Abstract
A number of biologics is being used for the treatment of psoriasis. Itolizumab is one such agent which has been approved in India. It is an anti-CD6 monoclonal antibody that acts by binding to scavenger receptor cysteine-rich (SRCR) distal domain 1 of CD6. Itolizumab has been found to be safe, with infusion reactions as the most common adverse effect. However, its advantages and disadvantages over other biologicals and immunosuppressants need to be established. Also, its utility in treating other immune-mediated disorders is being explored.
KEY WORDS: Anti-CD6 monoclonal antibody, itolizumab, psoriasis, treatment
What was known?
Itolizumab is a humanized recombinant IgG1 monoclonal antibody which targets the extracellular SRCR distal domain 1 of CD6.
Its recommended dose is 1.6 mg/kg body weight, once every 2 weeks for 12 weeks, then 1.6 mg/kg once in 4 weeks up to 24 weeks, administered as an intravenous infusion.
It was approved by the DCGI for the treatment of moderate-to-severe chronic plaque psoriasis in 2013.
Question 1: What is itolizumab?
Answer: Itolizumab is a humanized recombinant IgG1 monoclonal antibody that targets the extracellular, scavenger receptor cysteine-rich (SRCR) distal domain 1 of CD6. It is a novel biological agent that has been approved in India for the treatment of psoriasis.[1,2]
Question 2: What is the mechanism of action of itolizumab?
Answer: Itolizumab is an anti-CD6 monoclonal antibody. CD6 is a costimulatory molecule required for optimal T-cell stimulation by the antigen-presenting cells (APCs). This step is crucial in T-cell proliferation to form Th1 and Th17 cells, which play a major role in the pathogenesis of psoriasis.[1] The CD6 pathway contributes to Th1 activation and differentiation of T-cells, promoting a proinflammatory response. Itolizumab, thus, downregulates the gene transcription of proinflammatory cytokines and adhesion molecules. This leads to decreased levels of interferon-γ (IFN-γ), interleukin (IL)-6, and tumor necrosis factor-α (TNF-α), leading to reduction in the T-cell infiltration at the sites of inflammation.[1,3]
Interestingly, these immunomodulatory effects are not produced by inhibiting ligand binding and inducing T-cell depletion. They result from turning receptor binding nonproductive either by inhibiting new receptor formation or by stimulating the loss of existing receptors or by causing a blockage followed by internalization or downregulation of the receptors.[2]
Question 3: Discuss in detail about the CD6 molecule and its role in the pathogenesis of psoriasis?
Answer: CD6 is a 105–130 kDa surface glycoprotein found on mature T-cells, immature B-cells, the B1a subset of B-lymphocytes, and certain regions of the brain. It plays a pivotal role in cell proliferation, adhesion, differentiation, and survival.[1] Its extracellular region is composed of three SRCR domains.[4] The ligand of CD6, known as CD166 or activated leukocyte cell adhesion molecule (ALCAM), is expressed on T- and B-lymphocytes, APCs, thymocytes, spleen, lymph nodes, and skin and neuronal cells. The CD6 – ALCAM interaction facilitates stable adhesion between T-cells and APCs leading to T-cell differentiation, proliferation, and maturation. The binding site for ALCAM is the third, membrane-proximal domain (SRCR3) of CD6 [Figure 1].[1,12] Itolizumab, however, recognizes SRCR1. In vitro experiments have shown that itolizumab does not inhibit ALCAM binding to T-cells and does not cause T-cell depletion. However, it is found to inhibit T-cell proliferation induced in the presence of ALCAM and excess IL-2 and downregulates the phosphorylation of intracellular proteins implicated in the CD6-mediated activation pathways and reduces IFN-γ, IL-6, and TNF-α production.[1,2,4] Itolizumab, thus, downregulates the inflammatory cascade in a unique manner.
Figure 1.
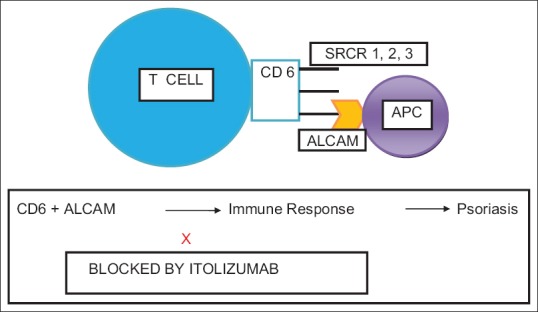
A schematic representation of CD6 molecule and its interaction with activated leukocyte cell adhesion molecule. APC = Antigen-presenting cell, ALCAM = Activated leukocyte cell adhesion molecule, SRCR = Scavenger receptor cysteine-rich domain
Question 4: Discuss the dose and administration of itolizumab?
Answer: The recommended dosage of itolizumab is 1.6 mg/kg body weight, once every 2 weeks for 12 weeks, then 1.6 mg/kg once in 4 weeks up to 24 weeks. It is administered as intravenous infusion made by mixing the required amount in 250 ml of normal saline. It must be infused over a period not <120 min administering approximately 50 ml in the 1st h and the remaining 200 mL in the next hour. It is commercially available as single-use vials containing 25 mg of itolizumab in 5 ml (5 mg/ml) solution.[5]
Question 5: What is the current regulatory status of itolizumab?
Answer: Itolizumab was approved in January 2013 for the treatment of moderate-to-severe chronic plaque psoriasis by the Drugs Controller General of India. It is also approved in Cuba for the treatment of psoriasis.[6]
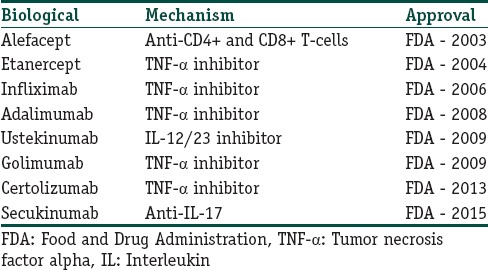
Question 6: What are the other biologicals approved for the treatment of psoriasis?
Answer: Various biological agents approved for the treatment of psoriasis are shown in Table 1.[6]
Table 1.
Other biologicals approved for the treatment of psoriasis
Question 7: Discuss the role of itolizumab in the treatment of psoriasis?
Answer: The results from a study[3] focusing on the immunological and histological effects of itolizumab on psoriasis suggested that itolizumab reduced T-cell proliferation capacity and decreased number of IFN-γ-secreting T-cells. On further analysis of cytokine profile, proinflammatory cytokines – IL-6, TNF and IFN-γ – showed a significant decrease after itolizumab treatment, while IL-10 levels did not change. Histopathological evaluation revealed a significant reduction in the epidermal hyperplasia and parakeratosis with shortening and increased thickness of the papillary ridges, and development of a well-defined granular layer, after eight doses of itolizumab.
Results from phase II study:[7] A phase II study conducted over forty patients who were randomized to eight groups of five patients each showed statistically significant improvement in mean psoriasis area severity index (PASI) score, physician's global assessment score, and psoriasis severity scale. The best therapeutic response was observed in the cohort receiving a dose of 1.6 mg/kg once every 2 weeks and treatment was continued up to 8 weeks.
Results from phase III study:[8] The efficacy of itolizumab was further established in a multicentric, double-blind, placebo-controlled, randomized, parallel-arm, one-way crossover study over 52 weeks including 225 patients with moderate-to-severe psoriasis. The patients were randomized (2:2:1) to two different itolizumab arms (A or B; A = 4-week loading dose of 0.4 mg/kg/week followed by 1.6 mg/kg every 2 weeks; B = 1.6/mg every 2 weeks) or placebo. At week 12, the placebo arm was switched to 1.6 mg/kg itolizumab every 2 weeks. The primary end point was the proportion of patients with at least 75% improvement in PASI score at week 12. At week 12, statistically significant improvement in PASI scores was noted in the treatment arms compared to placebo group (27% vs. 36.4% vs. 2.3%). After crossover of the placebo group to receive itolizumab at 12 weeks, rapid improvement was noted, and the PASI scores were comparable to the other treatment arms by week 20 (46.1% vs. 45.5% vs. 41.9%). The incidence of all adverse events was comparable across arms, and the incidence of infections was not greater than placebo.
Further follow-up of patients of this study revealed three subgroups of patients: good responders achieving long-term remission after controlled drug cessation, good responders achieving long-term remission with maintenance therapy, and late responders achieving PASI ≥75 after itolizumab reinitiation with the induction regimen. The authors concluded that itolizumab monotherapy provided significant benefit in patients with moderate-to-severe psoriasis, with long-term efficacy and safety, including an extended disease-free period.[9] In another case report from India, the patient was followed up for more than 4 years after stopping itolizumab and showed sustained response.[10]
Question 8: What are the adverse effects of itolizumab?
Answer: On the basis of published data, itolizumab seems to have better adverse effects profile than other biological agents. Safety data have been derived from two multicentric clinical trials on patients with psoriasis and another study on rheumatoid arthritis patients.[4,7,8,9] No treatment-related severe adverse effects necessitating discontinuation or reduction of drug dosage were noted in the above studies. The most common adverse effect noted was infusion reactions; the frequency and severity decreased with subsequent infusions. The symptoms included nausea, rash, urticaria, flushing, cough, wheezing, dyspnea, dizziness, and headache.[1]
The incidence of infections noted with itolizumab therapy was less compared to placebo group. Reactivation of tuberculous lymphadenitis was reported in a patient 4 weeks after therapy, and a single case of septic arthritis was noted. Other infections that were observed included upper respiratory tract and urinary tract infections.[1,4]
Regarding hematological effects, Krupashankar et al.[8] observed a small and transient decrease in mean absolute lymphocyte count, which was not found to be associated with any increase in incidence of infections. The white blood cell and absolute lymphocyte count were within normal limits throughout the study by Rodriguez et al.[4] Mild thrombocytosis and anemia were noted in a few patients but were not fully attributed to itolizumab, as both can be otherwise associated with rheumatoid arthritis.
The formation of anti-idiotypic antibodies:[4,8] Immunogenicity is one of the main limitations for the use of therapeutic antibodies. The development of anti-idiotypic antibodies leads to hypersensitivity reactions and the ineffectiveness of the treatment due to clearance of the molecule. It has been suggested to use concomitant methotrexate as a tool to reduce immunogenicity.[11] In the study[8] conducted by Krupashankar et al., antidrug antibodies (ADAs) were noted in 15.75% cases, although no clinical correlation between increased ADA titers and adverse events or decreased efficacy was established. While in another study[4] done on patients of rheumatoid arthritis, anti-idiotypic response was low, transient, and independent of the dose of administered drug.
On comparing itolizumab with other biologic agents, the incidence of infections reported for itolizumab was found to be lower, but at the same time, in terms of efficacy, itolizumab was found to be inferior than infliximab, adalimumab, etanercept, and ustekinumab.[1]
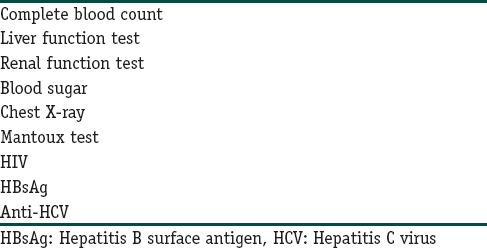
The recommended investigations before starting itolizumab therapy are enumerated in Table 2.
Table 2.
Recommended investigations before starting itolizumab
Question 9: Enumerate other uses of itolizumab?
Answer: Itolizumab is being studied in autoimmune diseases such as rheumatoid arthritis, Sjogren's syndrome, and multiple sclerosis. This is based on the increased expression of ALCAM in salivary gland epithelial cells in Sjogren's syndrome, synovium of rheumatoid arthritis patients, and blood–brain barrier endothelium of multiple sclerosis patients. Further, it is being explored for the treatment of B-cell chronic lymphocytic leukemia and cutaneous T-cell lymphoma.[1]
Question 10: Which aspects of itolizumab need further exploration?
Answer: Itolizumab is a newer biological agent approved in India for the treatment of psoriasis. Its following aspects need further studies:
Its efficacy, adverse effects, and cost-effectiveness in comparison to other immunosuppressive agents are useful in the treatment of psoriasis, particularly methotrexate, cyclosporine, retinoids, as well as other biologicals
Long-term adverse effects are to be watched for
Its safety and efficacy in special patient populations, that is, pregnant females, children and the elderly
The duration of remission achieved after completing the recommended treatment protocol
Its role in other dermatological and nondermatological diseases.
Key points of itolizumab

Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
CD6 pathway plays an important role in genesis of immune response, hence it is contributory to the pathogenesis of psoriasis as well as other immunemediated disorders.
Data from the clinical trials indicate itolizumab to have a good safety profile, with infusion reactions as the most common adverse effect.
Apart from psoriasis, itolizumab is likely to be beneficial in other autoimmune diseases like multiple sclerosis, rheumatoid arthritis and Sjogren's syndrome.
References
- 1.Menon R, David BG. Itolizumab - A humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2015;8:215–22. doi: 10.2147/CCID.S47784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Sharma YK, Deo K, Kothari P. Severe recalcitrant psoriasis treated with itolizumab, a novel anti-CD6 monoclonal antibody. Indian J Dermatol Venereol Leprol. 2016;82:459–61. doi: 10.4103/0378-6323.181466. [DOI] [PubMed] [Google Scholar]
- 3.Aira LE, López-Requena A, Fuentes D, Sánchez L, Pérez T, Urquiza A, et al. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs. 2014;6:783–93. doi: 10.4161/mabs.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez PC, Torres-Moya R, Reyes G, Molinero C, Prada D, Lopez AM, et al. A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis. Results Immunol. 2012;2:204–11. doi: 10.1016/j.rinim.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzumab™ (Itolizumab) Solution for IV Infusion [Prescribing Information] India: Biocon, Inc. 2013. [Last accessed on 2016 Jul 20]. Available from: http://www.biocon.com/docs/prescribing_information/immunotherapy/alzumab_pi.pdf .
- 6.Pai G, Sashidharan N. Biologic armamentarium in psoriasis. Asian J Pharm Clin Res. 2016;9:65–72. [Google Scholar]
- 7.Anand A, Assudani D, Nair P, Krishnamurthy S, Deodhar S, Arumugam M, et al. Safety, efficacy and pharmacokinetics of T1h, a humanized anti-CD6 monoclonal antibody, in moderate to severe chronic plaque psoriasis - Results from a randomized phase II trial. J Immunol. 2010;184:96.13. [Google Scholar]
- 8.Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: Results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71:484–92. doi: 10.1016/j.jaad.2014.01.897. [DOI] [PubMed] [Google Scholar]
- 9.Dogra S, Krupashankar DS, Budamakuntla L, Srinivas CR, Khopkar U, Gupta S, et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled study. J Am Acad Dermatol. 2015;73:331–3.e1. doi: 10.1016/j.jaad.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Budamakuntla L, Madaiah M, Sarvajnamurthy S, Kapanigowda S. Itolizumab provides sustained remission in plaque psoriasis: A 5-year follow-up experience. Clin Exp Dermatol. 2015;40:152–5. doi: 10.1111/ced.12509. [DOI] [PubMed] [Google Scholar]
- 11.Farhangian ME, Feldman SR. Immunogenicity of biologic treatments for psoriasis: Therapeutic consequences and the potential value of concomitant methotrexate. Am J Clin Dermatol. 2015;16:285–94. doi: 10.1007/s40257-015-0131-y. [DOI] [PubMed] [Google Scholar]
- 12.Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol. 2010;162:116–30. doi: 10.1111/j.1365-2249.2010.04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


