Abstract
Plasmacytoid dendritic cells (pDC) play a crucial role in host anti-viral immune response through the secretion of type I interferon. Interferon alpha (IFNα), a type I IFN, is critical for mounting the initial response to viral pathogens. A consequence of Human Immunodeficiency Virus-1 (HIV) infection is a decrease in both pDC number and function, but prolonged pDC activity has been linked with progression from HIV infection to the development of AIDS. HIV patients in the US routinely use cannabinoid-based therapies to combat the side effects of HIV infection and antiretroviral therapy (ART). However, cannabinoids, including Δ9-tetrahydrocannabinol (THC), are well-characterized immunosuppressants. Here we report that THC suppressed secretion of IFNα by pDC from both healthy and HIV+ donors through a mechanism involving impaired phosphorylation of interferon regulatory factor 7 (IRF-7). These results suggest that THC can suppress pDC function during the early host antiviral by dampening pDC activation.
Introduction
Plasmacytoid dendritic cells (pDC) compose a minor population (0.2–0.5%) of circulating peripheral blood mononuclear cells (PBMCs) that play a crucial role in bridging the innate and adaptive antiviral immune response1–4. Upon activation pDC secrete 1000-fold more type I interferon (IFN) than any other population of PBMCs to stimulate other leukocytes including NK cells5,6, B cells4, and T cells6,7.
Somewhat paradoxically, pDC number and function is suppressed in association with certain types of viral infections including hepatitis C virus (HCV) and HIV8,9. In a rhesus macaque model of HIV infection, using Simian Immunodeficiency Virus (SIV), the number of circulating pDC is reduced during the acute stage of SIV infection as pDC migrate to the gut10. In both HIV and SIV infection, gut lymphoid tissue is a key site of viral replication and, therefore, a target for pDC recruitment. However, pDC may be susceptible to productive HIV infection due to their expression of CD4. Specifically, infection by HIV may perturb pDC function resulting in reduced secretion of IFNα11. This reduced capacity for IFNα secretion during infection would hinder an appropriate host response, as evidenced by protection against HIV-mediated CD4+ T cell depletion in a humanized mouse model upon administration of IFNα12 and lead to an inability to appropriately control the infection13. HIV infected pDC may also directly facilitate the infection of CD4+ T cells during the acute phase of HIV infection14. Furthermore, the loss of pDC in circulation correlated with an increase in HIV viral serum titer such that fewer circulating pDC translated into a deficiency in antiviral response15. Collectively, these results have broader implications for the health of HIV+ patients as loss of pDC function could exacerbate susceptibility to opportunistic viral infection.
In 2015, the Centers for Disease Control and Prevention (CDC) estimated 1.2 million people were infected with HIV in the United States and 36.9 million globally. Anti-retroviral therapy (ART) is the primary therapy for HIV patients in the United States and has been since the mid 1990’s16. While effective, ART therapy can also induce nausea and reduced appetite17. Furthermore, HIV infection, even when properly controlled by ART, is associated with physical wasting18,19 and anxiety20,21, both of which can have deleterious effects on host immune response. The effects of both HIV infection and ART has led to a significant number of HIV patients utilizing cannabinoid-based therapies such as medical marijuana (Cannabis sativa) and dronabinol (marinol)22–24.
Δ9-Tetrahydrocannabinold (THC, aka Dronabinol or Marinol) is the primary psychoactive cannabinoid in marijuana and is a well characterized immune modulator25–27. In mouse models of herpes simplex virus Type II28,29, Listeria monocytogenes29, and influenza virus Type A30,31, THC administration exacerbated disease progression. While THC has been shown to have suppressive effects on the function of many different immune cell populations, THC-mediated suppression of interferon secretion was demonstrated in all the aforementioned models of disease32. Suppression of interferon (Type I and II) secretion by THC is likely a key mechanism by which viral infections are potentiated.
Currently the utilization of cannabinoid-based therapies in HIV infection is controversial. Utilization of cannabinoids has been found to reduce the concentration of circulating anti-retroviral drugs, and these studies indicated little effect of cannabinoids on retroviral therapy efficacy or immune cell function23,33. However, in these cases it is difficult to distinguish between the direct effects of the cannabinoids on leukocyte function and possible confounders. Furthermore, suppression of peripheral IFNα secretion via utilization of medicinal cannabinoids may reduce certain HIV-associated comorbidities, thereby lending potential support for cannabinoid based therapies. The objective of this study was to determine the effects of THC on IFNα production by pDCs utilizing leukocytes from HIV+ patients on ART and healthy donors as controls.
Materials and Methods
Peripheral Blood mononuclear cell (PBMC) isolation and cell identification
Leukocyte packs were purchased from the Gulf Coast Regional Blood Center (Houston, TX). Blood was diluted 1:1 with Hanks Balanced Salt Solution from Gibco™ (Grand Island, NY) and layered on 15 ml Ficoll Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA) in SepMate 50mL conical tubes by StemCell Technologies (Vancouver, BC, Canada). Leukocytes were centrifuged at 1300 × g for 25 min at 4°C. The leukocyte layer was re-suspended in RPMI Media from Gibco™ containing 5% Human AB Serum (Sigma-Aldrich, St. Louis, MO), 1% Penicillin-Streptomycin (Gibco™), and 0.035% β-mercaptoethanol. pDC were identified using mouse anti-human antibodies by Miltenyi Biotec GmgH© (Bergisch Gladbach, Germany) as CD303+ CD123+ cells.
pDC purification by Magnetic Activated Cell Sorting (MACS)
pDC were isolated by negative selection using MACs isolation kits from Miltenyi Biotec© per the manufacturer’s instructions. Briefly, PBMC cell concentrations were determined using a Coulter Cell Counter and the appropriate volume of non-pDC antibody cocktail was incubated with PBMC followed by washing and incubation with magnetic beads. Labeled PBMCs were then passed through a MACS depletion column affixed to a MACS magnet with unstimulated pDC being collected in the flow through. The number of PBMCs in a single leukocyte pack range from 3.0 – 11 × 108 total PBMC with an average of 6 × 108 total PBMC and 0.9 – 1 × 106 pDC per leukocyte pack containing 6 × 108 total PBMC when accounting for isolation efficiency.
Gene Expression Analysis
RNA was isolated using Qiagen© RNeasy™ kits (Germantown, MD) per the manufacturer’s instructions. Briefly, cells were lysed using lysing buffer containing β-mercaptoethanol and stored at −20°C. Lysates were then purified and treated with DNAse from Promega© ST Total RNA Isolation Kit™ (Madison, WI). RNA concentrations were determined by Nanodrop™ (Thermo-Fisher Scientific, Waltham, MA). RT-PCR was performed using High Capacity cDNA RT-PCR kit by Applied Biosystems™ (Foster City, CA). cDNA was frozen at −20°C. Gene analysis was determined by Real Time Quantitative PCR (Qt-PCR) using TaqMan™ probes for CNR1 (Hs00275634_m1) and CNR2 (Hs00275635_m1) by Life Technologies™ (Compendia Bioscience, Ann Arbor, MI) with 18sRNA as a loading control.
Treatment with Cannabinoids or Vehicle Control and Cell Stimulation
THC was supplied by the National Institute of Drug Abuse (NIDA). Purified, unstimulated pDC or PBMCs were treated with either Δ9-Tetrahydrocannabinol (THC), Cannabidiol (CBD), or Vehicle control (VC - 0.026% Ethanol). The appropriate concentration was prepared in Complete-RPMI. The prepared cell suspensions and appropriate treatments were added to flat bottom 96 well tissue culture plates. Cells were then incubated at 37°C and 5% CO2 for 30 min. Following incubation, cells were stimulated with CpG-ODN Type A 2216 (15 µg/ml) (InvivoGen©, San Diego, CA).
IFNα Capture Assay
Secretion of IFNα was determined using the IFNα Capture Assay by Miltenyi Biotec per the manufacturer’s directions. Treated cells were bound with IFNα capture reagent and placed into warm media and incubated under continuous motion for 30 min. Cells were then washed and incubated with IFNα detection antibody. Cells were fixed using CytoFix™ buffer by BD Biosciences (San Jose, CA) and IFNα secreting pDC were quantified by flow cytometry.
Phospho-IRF-7 Detection
Treated PBMCs were washed and pDCs were stained as described. pIRF7 levels were determined using Phosflow™ antibodies and the harsh detergent method by BD Biosciences©. In brief, cells were fixed using BD cytofix buffer for 10 min at 37°C then permeabilized using 1x of perm buffer IV™, stained for 1 hr under continuous motion using FACS buffer and 5% Human AB serum, washed 3X with 0.5x perm buffer, and analyzed by flow cytometry.
IFNΑ2 gene expression by PrimeFlow™
PrimeFlow™ RNA assay (eBiosciences©, San Diego, CA) was performed per manufacturer’s directions. Treated PBMCs were fixed, permeabilized, and bound with IFNΑ2 probe. The mRNA signal was then amplified and detected using Alexa Fluor 647 detection probes (Thermo-Fisher Scientific, Waltham, MA). Relative gene expression was determined via flow cytometry.
Measuring secreted IFNα
IFNα secretion was determined using the LegendPlex™ cytometric Bead array by BioLegend© per the manufacturer’s directions. Detection beads were sonicated and incubated with media from purified pDC. The BD Canto II™ was used for data acquisition and accompanying LegendPlex™ software was used for analysis.
Data Analysis
GraphPad© Prism 5.0™ was used for statistical analysis. Where appropriate, samples were normalized to 0µM THC + CpG, which was considered 100% maximum response for each individual donor and the appropriate statistical test was performed (See Figures 2-5). *=p<0.5,**=p<0.01,***=p<0.001.
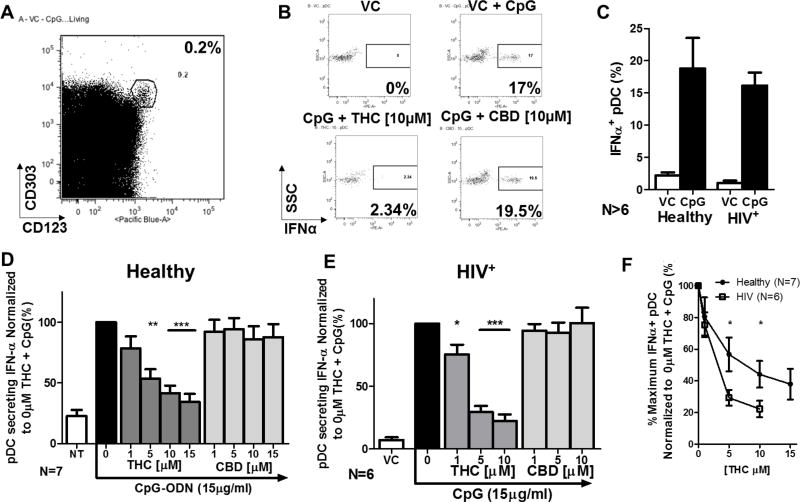
Figure 2. THC, but not CBD, suppresses IFNα secretion by pDC from healthy and HIV+ donors and pDC from HIV+ donors are more sensitive to THC mediated suppression than pDC from healthy donors.
Isolated human PBMCs were treated with either Vehicle control (VC; 0.026% Ethanol) or cannabinoid (THC or CBD) at 1, 5, 10, or 15 µM for 30 min, stimulated with CpG-ODN at 15µg/ml for 5 hrs, and utilized for the IFNα capture assay by Miltenyi Biotec. A) pDC population identified as CD303+/123+ cells. B) Example of IFNα+ pDCs with 10µM of THC and CBD. C) General profile of CpG-ODN induced IFNα in healthy (N=7) and HIV+ (N=6) donors. There was no statistical difference in the number of IFNα+ pDC in background (VC) or stimulated (CpG) when comparing between healthy and HIV+ donors. D) IFNα+ pDC in healthy donors normalized to 0µM THC + CpG group. E) IFNα+ pDC in HIV+ donors normalized to 0µM THC + CpG group. Asterisks indicate statistically significant differences in the number of IFNα+ pDCs compared to 0 THC with CpG group (1-way ANOVA with Dunnett’s Posttest). F) Inhibition curves comparing percent of IFNα+ pDC in healthy and HIV+ donors. Asterisks induce statistically significant (2-Way ANOVA with Bonferroni’s multiple comparison’s posttest).
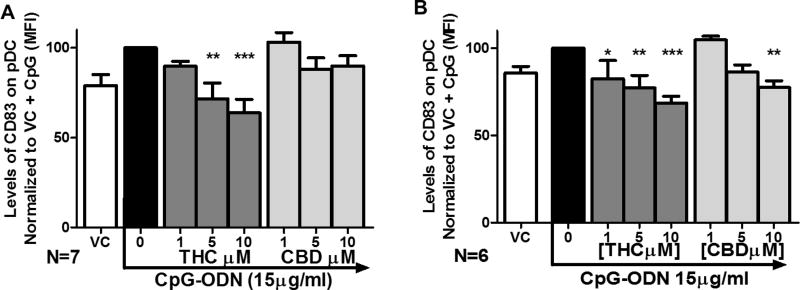
Figure 5. THC suppresses surface expression of CD83 in pDC from both healthy and HIV+ donors.
Healthy and HIV+ PBMCs were treated with THC at 1, 5, 10, 15 µM for 30 min and then stimulated with CpG-ODN for 5 hrs. pDCs were identified as CD303+/123+ cells and CD83+ pDCs were determined by flow cytometric analysis. A) THC concentration dependent suppression of CD83 surface expression in pDC from healthy donors. B) THC concentration dependent suppression of CD83 surface expression in pDC from HIV+ donors Asterisks indicate statistically significant differences in CD83 surface expression compared to 0 THC + CpG (1-Way ANOVA with Dunnett’s posttest).
HIV+ Donor recruitment and Data Management
HIV+ donors voluntarily enrolled in the Mid-Michigan HIV consortium (MMHC) under the IRB-approved protocol (IRB # 11-202) and into the MMHC Registry. Donors were recruited from clinics attended by Dr. Peter Gulick, HIV+ were males between the ages of 31 and 71 with an average age of 54.4 years. Donors received the standard of care and were not asked to change any lifestyle habits to participate. All subject questionnaires and their abstracted medical record data for the MMHC are managed using the Research Electronic Data Capture (REDCap) (Vanderbilt University), which supports 21 CFR Part 11 compliance for clinical research and trials data and HIPAA guidelines.
Results
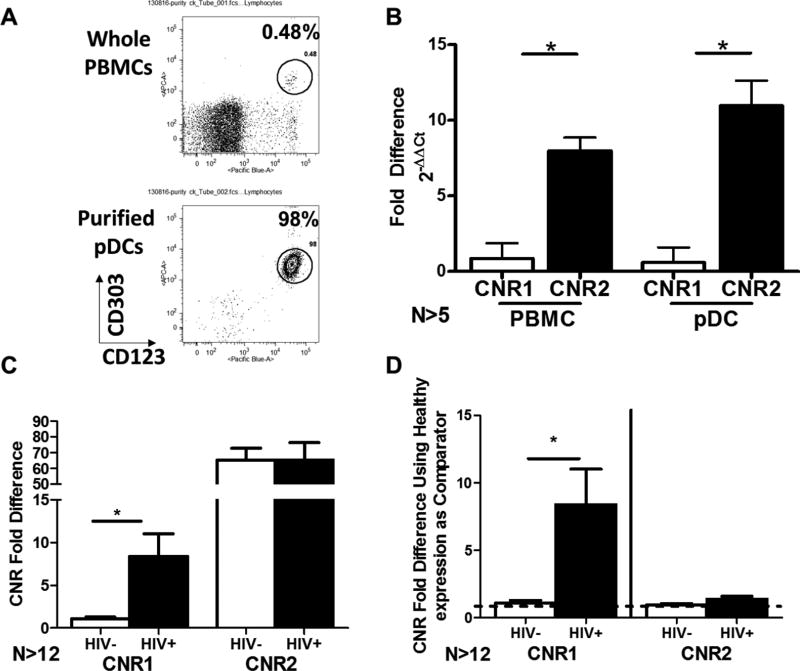
The profile of CNR1 and CNR2 expression in pDC and PBMC from HIV+ donors versus healthy donors
The profile of cannabinoid receptor (CNR1 and CNR2) expression has not previously been characterized in human pDC and was therefore investigated using purified pDC and compared to PBMC from healthy donors (Figure 1A). Purified pDC were found to exhibit a very similar profile of CNR1 and CNR2 expression compared to other PBMC such that CNR2 mRNA levels were more highly expressed than CNR1 (Figure 1B). These studies were extended to also quantify CNR1 and CNR2 levels in HIV+ donors. PBMC from HIV+ donors showed significantly augmented CB1 mRNA levels compared to healthy donors (Figure 1C and 1D). By contrast, CB2 mRNA levels were similar in PBMC from healthy versus HIV+ donors (Figures 1C and 1D). A sufficient amount of blood could not be collected from HIV+ donors to quantify CNR1 and CNR2 mRNA expression levels in purified pDC by RT/Qt-PCR.
Figure 1. pDCs exhibit the same expression pattern of cannabinoid receptors 1 and 2 as other PBMCs and the expression of CNR1, but not CNR2, is elevated in PBMC from HIV+ donors.
CNR1 (N=5) and CNR2 (N=6) gene expression was determined by qPCR from human PBMCs and highly purified (>95%) pDCs. A) Purification of pDCs using MACS isolation by Miltenyi Biotec. B) Fold expression of CNR1 and CNR2 in whole PBMCs and pDCs with CNR1 held as comparator. There was no statistically significant difference in CNR2 or CNR1 expression between isolated pDC and whole PBMC. C) Expression profiles of CNR1 and CNR2 in healthy (N=12) and HIV+ (N=15) PBMCs using CNR1 in healthy donors as comparator. D) Expression differences of CNR1 and CNR2 between healthy and HIV+ PBMC using expression of CNR1 and CNR2 in heathy donors as the respective gene comparator. Asterisks indicate statistically significant differences between healthy and HIV+ groups (Student’s T test).
pDCs from HIV+ donors are more sensitive to THC-mediated suppression of IFNα secretion compared to healthy donors
HIV infection reduces both the number of circulating pDC and the ability for the remaining pDC to secrete IFNα8,15,34. To extend the prior observations, PBMCs from HIV+ patients were treated with CpG-ODN and the number of IFNα secreting pDCs were quantified using the IFNα capture assay. THC is known to suppress interferon secretion in infection and inflammatory conditions32. Here the effects of THC on IFNα secretion were determined in CpG-ODN-induced human primary pDC..
pDC were identified as CD303+ CD123+ cells (Figure 2A) and secretion of IFNα was then quantified by flow cytometry (Figure 2B). The induction of IFNα+ pDC following CpG-ODN treatment from HIV+ donors was comparable to pDC from healthy donors (Figure 2C). Treatment of PBMCs with THC decreased the number of IFNα secreting pDC from both healthy and HIV+ donors (Figure 2D–2E). Conversely, the closely related cannabinoid congener cannabidiol (CBD), which possesses low affinity for both CB1 and CB2, produced no effect on the percentage of IFNα secreting cells in response to CpG-ODN activation (Figure 2D and 2E). Neither THC nor CBD exhibited cytotoxic effects on pDC at any of the concentrations used in these determinations.
HIV infection, and associated disease states, can cause prolonged stimulation of host immune cells and a chronic inflammatory state which can alter immune cell function. To determine possible differences in THC sensitivity of pDC between HIV+ and healthy donors, PBMCs from HIV+ donors were treated with THC and activated with CpG-ODN, as previously described. Treatment with THC significantly suppressed the number of IFNα secreting pDCs from HIV+ donors (Figure 2E), and the degree of suppression was greater than the suppression in pDC from healthy donors (Figure 2F), indicating more pronounced sensitivity to cannabinoid-mediated suppression in pDC from HIV+ donors.
Δ9-Tetrahydrocannabinol (THC) directly suppressed secretion of IFNα in Healthy donors
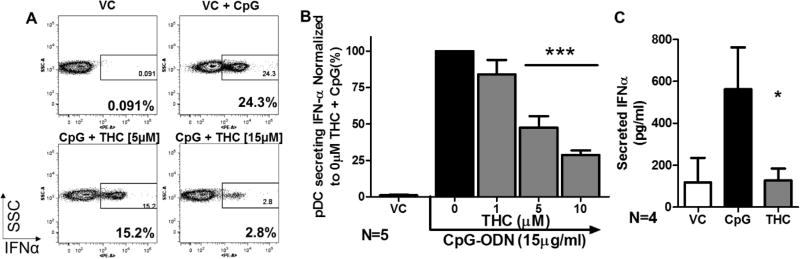
Given that pDC are a minor population within the PBMC (Figure 2A), studies were conducted to determine whether THC acts directly on pDC to suppress IFNα production or indirectly through bystander cell effects. The aforementioned studies were repeated using highly purified pDC (Figure 3A) which showed that treatment with THC decreased the percent of IFNα secreting pDCs in a manner comparable to that observed in the PBMC preparation (Figure 3B) indicating THC acts directly on pDC.
Figure 3. THC directly suppresses IFNα secretion in highly purified pDCs.
pDCs were isolated from PBMC via MACS (Mitenyi Biotec©). Highly purified pDCS (>95% purity) were then treated with 1, 5, 10, or 15µM THC for 30 min followed by stimulation with CpG-ODN for 5 hrs. A) FACS scatter plot of CpG-ODN induced IFNα and concentration dependent suppression by THC. B) IFNα+ pDC normalized to 0µM THC + CpG (N=5). Asterisks indicate significant differences compared to 0 µM THC + CpG (1-Way ANOVA with Dunnett’s Posttest). C) Amount of Secreted IFNα as determined by Legendplex™ secretion kit by BioLegend utilizing 1×105 isolated pDC (N=4) per treatment, treated with VC (0.026% EtOH), VC + CpG, or CpG+THC (15µM). Asterisks indicate statistically significant differences of treatment compared to 0 THC + CpG (1-way ANOVA with Dunnett’s posttest). *=p<0.5, **=p<0.01, ***=p<0.001
To determine if THC also suppressed the quantity of total secreted IFNα, Legendplex™ cytometric bead array was used to quantify the amount of IFNα in the cell-culture supernatants from purified healthy pDC preparations. THC treatment significantly suppressed the amount of IFNα secreted by the highly purified pDC (Figure 3C).
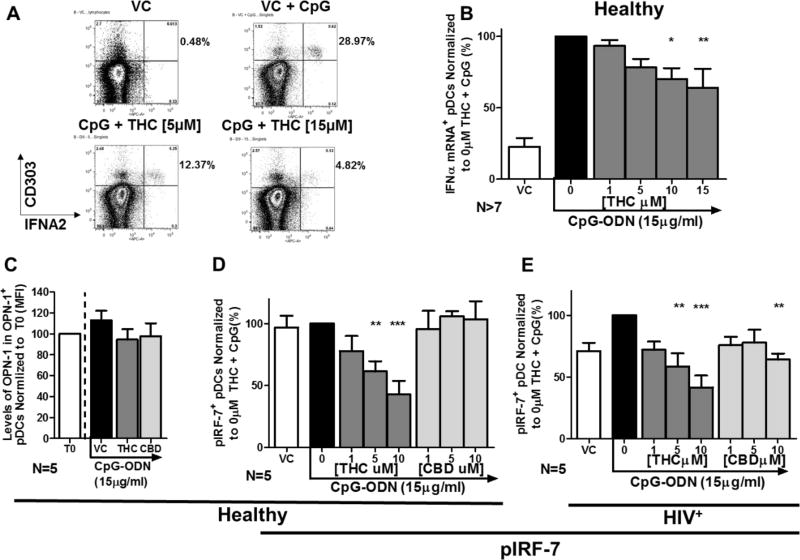
THC directly suppressed IFNα mRNA levels by impairment of Interferon Regulatory Factor 7 (IRF-7) phosphorylation
To determine if the suppression of IFNα by THC was tied to decreased IFNα mRNA levels, PrimeFlow™, a flow cytometry based method that allows quantification of gene specific mRNA levels on a per-cell basis, was employed (Figure 4A). THC suppressed the transcription of IFNΑ2, a member of the IFNα gene cassette, in healthy pDC in a manner that paralleled the decrease of secreted IFNα (Figure 4B).
Figure 4. IFNΑ2 expression and phosphorylation of IRF-7 (pIRF-7) are suppressed by THC in pDC from both healthy and HIV+ donors.
PBMCs were treated with THC at 1, 5, 10, 15 µM for 30 min and then stimulated with CpG-ODN for 5 hrs. IFNΑ2 gene expression was determined using PrimeFlow RNA assay by Affymetrix. pDCs were identified as CD303+/123+ cells. A) FACS scatter plot pDCs undergoing CpG-ODN induced upregulation of IFNΑ2 expression in pDCs and concentration dependent suppression by THC. B) pDC IFNΑ2 gene expression normalized to VC + CpG-ODN across multiple donors (VC & 0 µM: N=9; 1 & 5µM: N=8; 10 & 15µM: N=7). Asterisks indicate statistically significant differences (P<0.05) in IFNΑ2 expressing pDCs compared to 0 THC with CpG group (1-Way ANOVA with Dunnett’s posttest). Levels of Opteopontin (OPN) and pIRF-7+ pDCs were determined by flow cytometric analysis. pDCs were identified as CD303+/123+ cells. C) Osteopontin (OPN) levels in pDCs treated with THC and CBD at 10µM (N=5). D) Percent pIRF-7+ pDC in from healthy donors (N=5). E) Percent pIRF-7+ pDC from HIV+ donors (N=5). Asterisks indicate statistically significant differences in pIRF-7 expressing pDCs compared to the 0 THC + CpG group (1-Way ANOVA with Dunnett’s posttest).
Honda and coworkers demonstrated that phosphorylation of interferon regulatory factor 7 (IRF-7) is a master regulatory event of type I interferon responses35. In the present study, THC treatment suppressed the phosphorylation of IRF-7 in pDC from healthy and HIV+ donors in a concentration-dependent manner. Treatment with CBD had no effect healthy pDC but did suppress pIRF7 in pDC from HIV+ donors (Figure 4D and 4E). IFNα mRNA expression is dependent on nuclear translocation of pIRF-7, which is in turn controlled, at least in part, through osteopontin (OPN)36. Treatment with both THC and CBD treatment had no significant effect on OPN levels in pDC from healthy donors (Figure 4C).
THC suppressed TLR-9-mediated induction of co-stimulatory molecule CD83 on pDC from healthy and HIV+ donors
CD83 is a surface protein on myeloid lineage cells, including pDCs, which serves as a co-stimulatory molecule to drive other immune cell activation37–41. We found that CD83 is expressed early during pDC activation by CpG-ODN (within 6 hrs) and that THC suppressed the number of pDC expressing surface CD83 in both healthy and HIV+ donors (Figure 5A and 5B). Treatment with CBD did not alter CD83 expression by pDC from healthy donors (Figure 5A) or but did suppress CD83 expression in pDC from HIV+ donors (Figure B).
Discussion
Presented here is the first report of cannabinoid receptor expression and modulation by THC of pDC function. pDC expression of the canonical cannabinoid receptors (CNR1 and CNR2) was found to be comparable to other PBMC, with greater expression of CNR2 than CNR1. We also observed that treatment with THC, and not cannabidiol (CBD), caused a concentration-dependent suppression of IFNα secretion by pDC in healthy donors but did have an effect at higher concentrations in pDC from HIV+ donors. Because CBD has much lower affinity for both CB1 and CB2 than THC, suppression of pDC secretion of IFNα by THC suggests the involvement of cannabinoid receptors rather than non-specific mechanisms. Moreover, THC impaired IFNα secretion by purified pDC, ruling out the possibility for a bystander effect by other cell types. The direct suppression by THC of pDC-secreted IFNα is in agreement with previous findings showing pDC modulation by the endogenous cannabinoid, anandamide42.
The mechanism underlying the modulation of immune cell function by cannabinoids has been partially elucidated by our and other labs25,27,43. Here we provide evidence that THC suppresses the phosphorylation of IRF-7, the master regulator of IFNα secretion, in pDC and that this suppression results in the loss of IFNα gene transcription. IRF-7 can be phosphorylated by IRAK44, phosphoinositide 3-kinase (PI3K)45 and IκB kinase-α (IKK-α)46. PI3K signaling in particular has been identified in modulation of the innate immune-cell response and is a putative target for the development of therapeutics47. Activation of the cannabinoid receptors has been shown to directly modulate mTOR-AKT-PI3K signaling in neuronal cell differentiation and survival48,49 and disrupt T cell stimulation by keratinocytes through suppression of the same pathway50. Given the critical role of PI3K in IFNα secretion in pDC and the conservation of cannabinoid receptor-mediated suppression of mTOR-AKT-PI3K signaling across different cell types, the suppression of the mTOR-AKT-PI3K signaling axis is likely a means by which IFNα secretion is suppressed in pDC by THC. However, a comprehensive phosphoproteomic approach will be needed to elucidate the complexity surrounding the cannabinoid-mediated modulation of this signaling pathway.
pDC from HIV+ donors were found to be more sensitive to suppression by THC compared to pDC from healthy donors. This increased cannabinoid sensitivity may be linked to the significantly higher expression of CNR1 mRNA, and therefore CB1 receptors, in PBMC from HIV+ donors compared to healthy donors. The higher expression of CNR1 mRNA might be linked to the chronic inflammatory state experienced by many HIV+ patients as activation of T cells results in the upregulation of CNR1 and not CNR251. HIV patients, even those successfully treated by anti-retroviral therapy, experience a variety of inflammatory conditions (e.g. “Leaky Gut Syndrome”) that can lead to systemic inflammation and higher levels of circulating inflammatory cytokines52,53. It is tempting to speculate that higher levels of inflammatory cytokines lead to increased expression of CNR1, but pro-inflammatory cytokines can induce expression of both CNR1 and CNR254. Furthermore, it is noteworthy that in the current studies CB1 and CB2 expression was quantified solely at the mRNA level (CNR1 and CNR2 respectively). Additional studies will be needed to confirm these findings at the protein level.
pDC can stimulate other immune cells by secretion of IFNα and through the expression of costimulatory molecules (CD83, CD86, CD80, and HLA-DR)55. Expression of CD83 by pDC has been associated with stimulation of both T and B cells4. Here we show that THC can impair CD83 surface expression by pDC within 6 h post activation by CpG-ODN. Similarly, when CD83 signaling is ablated, dendritic cell induction of T cell expansion was significantly reduced38,39. Therefore, our results indicate that cannabinoid-based therapies may diminish pDC activation of the adaptive immune response by suppressing both the secretion of IFNα and the expression of a key costimulatory molecule, CD83. Future studies will reveal whether the suppression of CD83 by THC contributes to a functional deficit in pDC-mediated T cell effector function.
The use of cannabis remains controversial in both healthy and HIV+ populations. The results presented here suggest that THC directly impairs pDC function, which may further compromise HIV patients in responding to opportunistic viral infections. However, the actual implications of these results are mixed. HIV-Associated Neurocognitive Disorders (HAND) affect HIV patients56,57 regardless of ART and these neurocognitive deficits have been linked with a chronic neuroinflammatory state52,58. pDCs have been implicated in neuroinflammatory disease42,59–61 and elevated levels of IFNα in neuronal tissue have been associated with neuroinflammation and neurodegeneration62,63. Though the direct role of pDC on IFNα levels in the CNS is unclear, the suppression of pDC activation may be protective against neuroinflammation associated with prolonged HIV infection. Furthermore, and consistent with the premise of medicinal marijuana use as potentially neuroprotective, cannabinoids have been shown to help maintain the integrity of the blood brain barrier in HIV patients64, potentially reducing the migration of inflammatory cells from the periphery to the brain.
The data generated from HIV+ donors presented in this paper were generated using PBMC provided by male donors exclusively, which comprise 80% of HIV patients in the US. However, over 240,000 women are infected with HIV in the US and modulation of pDC activity is of particular interest for these patients. Women progress more quickly from the establishment of HIV infection to the development of Acquired Immune Deficiency syndrome (AIDS) than men65. Interestingly, pDC from women have an augmented IFN response compared to men when stimulated through TLR-766 and this difference may underlie the accelerated development of AIDS65. Collectively, the presented data imply that the use of cannabinoids may be also beneficial for suppressing the activity of the cells, which play a role in the persistent activation of the immune system of HIV patients that have been successfully treated by ART.
Acknowledgments
This work was supported by the National Institutes of Drug Abuse Grant DA07908 and the National Institutes of Environmental Health Sciences Training Grant T32 ES07255. We express our thanks to Linda Dale for coordinating blood collection from HIV+ donors.
Footnotes
Conflict of Interest
None of the authors report any conflict of interest.
References
- 1.Barchet W, Blasius A, Cella M, Colonna M. Plasmacytoid dendritic cells. Immunologic research. 2005;32(1–3):75–83. doi: 10.1385/IR:32:1-3:075. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu Y-J. Plasmacytoid dendritic cells in immunity. Nature immunology. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 3.Gilliet M, Cao W, Liu Y-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature Reviews Immunology. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 4.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nature Reviews Immunology. 2015;15(8):471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. The Journal of Immunology. 2005;174(2):727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 6.Megjugorac NJ, Young HA, Amrute SB, Olshalsky SL, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. Journal of leukocyte biology. 2004;75(3):504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 7.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. The Journal of Immunology. 2006;176(6):3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 8.Anthony DD, Yonkers NL, Post AB, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. The Journal of Immunology. 2004;172(8):4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 9.Cha L, Berry CM, Nolan D, Castley A, Fernandez S, French MA. Interferon-alpha, immune activation and immune dysfunction in treated HIV infection. Clinical & Translational Immunology. 2014;3(2):e10. doi: 10.1038/cti.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwa S, Kannanganat S, Nigam P, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118(10):2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101(11):4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 12.Lapenta C, Santini SM, Proietti E, et al. Type I Interferon Is a Powerful Inhibitor of in Vivo HIV-1 Infection and Preserves Human CD4+ T Cells from Virus-Induced Depletion in SCID Mice Transplanted with Human Cells. Virology. 1999;263(1):78–88. doi: 10.1006/viro.1999.9869. [DOI] [PubMed] [Google Scholar]
- 13.Feldman S, Stein D, Amrute S, et al. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clinical immunology. 2001;101(2):201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 14.Loré K, Smed-Sörensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. The Journal of experimental medicine. 2005;201(12):2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98(8):2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 16.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 17.Ammassari A, Murri R, Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2001;28(5):445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999) 2002;31(2):230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- 19.Wanke C, Silva M, Knox T, Forrester J, Speigelman D, Gorbach S. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2000;31(3):803–805. doi: 10.1086/314027. [DOI] [PubMed] [Google Scholar]
- 20.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 21.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences. 2006;1069(1):62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 22.Haney M, Gunderson EW, Rabkin J, et al. Dronabinol and marijuana in HIV-positive marijuana smokers: caloric intake, mood, and sleep. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;45(5):545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- 23.Abrams DI, Hilton JF, Leiser RJ, et al. Short-Term Effects of Cannabinoids in Patients with HIV-1 InfectionA Randomized, Placebo-Controlled Clinical Trial. Annals of Internal Medicine. 2003;139(4):258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- 24.Abrams DI. Potential interventions for HIV/AIDS wasting: an overview. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2000;25:S74–S80. doi: 10.1097/00042560-200010001-00012. [DOI] [PubMed] [Google Scholar]
- 25.Klein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Immunology today. 1998;19(8):373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- 26.Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Current pharmaceutical design. 2006;12(24):3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- 27.Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215(8):588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Mishkin E, Cabral G. Delta-9-tetrahydrocannabinol decreases host resistance to herpes simplex virus type 2 vaginal infection in the B6C3F1 mouse. Journal of general virology. 1985;66(12):2539–2549. doi: 10.1099/0022-1317-66-12-2539. [DOI] [PubMed] [Google Scholar]
- 29.Morahan P, Klykken P, Smith S, Harris L, Munson A. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infection and Immunity. 1979;23(3):670–674. doi: 10.1128/iai.23.3.670-674.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchweitz JP, Karmaus PW, Harkema JR, Williams KJ, Kaminski NE. Modulation of airway responses to influenza A/PR/8/34 by Δ9-tetrahydrocannabinol in C57BL/6 mice. Journal of Pharmacology and Experimental Therapeutics. 2007;323(2):675–683. doi: 10.1124/jpet.107.124719. [DOI] [PubMed] [Google Scholar]
- 31.Buchweitz JP, Karmaus PW, Williams KJ, Harkema JR, Kaminski NE. Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Δ9-tetrahydrocannabinol. Journal of leukocyte biology. 2008;83(3):785–796. doi: 10.1189/jlb.0907618. [DOI] [PubMed] [Google Scholar]
- 32.Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? Journal of neuroimmunology. 2005;166(1):3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Kosel BW, Aweeka FT, Benowitz NL, et al. The effects of cannabinoids on the pharmacokinetics of indinavir and nelfinavir. Aids. 2002;16(4):543–550. doi: 10.1097/00002030-200203080-00005. [DOI] [PubMed] [Google Scholar]
- 34.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. The Journal of Immunology. 2007;178(11):6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 35.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nature immunology. 2006;7(5):498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto Y, Tedder TF. CD83: a regulatory molecule of the immune system with great potential for therapeutic application. Journal of medical and dental sciences. 2006;53(2):85. [PubMed] [Google Scholar]
- 38.Hirano N, Butler MO, Xia Z, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107(4):1528–1536. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinho MP, Migliori IK, Flatow EA, Barbuto JAM. Dendritic cell membrane CD83 enhances immune responses by boosting intracellular calcium release in T lymphocytes. Journal of leukocyte biology. 2014;95(5):755–762. doi: 10.1189/jlb.0413239. [DOI] [PubMed] [Google Scholar]
- 40.Zhou L-J, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. The Journal of Immunology. 1995;154(8):3821–3835. [PubMed] [Google Scholar]
- 41.Lechmann M, Berchtold S, Steinkasserer A, Hauber J. CD83 on dendritic cells: more than just a marker for maturation. Trends in immunology. 2002;23(6):273–275. doi: 10.1016/s1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 42.Chiurchiu V, Cencioni MT, Bisicchia E, et al. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Annals of neurology. 2013;73(5):626–636. doi: 10.1002/ana.23875. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan BLF, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and-independent mechanisms of action in leukocytes. Journal of Pharmacology and Experimental Therapeutics. 2003;306(3):1077–1085. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- 44.Honda K, Yanai H, Mizutani T, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(43):15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guiducci C, Ghirelli C, Marloie-Provost M-A, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. The Journal of experimental medicine. 2008;205(2):315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshino K, Sugiyama T, Matsumoto M, et al. IκB kinase-α is critical for interferon-α production induced by Toll-like receptors 7 and 9. Nature. 2006;440(7086):949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 47.Weichhart T, Säemann M. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Annals of the rheumatic diseases. 2008;67(Suppl 3):iii70–iii74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- 48.Gomez O, Sanchez-Rodriguez A, Le M, Sanchez-Caro C, Molina-Holgado F, Molina-Holgado E. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. British journal of pharmacology. 2011;163(7):1520–1532. doi: 10.1111/j.1476-5381.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molina-Holgado E, Vela JM, Arévalo-Martín A, et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. The Journal of Neuroscience. 2002;22(22):9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiurchiù V, Rapino C, Talamonti E, et al. Anandamide Suppresses Proinflammatory T Cell Responses In Vitro through Type-1 Cannabinoid Receptor–Mediated mTOR Inhibition in Human Keratinocytes. The Journal of Immunology. 2016;197(9):3545–3553. doi: 10.4049/jimmunol.1500546. [DOI] [PubMed] [Google Scholar]
- 51.Börner C, Bedini A, Höllt V, Kraus J. Analysis of promoter regions regulating basal and interleukin-4-inducible expression of the human CB1 receptor gene in T lymphocytes. Molecular pharmacology. 2008;73(3):1013–1019. doi: 10.1124/mol.107.042945. [DOI] [PubMed] [Google Scholar]
- 52.Ipp H, Zemlin A. The paradox of the immune response in HIV infection: when inflammation becomes harmful. Clinica chimica acta. 2013;416:96–99. doi: 10.1016/j.cca.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Current opinion in neurology. 2009;22(3):315. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jean-Gilles L, Braitch M, Latif ML, et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiologica. 2015;214(1):63–74. doi: 10.1111/apha.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. European journal of immunology. 2001;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 56.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rumbaugh JA, Tyor W. HIV-associated neurocognitive disorders Five new things. Neurology: Clinical Practice. 2015;5(3):224–231. doi: 10.1212/CPJ.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Current opinion in neurology. 2011;24(3):275. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longhini AL, von Glehn F, Brandão CO, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. Journal of neuroinflammation. 2011;8(1):2. doi: 10.1186/1742-2094-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMahon EJ, Bailey SL, Miller SD. CNS dendritic cells: critical participants in CNS inflammation? Neurochemistry international. 2006;49(2):195–203. doi: 10.1016/j.neuint.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Pashenkov M, Huang Y-M, Kostulas V, Haglund M, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(3):480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 62.Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-α causes neuronal dysfunction in encephalitis. The Journal of Neuroscience. 2009;29(12):3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-α in the brain. Aids. 2007;21(16):2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- 64.Lu T-S, Avraham HK, Seng S, et al. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. The Journal of Immunology. 2008;181(9):6406–6416. doi: 10.4049/jimmunol.181.9.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor–mediated response of plasmacytoid dendritic cells to HIV-1. Nature medicine. 2009;15(8):955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-α production in females. The Journal of Immunology. 2006;177(4):2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]