Sir,
A 60-year-old Japanese man with a 30-year history of psoriasis vulgaris was referred to our hospital. His previous treatments, including topical corticosteroids, Vitamin D analogs, narrowband ultraviolet B, and cyclosporine, demonstrated poor disease control. At his first visit, he presented with scaly erythematous plaques on his trunk, extremities, and scalp, with considerable itching. At the time, his Psoriasis Area and Severity Index (PASI) score was 37, with an itch Numeric Rating Scale (itch NRS) score was 6 [Figure 1A].
Figure 1.
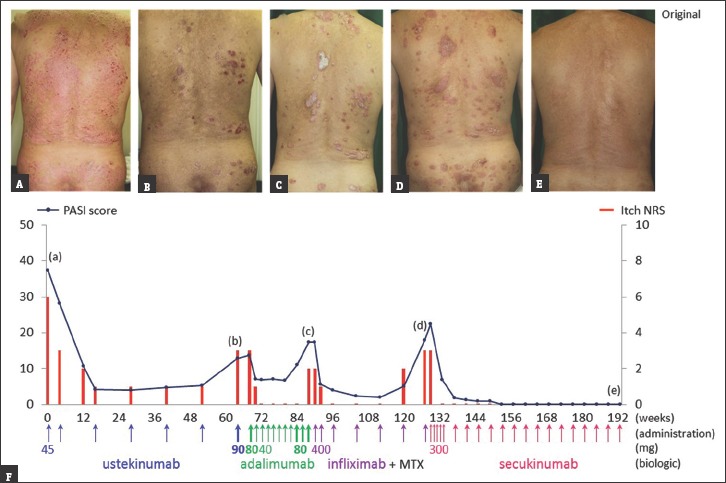
(a-e) Follow-up photographs taken at the corresponding time points indicated in the graph (F). (A) Before biologic treatment; (B-D) after secondary failure of biologics. (F) Changes in the Psoriasis Area and Severity Index score (line graph) and itch Numeric Rating Scale (bar graph)
Treatment was started with a 45 mg ustekinumab subcutaneous injection, which inhibits interleukin (IL)-12/23; it was initially effective against both psoriatic lesions and pruritus. However, clinical responsiveness was lost after 64 weeks [Figure 1B]. His dose was then doubled to 90 mg, but the skin condition did not improve.
Owing to the secondary failure with ustekinumab, it was switched to the tumor necrosis factor (TNF)-α inhibitor, adalimumab. Initially, an 80 mg subcutaneous injection was administered, followed by a 40 mg dose every other week. He showed no significant improvement with this regimen, and subsequently developed shoulder arthritis with psoriatic pruritus after 20 weeks, even after doubling the dose to 80 mg [Figure 1C].
To treat the symptom, a combination of another TNF-α inhibitor, infliximab (5 mg/kg, intravenous), and oral methotrexate (4 mg once weekly) was administered. Thereafter, arthralgia quickly resolved after 4 weeks, but headache and dyspnea developed during the seventh infusion of infliximab. Therefore, the treatment was discontinued [Figure 1D].
Finally, treatment was switched to secukinumab, which selectively targets IL-17A. Before the secukinumab treatment, the patient's PASI score was 18, with an itch NRS score of 3. Following the five initial subcutaneous secukinumab injections, his itch dissipated while the PASI score significantly dropped to 7. After 32 weeks, PASI 100 (clear skin) was achieved. The patient's condition is currently well controlled without arthralgia or pruritus, even after 64 weeks of receiving secukinumab [Figure 1E].
Our patient showed refractory psoriasis, which became unresponsive to several biologics. Antibodies against ustekinumab, adalimumab, and infliximab were reported in 3.8%–6%, 6%–45%, and 5.4%–43.6% of psoriasis patients, respectively.[1] Only 0.4% of patients developed anti- secukinumab antibodies over 52 weeks.[2] Further, secukinumab and ustekinumab resulted in lower numbers of T-cell epitopes and lower T-cell response rates in humans, than did adalimumab and infliximab,[3] indicating that secukinumab has a low immunogenic potential and is well tolerated.
Pruritus affects about 60%–90% psoriasis patients.[4] Recent studies have pointed to the important role of biologic agents in itch intensity reduction in participants suffering from psoriasis.[5] The correlation between PASI score and itch NRS is notable in the present case [Figure 1F]. Itching recurred when psoriasis started to flare because of poor responses to biologics. Therefore, psoriatic itch may be related to abnormal functioning of cytokines in the IL-12/23, TNF-α, and IL-17A pathways in psoriasis.
In conclusion, targeting IL-17A appears to be effective against psoriasis vulgaris with persistent pruritus, in patients with a history of secondary failure of other biologics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Dr. Osamu Negi, Dr. Utako Kimura, and Dr. Kaori Takeuchi of Department of Dermatology, Juntendo University Urayasu Hospital, Chiba, Japan, for their clinical assistance for following up of the patient.
References
- 1.Hsu L, Snodgrass BT, Armstrong AW. Antidrug antibodies in psoriasis: A systematic review. Br J Dermatol. 2014;170:261–73. doi: 10.1111/bjd.12654. [DOI] [PubMed] [Google Scholar]
- 2.Reich K, Blauvelt A, Armstrong A, Langley RG, Fox T, Huang J, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752–8. doi: 10.1111/bjd.14965. [DOI] [PubMed] [Google Scholar]
- 3.Karle A, Spindeldreher S, Kolbinger F. Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. MAbs. 2016;8:536–50. doi: 10.1080/19420862.2015.1136761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szepietowski JC, Reich A. Pruritus in psoriasis: An update. Eur J Pain. 2016;20:41–6. doi: 10.1002/ejp.768. [DOI] [PubMed] [Google Scholar]
- 5.Szepietowski JC, Reich A. Itch in psoriasis management. Curr Probl Dermatol. 2016;50:102–10. doi: 10.1159/000446050. [DOI] [PubMed] [Google Scholar]


