed] [Google Scholar]
- 35.Lupi O, Tyring SK, McGinnis MR. Tropical dermatology: Fungal tropical diseases. J Am Acad Dermatol. 2005;53:931–51. doi: 10.1016/j.jaad.2004.10.883. [DOI] [PubMed] [Google Scholar]
- 36.Bonifaz A, González-Silva A, Albrandt-Salmerón A, Padilla Mdel C, Saúl A, Ponce RM. Utility of helical computed tomography to evaluate the invasion of actinomycetoma; a report of 21 cases. Br J Dermatol. 2008;158:698–704. doi: 10.1111/j.1365-2133.2008.08435.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarris I, Berendt AR, Athanasous N, Ostlere SJ OSIRIS Collaborative Study Group. MRI of mycetoma of the foot: Two cases demonstrating the dot-in-circle sign. Skeletal Radiol. 2003;32:179–83. doi: 10.1007/s00256-002-0600-2. [DOI] [PubMed] [Google Scholar]
- 38.Czechowski J, Nork M, Haas D, Lestringant G, Ekelund L. MR and other imaging methods in the investigation of mycetomas. Acta Radiol. 2001;42:24–6. doi: 10.1080/028418501127346413. [DOI] [PubMed] [Google Scholar]
- 39.Welsh O, Sauceda E, Gonzalez J, Ocampo J. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443–8. doi: 10.1016/s0190-9622(87)70227-8. [DOI] [PubMed] [Google Scholar]
- 40.Gomez A, Saul A, Bonifaz A, Lopez M. Amoxicillin and clavulanic acid in the treatment of actinomycetoma. Int J Dermatol. 1993;32:218–20. doi: 10.1111/j.1365-4362.1993.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 41.Mahaisavariya P, Chaiprasert A, Sivayathorn A, Khemngern S. Deep fungal and higher bacterial skin infections in Thailand: Clinical manifestations and treatment regimens. Int J Dermatol. 1999;38:279–84. doi: 10.1046/j.1365-4362.1999.00681.x. [DOI] [PubMed] [Google Scholar]
- 42.Gugnani HC, Suselan AV, Anikwe RM, Udeh FN, Ojukwu JO. Actinomycetoma in Nigeria. J Trop Med Hyg. 1981;84:259–63. [PubMed] [Google Scholar]
- 43.Fahal AH. Mycetoma: A thorn in the flesh. Trans R Soc Trop Med Hyg. 2004;98:3–11. doi: 10.1016/s0035-9203(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 44.Smith MD, McGinnis MR. Subcutaneous fungal infections (chromoblastomycosis, mycetoma, and lobomycosis) In: Hospenthal DR, Rinaldi MG, editors. Diagnosis and Treatment of Human Mycoses. Totowa, NJ: Humana Press; 2008. pp. 383–92. [Google Scholar]
- 45.Chávez G, Estrada R, Bonifaz A. Perianal actinomycetoma experience of 20 cases. Int J Dermatol. 2002;41:491–3. doi: 10.1046/j.1365-4362.2002.01550.x. [DOI] [PubMed] [Google Scholar]
- 46.Damle DK, Mahajan PM, Pradhan SN, Belgaumkar VA, Gosavi AP, Tolat SN, et al. Modified Welsh regimen: A promising therapy for actinomycetoma. J Drugs Dermatol. 2008;7:853–6. [PubMed] [Google Scholar]
- 47.Ramam M, Garg T, D’Souza P, Verma KK, Khaitan BK, Singh MK, et al. A two-step schedule for the treatment of actinomycotic mycetomas. Acta Derm Venereol. 2000;80:378–80. doi: 10.1080/000155500459367. [DOI] [PubMed] [Google Scholar]
- 48.Ramam M, Bhat R, Garg T, Sharma VK, Ray R, Singh MK, et al. A modified two-step treatment for actinomycetoma. Indian J Dermatol Venereol Leprol. 2007;73:235–9. doi: 10.4103/0378-6323.32888. [DOI] [PubMed] [Google Scholar]
- 49.Khatri ML, Al-Halali HM, Fouad Khalid M, Saif SA, Vyas MC. Mycetoma in Yemen: Clinicoepidemiologic and histopathologic study. Int J Dermatol. 2002;41:586–93. doi: 10.1046/j.1365-4362.2002.01383.x. [DOI] [PubMed] [Google Scholar]
- 50.Sharma N, Mendiratta V, Sharma RC, Hemal U, Verma M. Pulse therapy with amikacin and dapsone for the treatment of actinomycotic foot: A case report. J Dermatol. 2003;30:742–7. doi: 10.1111/j.1346-8138.2003.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 51.Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47–71. [PubMed] [Google Scholar]
- 52.N’diaye B, Dieng MT, Perez A, Stockmeyer M, Bakshi R. Clinical efficacy and safety of oral terbinafine in fungal mycetoma. Int J Dermatol. 2006;45:154–7. doi: 10.1111/j.1365-4632.2004.02392.x. [DOI] [PubMed] [Google Scholar]
- 53.Mahgoub ES, Gumaa SA. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomii. Trans R Soc Trop Med Hyg. 1984;78:376–9. doi: 10.1016/0035-9203(84)90126-3. [DOI] [PubMed] [Google Scholar]
- 54.Venugopal PV, Venugopal TV. Treatment of eumycetoma with ketoconazole. Australas J Dermatol. 1993;34:27–9. doi: 10.1111/j.1440-0960.1993.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 55.Queiroz-Telles F, McGinnis MR, Salkin I, Graybill JR. Subcutaneous mycoses. Infect Dis Clin North Am. 2003;17:59–85. doi: 10.1016/s0891-5520(02)00066-1. viii. [DOI] [PubMed] [Google Scholar]
- 56.Porte L, Khatibi S, Hajj LE, Cassaing S, Berry A, Massip P, et al. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans R Soc Trop Med Hyg. 2006;100:891–4. doi: 10.1016/j.trstmh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339–46. doi: 10.1590/s0036-46652005000600006. [DOI] [PubMed] [Google Scholar]
- 58.Kloezen W, Meis JF, Curfs-Breuker I, Fahal AH, van de Sande WW. In vitro antifungal activity of isavuconazole against Madurella mycetomatis. Antimicrob Agents Chemother. 2012;56:6054–6. doi: 10.1128/AAC.01170-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed SA, Kloezen W, Duncanson F, Zijlstra EE, de Hoog GS, Fahal AH, et al. Madurella mycetomatis is highly susceptible to ravuconazole. PLoS Negl Trop Dis. 2014;8:e2942. doi: 10.1371/journal.pntd.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed AO, van de Sande WW, van Vianen W, van Belkum A, Fahal AH, Verbrugh HA, et al. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-Bis(2-methoxy-4-nitro-5- sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother. 2004;48:2742–6. doi: 10.1128/AAC.48.7.2742-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Sande WW, Fahal AH, Bakker-Woudenberg IA, van Belkum A. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother. 2010;54:2738–40. doi: 10.1128/AAC.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


