Abstract
Deregulated monoubiquitination of histone H2B (H2Bub1), mainly catalyzed by E3 ubiquitin-protein ligase RNF20/RNF40 complex, may play an important role in cancer. Here we investigate potential roles of H2Bub1 and the underlying mechanisms through which it contributes to cancer development and progression in lung adenocarcinoma. We show that downregulation of H2Bub1 through RNF20 knockdown dramatically decreases H3K79 and H3K4 trimethylation in both normal and malignant lung epithelial cell lines. Concurrently, global transcriptional profiling analysis reveals that multiple tumor-associated genes such as CCND3, E2F1/2, HOXA1, Bcl2 modifying factor (BMF), Met, and Myc; and signaling pathways of cellular dedifferentiation, proliferation, adhesion, survival including p53, cadherin, Myc, and anti-apoptotic pathways are differentially expressed or significantly altered in these lung epithelial cells upon downregulation of H2Bub1. Moreover, RNF20 knockdown dramatically suppresses terminal squamous differentiation of cultured bronchial epithelial cells, and significantly enhances proliferation, migration, invasion, and cisplatin resistance of lung cancer cells. Furthermore, immunohistochemistry analysis shows that H2Bub1 is extremely low or undetectable in > 70% of 170 lung adenocarcinoma samples. Notably, statistical analysis demonstrates that loss of H2Bub1 is significantly correlated with poor differentiation in lung adenocarcinoma (P = 0.0134). In addition, patients with H2Bub1-negative cancers had a trend towards shorter survival compared with patients with H2Bub1-positive cancers. Taken together, our findings suggest that loss of H2Bub1 may enhance malignancy and promote disease progression in lung adenocarcinoma probably through modulating multiple cancer signaling pathways.
Keywords: Lung adenocarcinoma, H2B monoubiquitination (H2Bub1), RNF20, global transcriptional profiling, differentiation, malignancy
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 About 85% of lung cancers are non-small cell lung cancer (NSCLC), while lung adenocarcinoma roughly accounts for about 50% of NSCLC.1 Although there have been advances in targeted therapies and immunotherapy, the 5-year survival rate for NSCLC patients remains only 15%.2 Therefore, novel therapeutic approaches are urgently needed. Recent studies have demonstrated that both genetic and epigenetic alterations play critical roles in disease progression and treatment resistance of cancer.3 Histone modification is emerging as an important regulator of gene expression in various human cancers.4 The regulation of monoubiquitination of the histone H2B at lysine 120 (H2Bub1) is complex, with at least four E3 ubiquitin ligases (E3s),4 and six deubiquitinases such as ubiquitin-specific protease 3 (USP3),5 USP7,6 USP22,7 and USP44 playing some role in H2B ubiquitination.8 Among these E3s, the ring finger protein complex RNF20/RNF40 is generally accepted as the main E3 for H2Bub1, while USP22 is the most important and intensively studied deubiquitinases for H2Bub1.4 H2Bub1 may functionally collaborate with histone methylation in regulation of gene expression. Several studies have indicated that H2Bub1 is a prerequisite for normal levels of methylation of histone H3 residues K4 (H3K4) and K79 (H3K79).9–11 However, other studies argue that H2Bub1 may be not a prerequisite for H3K4 methylation in cells like primary tumor cells where H2Bub1 is lost, but H3K4 trimethylation (H3K4me3) is retained.12,13 H2Bub1 participates in both transcriptional activation and repression, in controlling mRNA processing,14 and in DNA damage and repair.15 The role of H2Bub1 in DNA repair and transcriptional elongation are likely through cooperation with the FACT histone chaperone complex.16,17
Functionally, H2Bub1 and RNF20 may be tumor suppressors in cancer. Frequent hypermethylation of the promoter of RNF20 gene was found in breast cancers, and RNF20 depletion could enhance cell migration, and elicited transformation and tumorigenesis in mammary epithelial cells.13 Similarly, a recent study also demonstrated that heterozygous RNF20 knock-out (RNF20+/−) mice were predisposed to inflammation-associated colorectal cancer,18 and downregulation of RNF20/RNF40 and H2Bub1 were discovered in samples of human colorectal tumors.18 Moreover, loss of H2Bub1 was reported to correlate with a malignant phenotype. Prenzel et al. demonstrated that H2Bub1 was highly expressed in normal mammary epithelium and benign mammary tumors while undetectable in the overwhelming majority of malignant breast cancers.20 RNF20 and H2Bub1 were also decreased in human cancers including metastatic prostate cancer,19 seminoma,21 lung,22 colon,22 and gastric cancers.23 Furthermore, H2Bub1 pathway plays a critical role in proper differentiation of stem cells through regulating gene expression, and H2Bub1 levels are low in both embryonic and adult stem cells but increase during differentiation.8,26
Conversely, overexpression of USP22 is found in diverse human cancers including breast and colon cancers, and correlates with poor prognosis of cancer patients.24,25 Interestingly, Glinsky et al. reported that an 11-gene signature including USP22 mRNA is associated with aggressive growth, metastasis, and therapy resistance in a number of human cancers, including lung cancer.27 One study showed that knockdown of USP22 decreased cell proliferation in several cancer cell lines, suggesting that USP22 might be a novel therapeutic target in cancer.7
H2Bub1 is not well studied in lung adenocarcinoma. In addition, the precise mechanisms by which H2Bub1 affects cancer progression are largely unclear. In this study, we have for the first time demonstrated that loss of H2Bub1 is significantly associated with enhanced malignancy and poor differentiaton of lung adenocarcinoma. We have further identified critical downstream molecules and signaling pathways such as p53, cadherin, Myc, and anti-apoptotic signaling pathways that are altered with downregulation of H2Bub1 in lung epithelial cell lines, suggesting a possible role for these signaling pathways in H2Bub1-mediated regulation of lung adenocarcinoma growth and metastasis.
Material and Methods
Patients selection and clinical data collection
This study was reviewed and approved by the Institutional Review Board (IRB) of City of Hope National Medical Center. A total of 170 patients with lung adenocarcinoma who underwent surgical resection for curative intent between 2002 and 2014 without preoperative chemotherapy or radiation therapy were included. Tissue microarrays were created using cancer and matched normal tissues. The details of their demographic and survival data are presented in Supporting Information Table S1.
Immunohistochemistry analysis
Mouse monoclonal anti-H2Bub1 antibody clone 7B4 (MABE453,) was from EMD Millipore (Merck, KGaA, Darmstadt, Germany). The mouse monoclonal antibodies against Met, Myc, BMF, E2F2, p21, p53, ALDH1A1, total and cleaved caspase-3/PARP (Asp214), RNF20, Cyclin D3, H2B, trimethylated H3K4/K79 were purchased from Cell Signaling Technology (Beverly, CA USA) and Abcam (Cambridge, MA). IHC was performed as described previously.28 Expression levels of H2Bub1 in all clinical samples were scored based on the percentage of positively stained cells as described previously.28 H2Bub1 IHC staining was graded as negative (0), if < 1% cells displayed positive nuclear staining. Those cases with > 1% of tumor cells showing nuclear staining for H2Bub1 were classified as positive, and graded as 1+ (1– 5%), 2+ (5–24%), and 3+ (> 25% of the cells stained positive).
Analyzing RNF20 mRNA expression in lung adenocarcinoma using publicly available TCGA gene exphession data
RNF20 mRNA expression data for 517 lung adenocarcinoma (LUAD) and 59 normal lung tissues were accessed from The Cancer Genome Atlas (TCGA) public data portal (https://tcga-data.nci.nih.gov/tcga/). For data analysis, normalized RNA-Seq data (version 2, level 3) was used as gene expression values and the median was used to classify samples into high and low expression groups.
Cell culture, proliferation, and differentiation assays
Human lung cancer cell lines: A549, H1299, and H460 cells were purchased from American Type Culture Collection (ATCC). All cancer cells were cultured in DMEM or RPMI medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. For proliferation assessment, cells were seeded in 6-well plates in 3 replicates at densities of 2.0 × 105 cells per well, and were monitored at 72 hours using the trypan blue exclusion-based viable cell counting method by Vi-CELL® XR Cell Viability Analyzer (Beckman Coulter). BEAS-2B human bronchial epithelial cell was purchased from ATCC (CRL-9609), which retains the ability to undergo squamous differentiation. BEAS-2B cell was expanded in growth factor-supplemented medium (BEGM, Lonza) and differentiated in differentiation medium BEDM with 50 nM retinoic acid according to a previously published method.29 For induced differentiation to squamous cell, BEAS-2B cell (passage 3–5) was cultured in BEDM at a density of 5000 cells per well of a six-well tissue culture dish over 9 days in culture.29 Medium was replaced every 3 days.
siRNA transfection and RNA-seq data analysis
Two independent RNF20 siRNAs: Cat No.10620318 (5′-GGCAUCUCAGGAGGAUGCCAAUGAA-3′) and Cat No. 10620319 (5′-UUCAUUGGCAUCCUCCUGAGAUGCC-3′) purchased from Thermo Fisher Scientific Corporation (Carlsbad, CA) were applied to silence RNF20 expression using the protocol we described previously.30 At 72h post-transfection, total RNA was extracted for RNA-seq analysis. Transcriptome libraries and RNA sequencing analysis were performed according to the Illumina Genome Analyzer II (Illumina, San Diego, CA, USA) manufacturer’s instruction with minor modifications as we described previously.31 The matched high-quality reads were aligned to Human Refseq mRNA (NCBI). The mean log2 fold change [siRNF20/control siRNA] of each gene was calculated across all cell lines. The false discovery rate (FDR) of each gene was determined according to the previously reported method.32 Gene set enrichment analysis (GSEA) was used to examine pathways significantly modulated by the knockdown of RNF20.33 RNA-seq analysis showed a very similar global gene expression profiles in cells transfected with two independent siRNAs. Therefore, a RNF20 siRNA pool including equal amount of two siRNAs was used in the following experiments.
Quantitative real-time PCR
Total RNAs extraction and cDNA were generated as we previously described.30 The primer for involucrin (IVL) a squamous differentiation marker used were (5′-AAGCCTCTGCCTCAGCCTTACT-3′ (forward primer) and 5′-GAGGAGGAACAGTCTTGAGGAGC-3′ (reverse primer). Other primer sequences are available upon request. Beta-Actin gene was used as internal control for mRNA expression. Data were presented as the relative quantity of targets, normalized with respect to internal control, or relative to a calibrator control sample.
Migration and matrigel invasion assays
Test cells (5 × 104) in serum-free medium were plated in the Matrigel-coated inserts of a 24-well pate (8-mm pores; BD) and medium with 10% FBS was added into the bottom in accordance with the manufacturer’s protocol. After 12 hours, cells that migrated through and attached to the bottom membrane were fixed and stained with the Diff-Quick kit (Fisher Scientific). Invading cells in three different fields were counted under a light microscope with 100 X magnification after 24 h culture. Three replicate experiments were performed.
Cisplatin treatment and apoptosis assay
A549 and H460 cells were first transfected with either control siRNA (left panel) or RNF20 siRNA (right panel). At 24 h post-transfection, these cells were further treated for 72 h with 5–20 μM Cisplatin, and cisplatin-induced apoptosis was measured by flow cytometry analysis of Alexa Fluor 488-labeled Annexin-V and propidium iodide staining, according to the manufactory’s protocol. Total proteins were also extracted from these cells for Western blot analysis.
Statistical analysis
All experiments were performed in duplicates or triplicates and repeated at least two times in each experiment. Two group comparisons were analyzed for variation and significance using a Student’s t-test or Pearson χ2 test. All data shown are mean ± standard deviation (SD). Kaplan-Meier analysis and Log-rank test were used to compare patient overall survival and recurrence free survival between subgroups. Statistical significance was set at P < 0.05.
Results
Downregulation of RNF20 expression in lung adenocarcinoma tissues and its association with H2Bub1, H3K4, and H3K79 methylation in lung epithelial cell lines
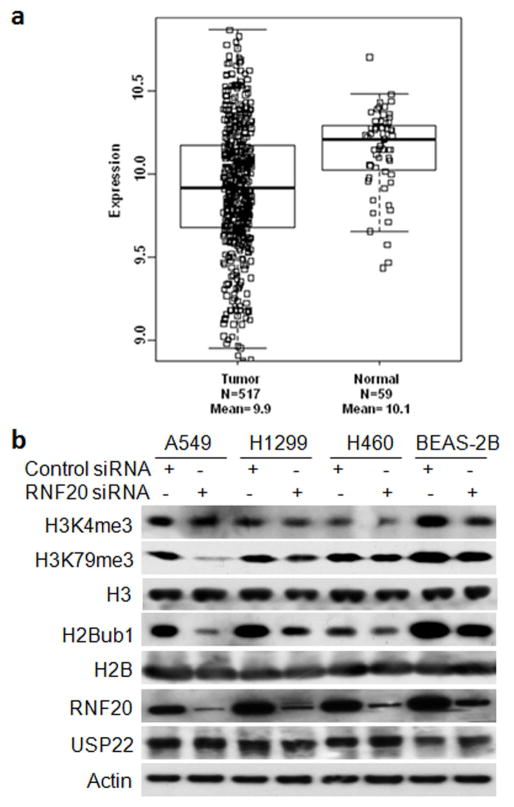
In the present study, we first mined lung cancer gene expression data from TCGA (https://tcga-data.nci.nih.gov/tcga/) to examine expression of RNF20 mRNA in lung adenocarcinoma tissues. As shown in Figure 1a, RNF20 mRNA level was substantially lower in at least 25% of lung adenocarcinoma samples than normal lung tissues. Statistical analysis revealed that in comparison to normal lung tissues, RNF20 mRNA was significantly reduced in lung adenocarcinoma tissues (ratio to normal = 0.86, P = 9.12 × 10−9).
Figure 1.
Downregulation of RNF20 mRNA in lung adenocarcinoma tissues and its association with histone modification in lung epithelial cell lines. (a) Box-and-Whisker plot of RNF20 mRNA level in lung adenocarcinoma (LUAD). Box represents first and third quartiles, thick band is median value, and bars extend to ± the interquartile range divided by the square root of the number of samples were applied to describe RNF20 gene expression values. Compared to normal lung tissues (Gr2, n=59), RNA20 mRNA was significantly decreased in LUAD (Gr1, n = 517) (fold change/FC = 0.86, and P= 9.12E-12). (b) Western blot analysis of RNF20, H2Bub1, H3K4/79-me3 and USP22 in lung epithelial cells at 72h post-transfection of control or RNF20 siRNA. Beta-actin served as the loading control.
Whether H2Bub1 regulates H3K4 and H3K79 methylation is controversial.9–13 We analyzed H3K4 and H3K79 methylation with H2Bub1 modulation in both normal (BEAS-2B) and malignant lung epithelial cancer cell lines (A549, H1299, and H460). Compared with normal lung epithelial BEAS-2B cells, RNF20 protein is slightly lower, while H2Bub1 and USP22 levels are markedly altered in cancer cells (Fig. 1b). Consistent with data of a previous study,34 we found that knockdown of RNF20 resulted in a moderate decrease of H2Bub1 in both normal and malignant lung epithelial cells (Fig. 1b). In agreement with another study,9 we also identified that RNF20 depletion and consequent reduction of H2Bub1 substantially decreased trimethylation of both H3K79 and H3K4 levels (Fig. 1b).
Changes in gene expression profile in the lung cancer cell lines upon downregulation of H2Bub1
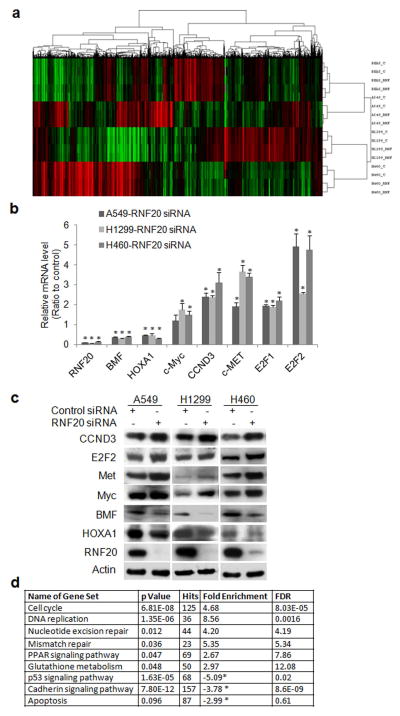
The effect of H2Bub1 on global gene transcription is not known in lung cancer. RNA sequencing (RNA-seq) analysis was utilized to characterize global gene expression change upon downregulation of H2Bub1 via RNAi-mediated knockdown of RNF20 in both normal and malignant epithelial cell lines. Unsupervised hierarchical clustering analysis of RNA-seq data depicts the changes in global gene expression profile in these lung epithelial cells upon downregulation of H2Bub1 (Fig. 2a). RNF20 knockdown significantly modulated about 8–10% of transcribed genes (1000–1500 genes) in each of these cells; with near half of them being down-regulated (Fig. 2a, red to green color) and the other half being up-regulated (Fig. 2a, green to red color), suggesting that H2Bub1 can both promote and suppress gene transcription. The original RNA-seq data is saved on NCBI GEO website with accession number GSE88943. Supporting Information Table S2 lists the differentially expressed genes in at least two of the lung cancer cell lines. Remarkably, knockdown of RNF20 had pronounced effects on the expression of important cancer-associated genes including CCND3 (Cyclin D3), E2F1/2, HOXA1, Bcl2 modifying factor (BMF), Met (c-Met), and Myc (c-Myc). Differential gene expression of these selected genes was validated by both qRT–PCR (Fig. 2b) and Western blot analysis Fig. 2c). A previous study showed that Myc was regulated by H2Bub1.13 In the study, we also observed that both mRNA and protein of Myc was upregulated in three cancer cells. Using GSEA, we identified multiple gene sets from the Kyoto Encyclopedia of Genes and Genomes (KEGG) that were significantly enriched in lung cancer cells upon downregulation of H2Bub1 (Fig. 2d) (P < 0.05). The top enriched gene sets in all three lung cancer cells were gene sets involved in DNA replication, DNA repair, cellular proliferation, and regulation of apoptosis; while p53 signaling pathway was significantly down-regulated in p53 wild-type A549 and H460 cells. (Fig. 2d), indicating that these pathways are potentially involved in H2Bub1-mediated lung cancer progression.
Figure 2.
Changes of gene expression profile and gene set in A549, H1299, H460 cells upon downregulation of H2Bub1. (a) Unsupervised hierarchical clustering analysis of global expression profiles in lung epithelial cells upon RNF20 knockdown. The two top rows represent two independent siRNA repeats, RNF is for RNF20 siRNA, and C is for control siRNA. (b) qRT–PCR and (c) Western blot analysis for selected genes. The level of each gene in cancer cell transfected with RNF20 siRNA is the average ratio of triplicate samples, and is presented as the ratio to control sample transfected with scramble siRNA (* P < 0.05, compared with control). (d). Selected gene sets that were enriched upon RNF20 knockdown in these lung cancer cells.
H2Bub1 downregulation promotes proliferation and inhibits in vitro differentiation of normal lung epithelial cell line
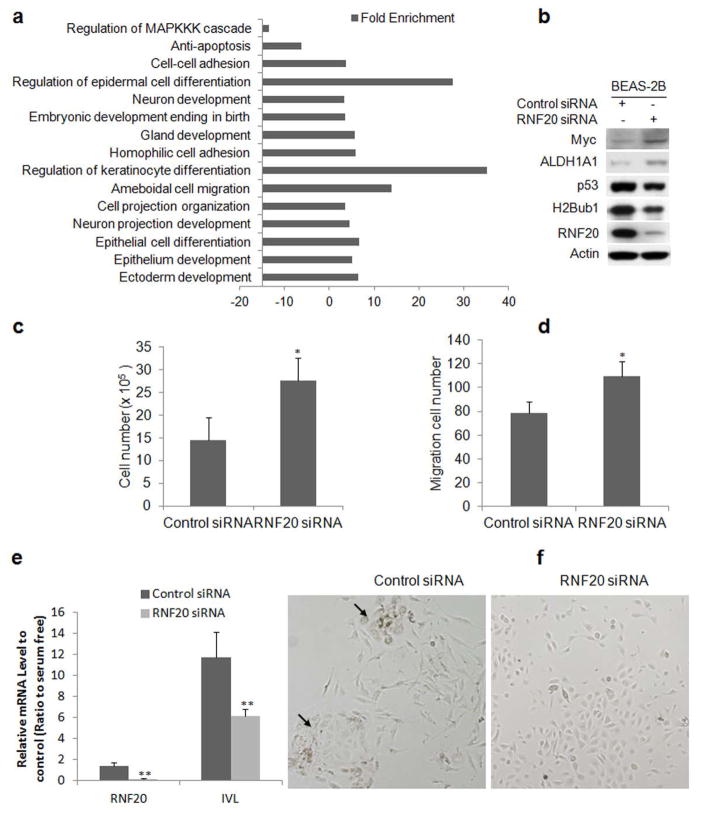
GSEA analysis revealed that multiple molecules and gene sets including these involved in proliferation, dedifferentiation, development, and apoptosis were also enriched in BEAS-2B cells upon downregulation H2Bub1 by knockdown of RNF20 (Fig. 3a; Supporting Information Table S3). Notably, among these modulated gene sets, Myc signaling pathway (Supporting Information Fig. S1a) was upregulated, while p53 signaling pathway (Supporting Information Fig. S1b) was downregulated in BEAS-2B cells upon RNF20 knockdown. Consistent with others’ findings, we also identified that knockdown of RNF20 led to a moderate upregulation of Myc, ALDH1A1, and downregulation of p53 in BEAS-2B cells (Fig. 3b) and significantly promoted both proliferation (Fig. 3c) and migration (Fig. 3d) of BEAS-2B cells.
Figure 3.
Knockdown of RNF20 enhances proliferation, migration and inhibits retinoic acid-induced squamous differentiation of BEAS-2B cells. (a) Enriched gene sets in BEAS-2B cells with silenced RNF20 and decreased H2Bub1 (P < 0.05, FDR < 5%). (b) Western blot for RNF20 and H2Bub1, p53, Myc, and ALDH1A1 proteins. (c) Enhanced cellular proliferation and (d) migration upon RNF20 knockdown in BEAS-2B cells. After 72 h of siRNA transfection, cell proliferation was measured. At 48 h post-transfection, 5 x 104 cells transfected with either control or RNF20 siRNA were further subjected to transwell migration assay for 12 h, and the number of migrated cells was counted (*P < 0.05, compared to control siRNA). (e) qRT–PCR analysis of IVL, a squamous differentiation marker, mRNA in BEAS-2B cells transfected with control or RNF20 siRNA and cultured in the differentiating medium BEDM, data were presented as ratio to non-differentiating medium control (*P < 0.05, ** P < 0.01, compared with control). (f) Micrographs show morphology changes of BEAS-2B cells after transfection with control siRNA (left panel) or RNF20 siRNA (right panel) and then subcultured in BEDM. Arrows point to “fried egg” morphology of squamous cells in control siRNA-transfected BEAS-2B cells.
RNA-seq and qRT-PCR analysis showed that downregulation of H2Bub1 significantly modulated these genes such as ELF3,35 TP63,36 KRT5,36 and ALDH1A1,37 participating in the dedifferentiation and stemness of lung epithelial cells (Supporting Information Fig. S2). Therefore, we hypothesized that downregulation of H2Bub1 may suppress normal differentiation of lung epithelial cells, leading to tumorigenesis. To prove this, we investigated the impact of H2Bub1 downregulation on the induced differentiation of BEAS-2B cells using IVL as a marker of terminal squamous epithelium differentiation.29,38,39 Tissue culture in the differentiation medium BEDM for 9 days induced a 15-fold increase in IVL mRNA in cells transfected with control siRNA, while cells transfected with RNF20 siRNA exhibited only a 6 fold increase in IVL mRNA (Fig. 3e, P < 0.01). We also observed that cells transfected with control siRNA grown in BEDM have increased size; seem less migratory as they grew in small, tightly associated colonies; and displayed a ‘fried egg’ appearance (Fig. 3f). However, these morphologic changes were less apparent in cells transfected with RNF20 siRNA cultured in the same medium, and most of the cells display normal, compact, migratory, and proliferative morphology (Fig. 3f). Therefore, these data indicated that the induced differentiation of BEAS-2B into squamous epithelium was significantly suppressed upon downregulation of H2Bub1.
H2Bub1 downregulation promotes proliferation, migration, and invasion of lung cancer cells
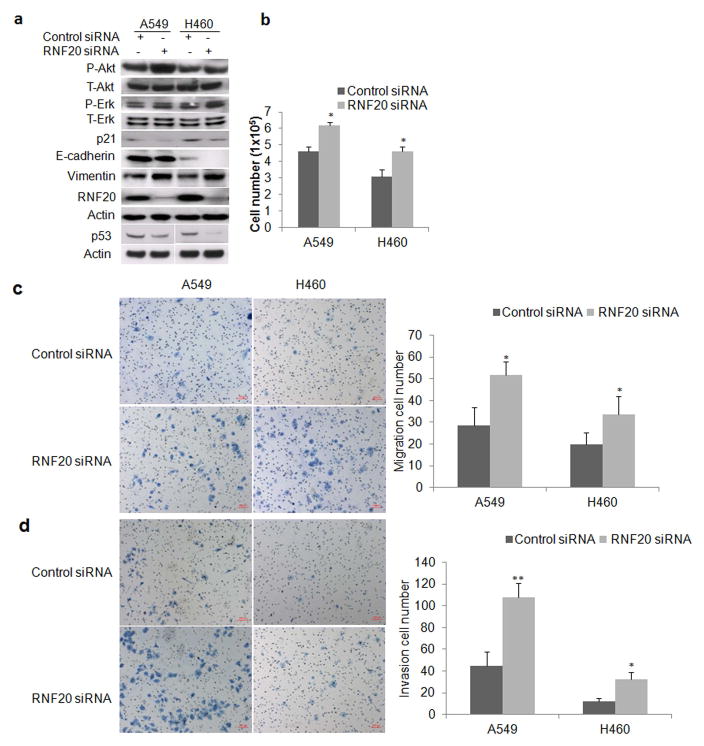
We examined the impact of downregulation of H2Bub2 on activation of MAPK and AKT pathways by Western blot analysis. The results showed a substantially increased phosphorylated AKT and ERK upon knockdown of RNF20 in cancer cells, while increase in phosphorylated AKT and ERK were more evident in H2Bub1-attenuated A549 and H460 respectively (Fig. 4a), indicating that knockdown of RNF20 may enhance activation of AKT and MAPK signaling pathways. In line with the downregulation of p53 signaling pathway we indentified by GSEA, we found that both p53 and p21, a target of p53 as a critical inhibitor of cell cycle progression at G1 and S phase, were monderately downregulated in lung cancer cells upon RNF20 knockdown (Fig. 4a). GSEA analysis also showed that the cadherin signaling pathway was significantly suppressed upon reduction of H2Bub1. Consistent with this finding, Western blot analysis showed that a moderate decrease of E-cadherin and increase of Vimentin in H2Bub1-attenuated A549 and H460 cells (Fig. 4a). Cellular proliferation assay demonstrated that attenuation of H2Bub1 resulted in a significant increase of in vitro cellular proliferation in both A549 and H460 cells at 72h post-transfection of RNF20 siRNA in comparison with control cells transfected with scramble siRNA (Fig. 4b).
Figure 4.
Impact of RNF20 knockdown on in vitro proliferation, migration, and invasion of lung cancer cell lines. (a) Western blot analysis of cell lysates of A549 and H460 cells transfected with either control or RNF20 siRNA and harvested at 72 h post-transfection. Actin served as loading control, total and phosphorylated Erk/Akt (T/P-Erk/Akt), p53, p21 E-Cadherin, and Vimentin were detected. The p53 blots were from separately developed blots for each cell, and the below Actin is its loading control. (b) Histogram of proliferation shows RNF20 knockdown increased the in vitro proliferation of both A549 and H460 cells over 72h (*P < 0.05, compared to control siRNA). (c) Representative photomicrographs of the migration chamber at the times indicated in control or RNF20 siRNA-transfected cells is shown on the left, with quantitative analysis of migrated cells shown on the right (N = 3 replicates per cell type). Error bars show SD (*P < 0.05, compared with control siRNA). (d) Lower surface of Matrigel transwell membranes seeded with cancer cells previously transfected with indicated siRNAs, 24 h after incubation (left), and corresponding quantitative analysis of invading cells (right). Data are shown as mean values graphed for indicated cells on the right (N = 3 replicates per cell type). Error bars show SD (*P < 0.05, **P < 0.01, compared with control siRNA).
Decreased adhesion may enhance cancer cells’ migration and proliferation, leading to invasion and metastasis.40 We herein investigated the effects of H2Bub1 on the in vitro migration and invasion of lung cancer cells. We found that downregulation of H2Bub1 significantly increased the migration potential of A549 and H460 cells in a migration chamber assay (Fig. 4c), and likewise enhanced their invasive potential as observed in the Matrigel invasion assay (Fig. 4d, P < 0.05). Taken together, the above data strongly indicated that loss of H2Bub1 enhanced cellular proliferation, migration, and invasion in both A549 and H460 lung cancer cells probably by modulating multiple signaling pathways including increased ERK and decreased p53 and Cadherin as well as other signaling pathways.
Downregulation of H2Bub1 enhances cisplatin resistance in lung cancer cells
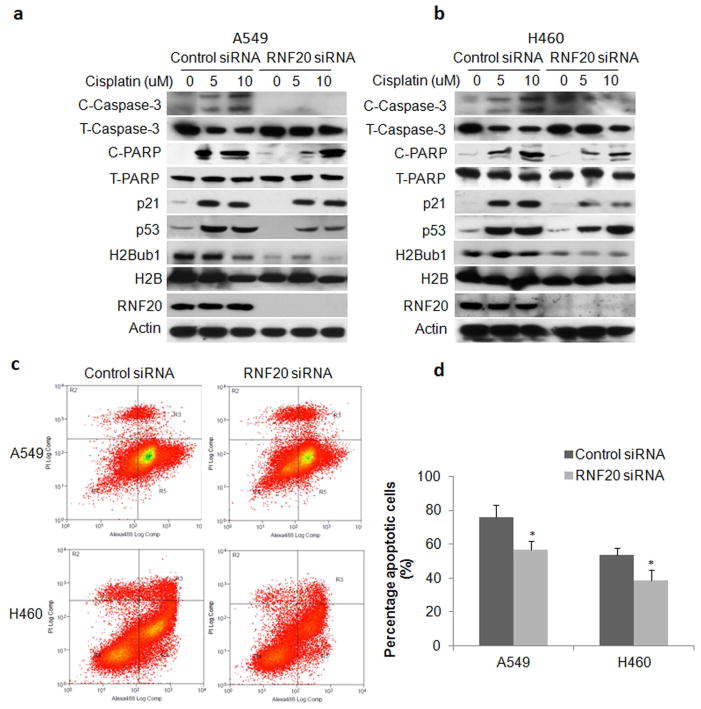
RNA-seq and GSEA analysis showed that attenuation of H2Bub1 significantly altered molecules and pathways involved in regulation of apoptosis such as BMF and p53. Therefore, we further investigated the effect of downregulation of H2Bub1 on cisplatin-induced apoptosis and p53 activation in lung cancer cells. Western blot analysis showed that cleaved caspase-3/PARP in A549 (Fig. 5a) and H460 (Fig. 5b) cells treated with 5 – 20 μM cisplatin for 72 h were substantially decreased upon downregulation of H2Bub1, indicating less apoptosis was induced. Notably, induction of p53 and p21 was also dramatic lower in A549 (Fig. 5a) and H460 (Fig. 5b), indicating that p53-mediated response to genetoxic stress was also attenuated in these cancer cells upon knockdown of RNF20. The impact of H2Bub1 downregulation on apoptosis was further validated by Annexin-V flow cytometry analysis of apoptotic cells in A549 and H460 cells treated with 20 μM cisplatin for 72 hours. Representative flow cytometry plots show that A549 cells (Fig. 5c, upper panel) and H460 cells (Fig. 5c, lower panel) cells transfected with control siRNA (Fig. 5c, left panels) were more sensitive to cisplatin than cells transfected with RNF20 siRNA (Fig. 5c, right panels). Statistical analysis further revealed a significant decrease in the percentages of apoptotic cells in A549 transfected with RNF20 siRNA compared to control siRNA (70.33 ± 10.70 vs. 53.97 ± 11.02%; P < 0.05). The same effect was observed in H460 with attenuated H2BuB1 (control siRNA: 56.46 ± 6.2 vs. siRNF20: 38.23 ± 3.72%; P < 0.05) (Fig. 5d). Similar results were also observed in A549 and H460 treated with lower concentration of cisplatin. In all, the above data strongly suggested that downregulation of H2Bub1 significantly enhanced resistance of lung cancer cells to cisplatin-induced apoptosis.
Figure 5.
Downregulation of H2Bub1 enhanced resistance to cisplatin in lung cancer cells. The levels of RNF20, H2Bub1, p21, p53, and apoptotic markers caspase-3/PARP cleaved products (T/C- Caspase-3/PARP for total and cleaved proteins) in (a) A549 and (b). H460 cells treated with cisplatin were analyzed by Western blot. Beta-actin was used as loading control. (c) Representative flow cytometry profile of A549 and H460 cells transfected with either control (left panel) or RNF20 siRNA (right panel). After cells were treated for 72 h with 20 uM Cisplatin, apoptosis was measured by flow cytometry analysis of Annexin-V (labeled with Alexa Fluor 488) staining in X axis and propidium iodide staining in Y axis. (d) Quantitative analysis of the experiments shows that apoptotic cells were significantly reduced in both A549 and H460 cells upon downregulation of H2Bub1. The experiment was repeated three times and data represent the average of the early apoptotic and late apoptotic cells (* P < 0.05; ** P < 0.01).
Loss of H2Bub1 is associated with poor differentiation in primary lung adenocarcinoma
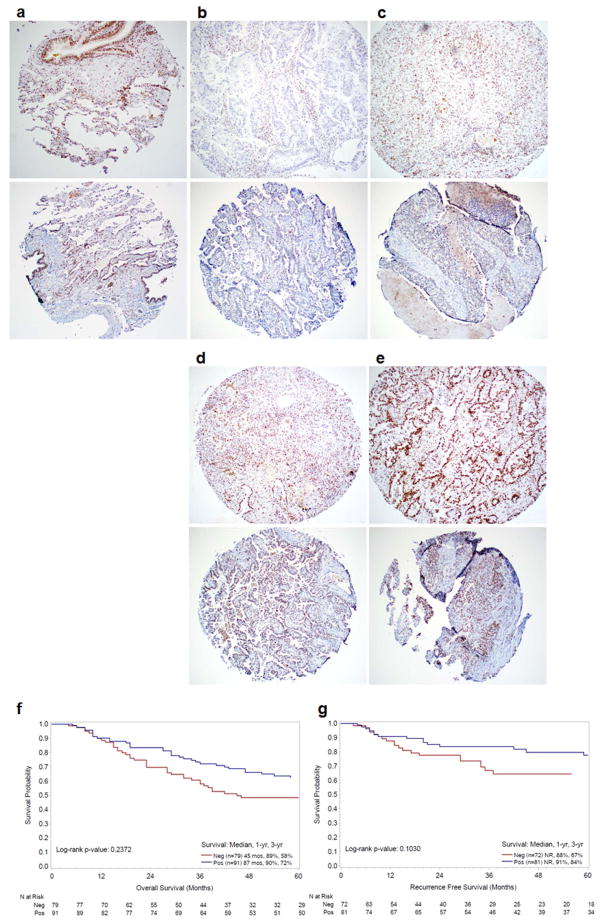
To investigate the status of H2Bub1 pathway in lung adenocarcinoma, we examined H2Bub1protein in 170 cancer and matched adjacent normal patient tissue samples by immunohistochemical analysis. Representative H2Bub1 immunostaining images of normal lung tissue (Fig. 6a, upper panel) and lung adenocarcinoma tissues (Fig. 6b to 6e, upper panel) reveal a nuclear staining pattern in both normal and tumor tissues. IHC analysis showed that H2Bub1 was absent (scored as 0) in 46.5% (79/170), while H2Bub1 levels in 25.3% (43/170), 17.1% (29/170), and 11.2% (19/170) of lung adenocarcinoma cases were scored as 1 +, 2 +, 3 + respectively. In contrast, total H2B levels were the same in both normal and lung adenocarcinoma tissues with different H2Bub1 levels (Fig. 6a to 6e, lower panel). Statistical analysis showed that the protein levels of H2Bub1 were significantly associated with poor cellular differentiation (P = 0.0134) (Supporting Information Table S1) in these lung adenocarcinoma tissues. The percentages of H2Bub1 loss in the poorly, moderately, and well differentiated groups were 54.2% (32/59), 46.1% (35/76), and 31.0% (9/28), respectively. Although Log-rank test analysis showed that the overall survival (Fig. 6f) and recurrence free survival (Fig. 6g) between patients with H2Bub1-negative adenocarcinoma (Neg, N = 79, IHC -) and patients with H2Bub1-positive adenocarcinoma (Pos, N = 91, IHC 1 to 3+) were not significantly different (P = 0.225; P = 0.103, respectively), patients with H2Bub1-positive cancers had a trend towards longer survival than patients with H2Bub1-negative cancers. The 5-year overall/recurrence free survival rates for patients with H2Bub1-negative and H2Bub1-positive adenocarcinoma were 55%/42% and 37%/25%, respectively. These findings suggest that H2Bub1 loss is a possible prognostic marker in lung adenocarcinoma patients.
Figure 6.
Loss of H2Bub1 and its association with cellular differentiation and survival of lung adenocarcinoma patients. Photomicrographs of H2Bub1 (upper panel) and total H2B (lower panel) in (a) normal lung tissue, and four representative lung adenocarcinoma tissues scored as (b) 0, (c) 1+, (d) 2+, (e) 3+ (Magnification × 100). (f). Kaplan–Meier analysis for overall survival of patients with H2Bub1 protein (Positive, n=91) or without H2Bub1 protein (Negative, n=79) (Neg v.s Pos, P = 0.237). (g). Kaplan–Meier analysis for recurrence free survival of lung adenocarcinoma patients with H2Bub1 protein (Positive, n=81) or without H2Bub1 protein (Negative, n=72) (Neg V.S Pos, P=0.103).
Discussion
Loss of H2Bub1 has been shown to play a role in a number of cancers.13,20,24,25 Several studies have indicated that both H2Bub1 and its E3 ligase RNF20 appear to act as tumor suppressors in mammalians cells.4,13 Reduction of RNF20 has been observed in seminoma,21 and due to promoter hypermethylation in breast cancers;13 and it has been associated with increased in vitro cell migration, growth and tumorigenicity.42 Several studies also showed that RNF20 downregulation promoted proliferation, migration, anchorage independence, and tumorigenesis in mammalian cells.7,13,43 Consistent with these observations, we found that RNF20 mRNA was significantly reduced in lung adenocarcinoma samples compared with normal lung tissues and that knockdown of RNF20 resulted in dramatic downregulation of H2Bub1 and increased cell proliferation in lung epithelial cells. We further identified that H2Bub1 was frequently decreased or even undetectable in lung adenocarcinoma tissues and is correlated with poor-differentiation in human lung cancer samples.
The precise mechanisms by which H2Bub1 loss affects cancer progression are largely unclear. H2Bub1 is thought to transcriptionally regulates global gene expression in large part through histone cross-talk with H3K4 and H3K79.9–13 Several experimental and clinical studies recently showed that high level of H2Bub1 could suppress transcription of several proto-oncogenes, including c-myc and c-Fos, and could augment expression of tumor suppressor genes, notably p53.13 In agreement with these findings, we found that knockdown of RNF20 significantly upregulated gene expression of growth-promoting oncogenes including, c-Myc, c-Met, and E2F1/2, CCND3 (Cyclin D3). Signaling pathways including p53, c-Myc, cellular proliferation, differentiation, and apoptotic pathways in lung normal epithelial and malignant cell lines were also altered upon downregulation of H2Bub1. In breast cancer cells, Prenzel et al showed that knockdown of the H2B ubiquitin ligase RNF40 decreased ERα-induced gene transcription, but supported estrogen-independent cell proliferation and activation of cell survival signaling pathways.20 Very recently, Bedi et al. showed that that H2Bub1 decrease led by downregulation of suppressor of Ty homologue-6 (SUPT6H) was associated with poor differentiation and enhanced malignancy in human breast cancer.44 Interestingly, depletion of RNF20/40 strongly retarded the growth of LNCaP cells, due to decreased expression of several cell cycle promoters.19
One of the hallmarks of cancer is acquisition of resistance to apoptosis, resulting in cells refractory to therapy.45 We found that knockdown of RNF20 dramatically decreased BMF, a critical pro-apoptotic protein. Downregulation of BMF in lung cancer cells is associated with resistance to drug-induced apoptosis, in particular to platinum chemotherapy, the most commonly used chemotherapeutic for lung cancer.41,46 In our study, we found that lung cancer cells with low levels of H2Bub1 were more resistant to cisplatin-induced apoptosis, and had higher rates of proliferation compared to cells with high H2Bub1 levels. We also found that knockdown of RNF20 also moderately increased phosphorylated AKT, and suppressed wild-type p53 signaling pathways. Considering of the important roles of these molecules and signaling pathways in apoptosis, we proposed that the reduction of BMF, p53, and increased AKT and modulated other pro-apoptotic proteins may have a combined contribution to enhanced cisplatin resistance in the lung cancer cells upon downregulation of H2Bub1.
In addition, H2Bub1 participates in maintaining stem cell multipotency,26,47 H2Bub1 increases during induced differentiation of human and mouse embryonic and carcinoma stem cells.8 In the study, we found that FCS-induced squamous differentiation of BEAS-2B cells was also suppressed upon H2Bub1 depletion, suggesting it has a role in maintaining stemness in lung tissue. Interestingly, Bedi et al very recently showed that downregulation of H2Bub1 led by SUPT6H a histone chaperone was associated with poor differentiation and enhanced malignancy in breast cancer.44 Therefore, we propose that H2Bub1 is involved in terminal differentiation and suppression of genes involved in cell proliferation. Conversely, when H2Bub1 is lost, cancer cells proliferate more readily, and de-differentiation of normal epithelial cells may occur.
Overexpression of USP22 protein has been reported in numerous cancers including lung adenocarcinoma.4,24,25,48 A variety of studies suggest that elevation of USP22 correlates epithelial–mesenchymal transition (EMT) acquisition with highly malignant clinical behavior, drug resistance and poor prognosis. USP22 has been implicated in a number of cancers, including lung cancer, as a “death by cancer” gene associated with an aggressive, highly metastatic and therapy-resistant phenotype.24,25,27 Given the association of USP22 with stem cell-like features in many cancers, it is possible that decreased H2Bub1 negatively regulates differentiation of human lung epithelial cells, thereby promoting tumorigenesis. Finally, it should be noted that RNF20 has other targets and H2Bub1-independent functions; nevertheless, a key mechanism of action for some of these changes led by RNF20 knockdown is probably through downregulation of H2Bub1. Additional research on the mechanisms responsible for H2Bub1 loss in lung cancer should be carried out. Moreover, given our results, research on targeting H2Bub1 levels as a therapy for lung cancer are warranted.
In summary, we for the first time demonstrated that frequent H2Bub1 loss is correlated with poor-differentiation and enhanced malignancy in lung adenocarcinoma probably through regulating multiple important cancer signaling pathways such as p53, Myc, cadherin, indicating deregulated H2Bub1 pathway may offer new opportunities for targeting of the epigenome for the treatment of lung adenocarcinoma patients, which warrants further investigation.
Supplementary Material
What’s new?
This study has for the first time demonstrated that loss of H2Bub1 is associated with enhanced malignancy and poor differentiaton of lung adenocarcinoma. The study has further uncovered these potential molecules and signaling pathways underlying loss of H2Bub1 in lung adenocarcinoma. The study has indicated that loss of H2Bub1 plays an oncogenic role in lung adenocarcinoma via modulating multiple cancer signaling pathways, and represents a prognostic biomarker and therapeutic target for lung adenocarcinoma.
Acknowledgments
Financial support: This study is supported by the V Foundation, and NIH 5K12CA001727-20 and P30CA33572 through the use of several City of Hope Core Facilities.
Research reported in this publication is supported by the V Foundation (DR) and the National Cancer Institute of the National Institutes of Health under award numbers NIH 5K12CA001727-20 (DR) and P30CA33572 through the use of several core facilities. We acknowledge the generous support of the Baum Family Foundation in support of this research. The authors thank Michael Lewallen of the pathology core at Department of Pathology for IHC staining; Loren Quintanar at Light Microscope core of City of Hope for imaging IHC slides, and Dr. Indra Mahajan at Department of Surgery for language edition.
Footnotes
Disclosure of Conflicts of Interest: No conflicts are declared by authors.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contributions
K Zhang and D Raz conceived the study, interpreted data, wrote, and revised the manuscript. K Zhang, J Wang, and T Tong developed methods and performed experiments. X Wu did RNAseq data analysis. R Nelson did statistic analysis. Y Yuan and Z Liu performed TCGA data analysis. X Yun and T Reno provided technical and material support. J Kim and R Salgia critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Wilting RH, Dannenberg JH. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist Updat. 2012;15:21–38. doi: 10.1016/j.drup.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Cole AJ, Clifton-Bligh R, Marsh DJ. Histone H2B monoubiquitination: roles to play in human malignancy. Endocr Relat Cancer. 2015;22:T19–33. doi: 10.1530/ERC-14-0185. [DOI] [PubMed] [Google Scholar]
- 5.Nicassio F, Corrado N, Vissers JH, et al. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol. 2007;17:1972–7. doi: 10.1016/j.cub.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Van der Knaap JA, Kumar BR, Moshkin YM, et al. GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol Cell. 2005;17:695–707. doi: 10.1016/j.molcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XY, Varthi M, Sykes SM, et al. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102–11. doi: 10.1016/j.molcel.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs G, Shema E, Vesterman R, et al. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol Cell. 2012;46:662–73. doi: 10.1016/j.molcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Guermah M, McGinty RK, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–71. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng HH, Xu RM, Zhang Y, et al. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J Biol Chem. 2002;277:34655–7. doi: 10.1074/jbc.C200433200. [DOI] [PubMed] [Google Scholar]
- 11.Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418:104–8. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- 12.Hahn MA, Dickson KA, Jackson S, et al. The tumor suppressor CDC73 interacts with the ring finger proteins RNF20 and RNF40 and is required for the maintenance of histone 2B monoubiquitination. Hum Mol Genet. 2012;21:559–68. doi: 10.1093/hmg/ddr490. [DOI] [PubMed] [Google Scholar]
- 13.Shema E, Tirosh I, Aylon Y, et al. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev. 2008;22:2664–76. doi: 10.1101/gad.1703008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirngruber J, Shchebet A, Schreiber L, et al. CDK9 directs H2B monoubiquitination and controls replication-dependent histone mRNA 3′-end processing. EMBO Rep. 2009;10:894–900. doi: 10.1038/embor.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergink S, Salomons FA, Hoogstraten D, et al. DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A. Genes Dev. 2006;20:1343–52. doi: 10.1101/gad.373706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavri R, Zhu B, Li G, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–17. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Chernikova SB, Dorth JA, Razorenova OV, et al. Deficiency in Bre1 impairs homologous recombination repair and cell cycle checkpoint response to radiation damage in mammalian cells. Radiat Res. 2010;174:558–65. doi: 10.1667/RR2184.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarcic O, Pateras IS, Cooks T, et al. RNF20 Links Histone H2B Ubiquitylation with Inflammation and Inflammation-Associated Cancer. Cell Rep. 2016;14:1462–76. doi: 10.1016/j.celrep.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jääskeläinen T, Makkonen H, Visakorpi T, et al. Histone H2B ubiquitin ligases RNF20 and RNF40 in androgen signaling and prostate cancer cell growth. Mol Cell Endocrinol. 2012;350:87–98. doi: 10.1016/j.mce.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Prenzel T, Begus-Nahrmann Y, Kramer F, et al. Estrogen-dependent gene transcription in human breast cancer cells relies upon proteasome-dependent monoubiquitination of histone H2B. Cancer Res. 2011;71:5739–53. doi: 10.1158/0008-5472.CAN-11-1896. [DOI] [PubMed] [Google Scholar]
- 21.Chernikova SB, Razorenova OV, Higgins JP, et al. Deficiency in mammalian histone H2B ubiquitin ligase Bre1 (Rnf20/Rnf40) leads to replication stress and chromosomal instability. Cancer Res. 2012;72:2111–9. doi: 10.1158/0008-5472.CAN-11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urasaki Y, Heath L, Xu CW. Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLoS One. 2012;7:e36775. doi: 10.1371/journal.pone.0036775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZJ, Yang JL, Wang YP, et al. Decreased histone H2B monoubiquitination in malignant gastric carcinoma. World J Gastroenterol. 2013;19:8099–107. doi: 10.3748/wjg.v19.i44.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Yao L, Zhang X, et al. Elevated expression of USP22 in correlation with poor prognosis in patients with invasive breast cancer. J Cancer Res Clin Oncol. 2011;137:1245–53. doi: 10.1007/s00432-011-0998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YL, Yang YM, Xu H, et al. Aberrant expression of USP22 is associated with liver metastasis and poor prognosis of colorectal cancer. J Surg Oncol. 2011;103:283–9. doi: 10.1002/jso.21802. [DOI] [PubMed] [Google Scholar]
- 26.Karpiuk O, Najafova Z, Kramer F, et al. The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. Mol Cell. 2012;46:705–13. doi: 10.1016/j.molcel.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–21. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Chu K, Wu X, et al. Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 2013;73:1298–307. doi: 10.1158/0008-5472.CAN-12-2086. [DOI] [PubMed] [Google Scholar]
- 29.Wadsworth SJ, Nijmeh HS, Hall IP. Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol. 2006;26:376–87. doi: 10.1007/s10875-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Gao H, Wu X, et al. Frequent overexpression of HMGA2 in human atypical teratoid/rhabdoid tumor and its correlation with let-7a3/let-7b miRNA. Clin Cancer Res. 2014;20:1179–89. doi: 10.1158/1078-0432.CCR-13-1452. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Zhang K, Wang J, et al. Underexpression of LKB1 tumor suppressor is associated with enhanced Wnt signaling and malignant characteristics of human intrahepatic cholangiocarcinoma. Oncotarget. 2015;6:18905–20. doi: 10.18632/oncotarget.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyal L, Lerenthal Y, Gana-Weisz M, et al. Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol Cell. 2011;41:529–42. doi: 10.1016/j.molcel.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver JR, Kushwah R, Wu J, et al. Elf3 plays a role in regulating bronchiolar epithelial repair kinetics following Clara cell-specific injury. Lab Invest. 2011;91:1514–29. doi: 10.1038/labinvest.2011.100. [DOI] [PubMed] [Google Scholar]
- 36.Zuo W, Zhang T, Wu DZ, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–20. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koren A, Rijavec M, Kern I, et al. BMI1, ALDH1A1, and CD133 Transcripts Connect Epithelial-Mesenchymal Transition to Cancer Stem Cells in Lung Carcinoma. Stem Cells Int. 2016;2016:9714315. doi: 10.1155/2016/9714315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson T, Vuong H, Liaw YS, et al. Mechanism of repression of squamous differentiation marker, SPRR1B, in malignant bronchial epithelial cells: role of critical TRE-sites and its transacting factors. Oncogene. 2001;20:634–44. doi: 10.1038/sj.onc.1204134. [DOI] [PubMed] [Google Scholar]
- 39.Walts AE, Said JW, Siegel MB, et al. Involucrin, a marker of squamous and urothelial differentiation. An immunohistochemical study on its distribution in normal and neoplastic tissues. J Pathol. 1985;145:329–40. doi: 10.1002/path.1711450406. [DOI] [PubMed] [Google Scholar]
- 40.Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–91. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinon JD, Labi V, Egle A, et al. Bim and Bmf in tissue homeostasis and malignant disease. Oncogene. 2008;27(Suppl 1):S41–52. doi: 10.1038/onc.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shema E, Field Y, Schuster M, et al. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–8. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 43.Shema E, Kim J, Roeder RG, et al. RNF20 inhibits TFIIS-facilitated transcriptional elongation to suppress pro-oncogenic gene expression. Mol Cell. 2011;42:477–88. doi: 10.1016/j.molcel.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedi U, Scheel AH, Hennion M, et al. SUPT6H controls estrogen receptor activity and cellular differentiation by multiple epigenomic mechanisms. Oncogene. 2015;34:465–73. doi: 10.1038/onc.2013.558. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Kepp O, Gottschalk K, Churin Y, et al. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS Pathog. 2009;5:e1000348. doi: 10.1371/journal.ppat.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buszczak M, Paterno S, Spradling AC. Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science. 2009;323:248–51. doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ning J, Zhang J, Liu W, et al. Overexpression of ubiquitin-specific protease 22 predicts poor survival in patients with early-stage non-small cell lung cancer. Eur J Histochem. 2012;56:e46. doi: 10.4081/ejh.2012.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.