Abstract
Introduction:
Dermatophytosis are the most common fungal infections globally. Terbinafine is considered to have good potency against dermatophytes, but resistance to terbinafine is on the rise.
Objective:
The objective of this study was to evaluate the efficacy and safety of terbinafine 500 mg given once daily in treatment of patients with superficial dermatophytosis.
Materials and Methods:
It was a retrospective questionnaire-based survey. Each doctor was given survey questionnaire booklet containing survey forms. Clinical response was graded according to the improvement in the affected lesion. Mycological cure was defined as negative microscopy under potassium hydroxide examination and a negative culture in Sabouraud's dextrose agar. Patients were divided into three groups depending on the duration of therapy, Group A – terbinafine 500 mg for 2 weeks, Group B – terbinafine 500 mg for 4 weeks, and Group C – terbinafine 500 mg for 6 weeks.
Results:
Total 50 doctors completed the survey involving 440 patients. In Group A, out of 194 patients, 87% (n = 169) patients showed very good response. In Group B, out of 211 patients, 92% (n = 194) of the patients showed very good response with >75% improvement in their lesion. In Group C, out of 35 patients, 80% (n = 30) patients showed very good response. Adverse drug reactions of mild to moderate intensity related to terbinafine were seen in 57 patients.
Conclusion:
Our survey indicates that terbinafine in a dose of 500 mg given once daily was efficacious and safe in the treatment of patients with dermatophytosis.
KEY WORDS: Antifungal resistance, dermatophytosis, terbinafine
What was known?
Terbinafine is considered to have good potency against tinea, but incomplete cure is common with current terbinafine 250 mg/day therapy.
Introduction
Dermatophytosis are the most common fungal infections globally. According to the World Health Organization, the prevalence rate of superficial mycotic infection worldwide has been found to be 20%–25%.[1] Hot and humid climate in the tropical and subtropical countries like India makes dermatophytosis a very common superficial fungal infection.[2]
Various antifungal agents both topical and systemic have been introduced into clinical practice for effectively treating dermatophytic conditions. The commonly used drugs include azoles, allylamines, and griseofulvin.
Terbinafine is the orally available allylamine antifungal. With a favorable mycological and pharmacokinetic profile, terbinafine is considered to be a first-line drug for the treatment of tinea corporis and cruris.[3] Terbinafine acts by inhibiting the enzyme squalene epoxidase thus inhibits the synthesis of ergosterol, an important component of fungal cell membrane leading to fungal cell wall disintegration.[4] The drug has shown consistent efficacy against dermatophytes achieving more than 90% cure rates at a dose of 250 mg/day when administered for 2 weeks.[3,4]
However, recently, clinical failure and relapses have been observed with terbinafine in patients with tinea infections[5] with increase in incidence of terbinafine resistance.[6,7,8] The principle reasons may include low plasma concentration[9] and incomplete cure[5] which are very common following 2 weeks therapy with 250 mg/day of terbinafine. The increased use, inappropriate prescribing and over the counter sale of antifungal agents has also added in the development of resistance to these drugs.[10,11]
Hence, there was need for different treatment strategy while using terbinafine. We conducted this survey with the aim of evaluating the efficacy and safety of terbinafine 500 mg given once daily in treatment of patients with dermatophytosis.
Materials and Methods
A survey was conducted using a pretested questionnaire. The questionnaire was designed to assess the efficacy and safety of terbinafine 500 mg in the treatment of superficial fungal infections. Survey period was from July 2016 to October 2016. Dermatologists and general physicians involved in the management of superficial fungal infections were identified through “SCRIP intelligence” database. Among these 50 doctors who were maintaining the patients’ clinical record were selected across 4 zones (east, south, west, and north) each by convenient sampling to have uniform representation of population across country.
Each doctor was given survey questionnaire booklet containing survey forms. The questionnaires’ booklets were collected after the end of survey period and data from all the patients were assessed to evaluate the efficacy and safety of terbinafine 500 mg. In this survey, we included patients who underwent mycological examination before and after the treatment and who had taken terbinafine therapy for 2, 4, and 6 weeks. Patients were divided into three groups depending on the duration of therapy for final evaluation. Patients included in Group A received terbinafine for duration of 2 weeks, patients included in Group B received terbinafine for 4 weeks, and patients included in Group C received terbinafine for 6 weeks.
Efficacy evaluation
Clinical cure
Clinical response was graded according to the changes observed in clinical signs and was divided into four categories: Grade I - when the improvement was >75% in the affected lesion (very good response); Grade II - when the improvement was between 51% and 75% (good response); Grade III - when the improvement was between 26% and 50% (poor response); and Grade IV - When the improvement was ≤25%. The clinical cure was defined as Grade I response in improvement.[12]
Mycological cure
Mycological cure was defined as negative microscopy under potassium hydroxide (KOH) examination and a negative culture in Sabouraud's dextrose agar at the end of the follow-up period.
Safety evaluation
All adverse events (AEs) were assessed for severity and relationship to terbinafine at each visit. Multiple occurrences of the same AE were only counted once for each patient. Laboratory measurements such as liver function tests were also included for safety evaluation.
Results
Total fifty doctors participated in the survey, from whom a total of 500 survey forms were collected at the end of 4 months period. Four hundred and forty completed survey questionnaire forms were included for further evaluation. The baseline demographic parameters from the evaluable survey forms representing gender, age, medical history among the patients are shown in Table. 1.
Table 1.
Demographic characters of patients
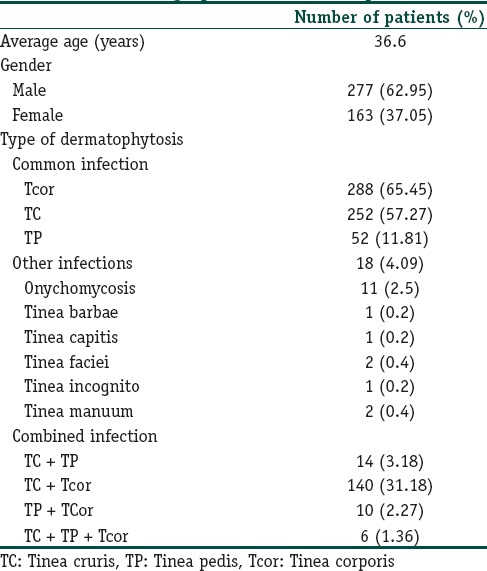
The average age of the population was 36.6 years out of total 440 patients evaluated, 62.95% (n = 277) were male while 37.05% (n = 163) were female patients.
In this survey, tinea corporis was the most common infection in 65.45% patients. Tinea cruris was the second most common infection in 57.27% patients. The less common infections were tinea pedis (11.81%), onychomycosis (2.5%), tinea barbae (0.2%), tinea faciei (0.4%), tinea capitis (0.2%), tinea incognito (0.2%), and tinea manuum (0.4%). Out of 440 patients evaluated, 170 were suffering from associated dermatophytes infections at multiple sites. The most common combination being tinea corporis and tinea cruris in 31.18% (n = 140) of patients [Table 1].
In the current survey, it has been observed that in 44.09% (n = 194) patients, terbinafine 500 mg was given OD for 2 weeks (Group A). In 47.95% (n = 211) patients, terbinafine 500 mg OD was given for 4 weeks (Group B), while in 7.95% (n = 35) patients, the therapy was given for 6 weeks (Group C) [Table 2].
Table 2.
Number of patients according to duration of therapy

In this survey, it has been observed that majority of patients in Group A had infection of single site with mild to moderate intensity. Group B patients were mainly having infections of multiple sites with severe intensity. The patients in Group C were mainly having resistant infections such as onychomycosis and infections of multiple sites.
Efficacy evaluation
In Group A, out of 194 patients treated with terbinafine, 87% (n = 169) patients showed very good response (>75% improvement in lesion) at the end of the therapy while 10% (n = 19) patients showed good response (51%–75% improvement in lesion) [Figure 1].
Figure 1.
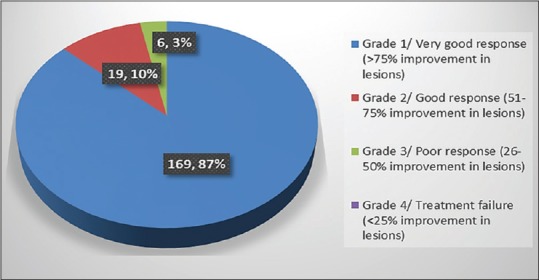
Group A – Number of patients showing clinical improvement
In Group B, it has been observed that patients visited doctor twice initially after 2 weeks and again after 4 weeks. At the end of 2nd week, 73% (n = 154) patients showed improvement of around 51%–75% in their lesions and 20% (n = 43) patients showed poor response (25%–50% improvement in lesion).
At the end of 4 weeks, there was much more improvement in lesions of the patients. Out of 211 patients, 92% (n = 194) of the patients showed very good response to the treatment with >75% improvement in their lesion, whereas 17 patients showed good response [Figure 2].
Figure 2.
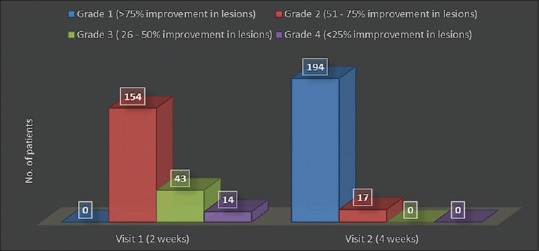
Group B – Number of patients showing clinical improvement
It has been observed that in Group C, patients visited doctor thrice at interval of 2 weeks. At the end of 2nd week, 48% (n = 17) patients showed Grade 4 response while 52% (n = 18) patients showed Grade 3 response. After 4 weeks, 72% (n = 25) patients showed Grade 2 response while remaining 28% (n = 10) patients showed around 20%–50% improvement in their lesions. At the end of 6 weeks, 80% (n = 27) patients showed very good response and remaining 20% (n = 8) did not responded to the treatment [Figure 3].
Figure 3.
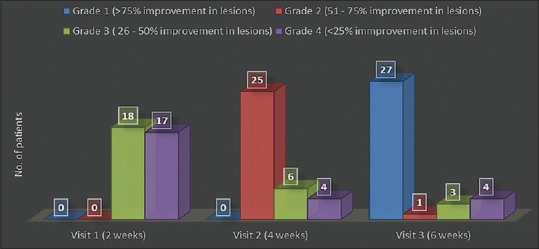
Group C – Number of patients showing clinical improvement
KOH and culture positivity were recorded in 80.0 and 65.5% cases, respectively. Trichophyton rubrum was the most common isolate in 60% cases, trichophyton mentagrophytes in 13.3% patients. Mycological cure rates were 83%, 90%, and 78% in Group A, B, and C, respectively.
Safety evaluation
Adverse effects due to terbinafine were observed in 57 patients [Figure 4]. Majority of these events were observed during initial few days after start of therapy. All the side effects disappeared with time without any treatment. The AEs were of mild to moderate in intensity. Discontinuation of therapy due to AEs was not required in any of the patient. Adherence to the treatment in all the groups was excellent with all the patients completing the treatment.
Figure 4.
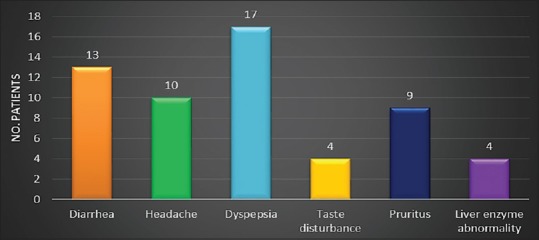
Adverse event profile
Discussion
Dermatophytosis is the most common superficial mycoses globally.[1] Number of antifungal agents have been introduced for treating this condition and more are underway. Terbinafine is one of the most commonly used antifungal drug used in the treatment of superficial fungal infections because of its broad-spectrum fungicidal activity. The drug has shown consistent efficacy against dermatophytes achieving more than 90% cure rates at a dose of 250 mg/day when administered for 2 weeks.[3,4]
However, recently, clinical failure and relapses have been observed with terbinafine in patients with tinea infections with increase in incidence of terbinafine resistance.[5] Although resistance to terbinafine in dermatophytosis is not common in clinical practice, it has been reported in clinical isolates by few authors.[6,7,8] Mukherjee et al. in 2003 reported first confirmed report of terbinafine resistance in dermatophytes.[8] Majid et al. in their study reported that at the end of 12 weeks, there were only 43 cases out of the total 100 cases enrolled who were able to maintain a long-term clinical and mycological cure after 2 weeks of oral terbinafine treatment. Authors concluded that incomplete mycological cure as well as relapse was very common after standard (2-week) terbinafine therapy in patients of tinea cruris/corporis.[5]
One of the principle mechanisms of antifungal resistance is decrease in effective drug concentration,[13] which in case of terbinafine is quite known feature following standard dosing regimen of 250 mg daily due to extensive accumulation in skin and adipose tissue.[9] This clearly shows that the current standard terbinafine therapy with 250 mg/day dose is not sufficient in current scenario where fungal resistance is further aggravated by increased use, inappropriate prescribing and over the counter sale of antifungal agents.[10,11]
Even though there is no clear evidence as to what strategy should be used to best avoid resistance,[14] the most commonly suggested measures in the past include prudent use of antifungals and appropriate dosing with special emphasis on avoiding treatment with low antifungal dosage.[15]
Using higher dosages of terbinafine thus seems useful strategy in countering these problems. Dolton et al. in their study reported that higher dose of terbinafine was associated with highest trough and peak concentrations and area under the curve values in plasma which in turn were associated with lower minimum inhibitory concentration values and higher antifungal activity.[16]
We conducted this survey to find out the efficacy and safety of higher dose of terbinafine, i.e., 500 mg once day in patients with dermatophytosis. In our study, 87% patients in 2 weeks group, 92% patients in 4 weeks group, and 80% patients in 6 weeks group achieved clinical cure. These results were in accordance with previous results reported by various authors. Cole et al. in their study reported 87% clinical cure rate in patients with tinea corporis treated with terbinafine 500 mg/day.[17] Similarly, Hay et al. reported 100% cure rates in patients with tinea pedis treated with terbinafine 250 mg twice daily.[18] Arca et al. in their study in patients with onychomycosis reported 81.3% clinical cure rate and 75% mycological cure rate with terbinafine.[19] In our survey, terbinafine given for 4 weeks was found to be most effective treatment strategy against superficial dermatophytosis.
The mycological cure rate in our survey was 83%, 90%, and 78% in Group A, B, and C, respectively, at the end of follow-up period. These results were in accordance with the previous reports.[17,18,19,20]
In this survey, terbinafine was well tolerated by the patients with only 12% patients reporting the AEs. All the AEs were of mild to moderate intensity and none of the patient discontinued the treatment due to any event. Adherence to the treatment in all the group was excellent.
This survey has certain limitations. Because of the observational and design of the survey, the possibility of selection bias cannot be ruled out. Treatment with other antifungal such as topical agents was not taken into consideration which may have impacted the final outcome. Long-term comparative studies to address the shortcomings of the present study are warranted.
Conclusion
Even though the resistance to terbinafine is not common in clinical practice, its incidence is on the rise. Incomplete cure and relapses are very common with standard terbinafine therapy. Our study indicates that terbinafine in a dose of 500 mg given once daily was efficacious and safe in the treatment of patients with superficial dermatophytosis.
Financial support and sponsorship
Sponsorship and financial support was provided by Glenmark Pharmaceuticals Limited.
Conflicts of interest
There are no conflicts of interest.
What is new?
This study highlights the fact that incidence of terbinafine resistance is on the rise, terbinafine 250 mg/day for 2 weeks is not sufficient for treatment of dermatophytosis and use of higher dose terbinafine, i.e., terbianafine 500 mg for extended periods is associated with good efficacy and safety.
Acknowledgments
We would like to acknowledge the contribution of the doctors all over India who provided data for this survey.
References
- 1.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Beena PM. Comparative study of different microscopic techniques and culture media for the isolation of dermatophytes. Indian J Med Microbiol. 2003;21:21–4. [PubMed] [Google Scholar]
- 3.Newland JG, Abdel-Rahman SM. Update on terbinafine with a focus on dermatophytoses. Clin Cosmet Investig Dermatol. 2009;2:49–63. doi: 10.2147/ccid.s3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan KJ, Wiseman LR, Markham A. Terbinafine. An update of its use in superficial mycoses. Drugs. 1999;58:179–202. doi: 10.2165/00003495-199958010-00018. [DOI] [PubMed] [Google Scholar]
- 5.Majid I, Sheikh G, Kanth F, Hakak R. Relapse after oral terbinafine therapy in dermatophytosis: A clinical and mycological study. Indian J Dermatol. 2016;61:529–33. doi: 10.4103/0019-5154.190120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne CS, Leitner I, Favre B, Ryder NS. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother. 2005;49:2840–4. doi: 10.1128/AAC.49.7.2840-2844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne CS, Leitner I, Hofbauer B, Fielding CA, Favre B, Ryder NS. Biological, biochemical, and molecular characterization of a new clinical Trichophyton rubrum isolate resistant to terbinafine. Antimicrob Agents Chemother. 2006;50:2234–6. doi: 10.1128/AAC.01600-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47:82–6. doi: 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseini-Yeganeh M, McLachlan AJ. Physiologically based pharmacokinetic model for terbinafine in rats and humans. Antimicrob Agents Chemother. 2002;46:2219–28. doi: 10.1128/AAC.46.7.2219-2228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PK Nigam. Antifungal drugs and resistance: Current concepts. Our Dermatol Online. 2015;6:212–221. [Google Scholar]
- 11.Klepser ME, Ernst EJ, Pfaller MA. Update on antifungal resistance. Trends Microbiol. 1997;5:372–5. doi: 10.1016/S0966-842X(97)01108-6. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal A, Sharma RP, Garg AP. An open randomized comparative study to test the efficacy and safety of oral terbinafine pulse as a monotherapy and in combination with topical ciclopirox olamine 8% or topical amorolfine hydrochloride 5% in the treatment of onychomycosis. Indian J Dermatol Venereol Leprol. 2007;73:393–6. doi: 10.4103/0378-6323.37056. [DOI] [PubMed] [Google Scholar]
- 13.Sanglard D. Emerging threats in antifungal-resistant fungal pathogens. Front Med (Lausanne) 2016;3:11. doi: 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum MA, Rice LB. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–17. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolton MJ, Perera V, Pont LG, McLachlan AJ. Terbinafine in combination with other antifungal agents for treatment of resistant or refractory mycoses: Investigating optimal dosing regimens using a physiologically based pharmacokinetic model. Antimicrob Agents Chemother. 2014;58:48–54. doi: 10.1128/AAC.02006-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537–9. [PubMed] [Google Scholar]
- 18.Hay RJ, Logan RA, Moore MK, Midgely G, Clayton YM. A comparative study of terbinafine versus griseofulvin in ‘dry-type’ dermatophyte infections. J Am Acad Dermatol. 1991;24(2 Pt 1):243–6. doi: 10.1016/0190-9622(91)70035-z. [DOI] [PubMed] [Google Scholar]
- 19.Arca E, Tastan HB, Akar A, Kurumlu Z, Gür AR. An open, randomized, comparative study of oral fluconazole, itraconazole and terbinafine therapy in onychomycosis. J Dermatolog Treat. 2002;13:3–9. doi: 10.1080/09546630252775171. [DOI] [PubMed] [Google Scholar]
- 20.Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol. 1994;131:684–6. doi: 10.1111/j.1365-2133.1994.tb04983.x. [DOI] [PubMed] [Google Scholar]


