Abstract
The American Joint Committee for Cancer (AJCC) has adopted a size-based T stage system (8th edition) for pancreatic ductal adenocarcinoma (PDAC), defined as follows: pT1 ≤ 2 cm (pT1a ≤ 0.5 cm, pT1b > 0.5 cm and < 1 cm, and pT1c 1–2 cm); pT2 > 2 cm and ≤ 4 cm; and pT3 > 4 cm. However, the prognostic value of this new T staging system has not been validated in patients who underwent pancreaticoduodenectomy (PD) after neoadjuvant therapy. In this study, we analyzed 398 PDAC patients who underwent neoadjuvant therapy and PD at our institution from 1999 to 2012. The results were correlated with clinicopathologic parameters and survival. The new T stage correlated with lymph nodes metastasis (p<0.001), tumor regression grade (p<0.001), disease-free survival (DFS, p<0.001) and overall survival (OS, p<0.001). None of the patients with ypT0 had recurrence or died of disease. Among the patients with ypT1 disease, patients with ypT1a and ypT1b had better DFS (p=0.046) and OS (p=0.03) than those with ypT1c. However, there was no significant difference in either DFS or OS between ypT1c and ypT2 or between ypT2 and ypT3 groups (p>0.05). In multivariate analysis, new ypT3 stage was associated with shorter OS (p=0.04), but not DFS (p=0.16). Our results show that the new ypT stage better stratify survival than the ypT stage in AJCC 7th edition for PDAC patients who received PD after neoadjuvant therapy, and that tumor size cut off of 1.0 cm work better for ypT2 than the proposed tumor size cut off of 2.0 cm in this group of patients.
Keywords: Pancreatic cancer, neoadjuvant chemotherapy, tumor stage, survival
Introduction
The incidence of pancreatic cancer is estimated to rise over the next few decades (1), ranking third in the leading causes of cancer death. In spite of developments in treatment strategies, pancreatic ductal adenocarcinoma (PDAC) remains a highly lethal disease, with a mortality rate that closely parallels its incidence. Surgical resection is regarded as the only potentially curative treatment (2). However, the tumor is mostly asymptomatic in early stages of the disease, and consequently, the majority of PDAC patients present late in the disease course when the tumor is locally advanced and unresectable.
Neoadjuvant therapy is a relatively new strategy in the treatment of PDAC. It improves resectability in borderline resectable cases and provides a survival benefit in patients with advanced stages of PDAC (3). Resection of the primary tumor is attempted only in patients who have no evidence of disease progression/metastasis on post-therapy restaging imaging, and who have a performance status and comorbidity profile that is appropriate for major surgery. As such, neoadjuvant therapy strategy helps to select the patients who may benefit the most from pancreatic resection. In our previous studies of large cohorts of PDAC patients who underwent pancreatectomy after completion of neoadjuvant therapy, we showed that histologic tumor regression grade (4–6) and histopathologic parameters such as vascular invasion, perineural invasion, lymph node status, and resection margin status are significant prognostic factors in this group of PDAC patients (7–13).
According to the tumor (T) staging protocol for PDAC by the AJCC 7th edition (14), a pathologic stage pT3 tumor is defined as one that has extended beyond the pancreas into peripancreatic soft tissue or the surrounding structures, but does not involve celiac axis or the superior mesenteric artery, irrespective of tumor size. It is only for tumors confined to the pancreas, that the T-staging involves a cut-off size of 2 cm (≤ 2 cm is classified as T1, and > 2 cm is T2) (14). The underlying basis of this classification is that, extrapancreatic extension is prognostically more important than is tumor size. However, since the pancreas does not have a true capsule, the assessment of extrapancreatic extension has been a matter of significant subjectivity.
Although the current AJCC staging system (7th edition) has been shown to be of prognostic significance in PDAC patients (15), it falls short in serving its purpose and has been found to be inadequate in other studies (16–21). In the 8th edition of the AJCC staging system (to be implemented in January 2018), the pT stage of PDAC has been revised and formulated as follows: pT1 tumor ≤ 2 cm in maximum dimension (subdivided into pT1a tumor ≤ 0.5 cm, pT1b tumor > 0.5 cm and < 1 cm, and pT1c tumor ≥ 1 cm and ≤ 2 cm), pT2 tumor > 2 cm and ≤ 4 cm, pT3 tumor > 4 cm. The criteria for pT4 tumor remain the same as the 7th edition, defined as tumor involving the celiac axis, superior mesenteric artery and/or common hepatic artery, irrespective of tumor size (22). The studies that formed the basis of this new classification system included pancreatic resections in patients who had not undergone neoadjuvant therapy. The significance of this new pT grouping in PDAC patients who underwent surgical resection after receiving neoadjuvant therapy has not been validated.
In this retrospective study, we examined the prognostic significance of ypT stage based on the AJCC Staging Manual, 7th edition and the new ypT stage based on the 8th edition in 398 PDAC patients who underwent pancreaticoduodenectomy after receiving neoadjuvant therapy. The results were correlated with the clinicopathologic parameters, and disease-free and overall survival. Our results showed that the new ypT stage is a significant prognostic factor for both disease-free and overall survival and better stratifies patients prognostically, than the current ypT stage, in patients who underwent pancreaticoduodenectomy after receiving neoadjuvant therapy.
Patients and Methods
Patient population and follow-up
After obtaining approval from the institutional review board, we retrospectively analyzed 398 patients who had undergone pancreaticoduodenectomy (PD), after neoadjuvant therapy for PDAC at University of Texas MD Anderson Cancer Center between January 1999 to December 2012. They were identified from a database that is prospectively maintained by the Department of Surgical Oncology. A waiver of consent was granted for the use of their specimen information for research. We excluded patients who had undergone distal pancreatectomy and those who had undergone PD and neoadjuvant therapy for other neoplasms. The clinical and follow-up information such as date of diagnosis, date and site of recurrence, or date and cause of death, when applicable, was verified by reviewing patients’ medical records and the U.S. Social Security Index. Disease recurrence or metastasis was determined on the basis of radiographic and clinical suspicion, as often, biopsy confirmation had not been obtained.
Our study population consisted of 222 male and 176 female patients, with ages ranging from 34.5 years to 85.4 years (median age, 64.1 years). Seventy-four patients (18.6%) had undergone neoadjuvant fluoropyrimidine-based chemoradiation therapy (group 1), 101 (25.4%) neoadjuvant gemcitabine-based chemoradiation therapy (group 2), 102 (25.6%) systemic chemotherapy followed by gemcitabine-based chemoradiation therapy (group 3), 103 (25.9%) systemic chemotherapy followed by fluoropyrimidine-based chemoradiation therapy (group 4), and 18 (4.5%) neoadjuvant systemic chemotherapy alone (group 5, Table 1).
Table 1.
Correlations between the Post-therapy Primary Tumor Stage by the AJCC 8th Edition and Clinicopathologic Factors
| Characteristic | Primary Tumor Stages by AJCC 8th Edition
|
p value | |||
|---|---|---|---|---|---|
| ypT0 | ypT1 | ypT2 | ypT3 | ||
| Age, years | 0.99 | ||||
| ≤ 65 | 5 | 84 | 111 | 18 | |
| > 65 | 4 | 68 | 92 | 16 | |
| Sex | 0.51 | ||||
| Female | 6 | 67 | 90 | 13 | |
| Male | 3 | 85 | 113 | 21 | |
| Neoadjuvant therapy group** | 0.17 | ||||
| Group 1 | 3 | 26 | 41 | 4 | |
| Group 2 | 2 | 45 | 47 | 7 | |
| Group 3 | 4 | 38 | 55 | 5 | |
| Group 4 | 0 | 37 | 50 | 16 | |
| Group 5 | 0 | 6 | 10 | 2 | |
| Differentiation | 0.80 | ||||
| Well-moderate | NA* | 95 | 125 | 23 | |
| Poor | NA | 57 | 78 | 11 | |
| ypT stage by AJCC# 7th edition | <0.001 | ||||
| ypT0 | 9 | 0 | 0 | 0 | |
| ypT1 | 0 | 23 | 0 | 0 | |
| ypT2 | 0 | 0 | 8 | 0 | |
| ypT3 | 0 | 129 | 195 | 34 | |
| Margin status | 0.2 | ||||
| Negative | 9 | 144 | 185 | 29 | |
| Positive | 0 | 8 | 18 | 5 | |
| Treatment response | <0.001 | ||||
| CAP score 0 and 1 | 9 | 32 | 20 | 2 | |
| CAP score 2 and 3 | 0 | 120 | 183 | 32 | |
| ypN stage by AJCC 7th edition | <0.001 | ||||
| Negative (ypN0) | 8 | 82 | 85 | 8 | |
| Positive (ypN1) | 1 | 70 | 118 | 26 | |
| ypN stage by AJCC 8th edition | 0.001 | ||||
| Negative (ypN0) | 8 | 82 | 85 | 8 | |
| 1–3 positive nodes (ypN1) | 1 | 50 | 71 | 20 | |
| ≥ 4 positive nodes (ypN2) | 0 | 20 | 47 | 6 | |
Abbreviations: NA, not applicable
AJCC: American Joint Committee for Cancer
Neoadjuvant therapy groups: Group 1: Fluoropyrimidine-based chemoradiation therapy; group 2: Gemcitabine-based chemoradiation therapy; group 3: Systemic chemotherapy followed by gemcitabine-based chemoradiation therapy; group 4: Systemic chemotherapy followed by fluoropyrimidine-based chemoradiation therapy; group 5: neoadjuvant systemic chemotherapy alone.
Pathologic examination of PD specimens
A standardized scheme for histologic evaluation and pathologic reporting of PD specimens was set up and has been used at our institution since 1990 which included tumor location, size, tumor type, differentiation, histologic tumor response grade, tumor involvement of extrapancreatic tissue, presence of lymphovascular or perineural invasion, number of positive and total lymph nodes, and margin status. The ypTNM was grouped according to the American Joint committee on Cancer (AJCC) Cancer Staging Manual, 7th edition (14) and 8th edition (22). The pretreatment pathologic diagnosis of PDAC was confirmed in all cases by reviewing the fine needle aspiration cytology and/or biopsies.
Tumor size assessment
Neoadjuvant treatment is known to shrink the tumor and pancreas due to therapy-induced chronic pancreatitis and fibrosis. After treatment, the tumor size is often difficult to assess because the tumor bed may become extremely fibrotic, which merges with the therapy-induced diffuse fibrosis in the adjacent non-neoplastic pancreatic parenchyma and thus makes the boundary of the treated tumor bed difficult to identified on the gross examination (23). Moreover, there is also a decrease in overall cellularity of the tumor with neoadjuvant therapy, and the response is often heterogeneous, leading to islands and nests of surviving tumor, with stretches of tumor-free fibrotic tumor bed in between. In this study, the tumor size measurement was performed using the method in the standardized pathologic evaluation of post-neoadjuvant specimens of breast cancer as recommended by an international working group (24). The tumor size was measured by gross assessment, validated by histology and recorded in the final pathologic report when there was a grossly identifiable mass lesion or treated tumor bed.
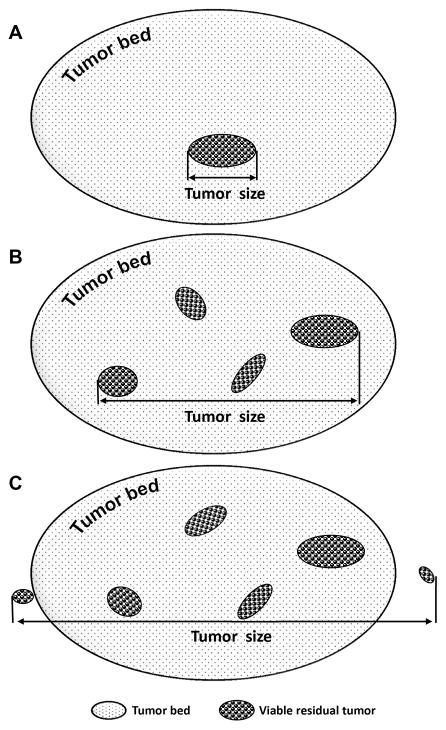
The schematic drawing of the tumor size measurement is shown in Figure 1. For cases which had no grossly identifiable mass or treated tumor bed, the entire head of pancreas, common bile duct and ampulla of Vater were systematically submitted for histologic examination. Particular attention was paid to careful mapping of the sections taken, so that the tumor size could be measured microscopically on the hematoxylin & eosin (H & E) stained slides. For the cases which had only single a microscopic focus of viable residual PDAC, the largest linear dimension of the viable tumor focus on H & E slide was used as the final tumor size (Figure 1A). For the cases which had more than one microscopic foci (multifocal) of residual tumor, the largest linear dimension of the area involved by all islands of viable residual PDAC and the intervening fibrotic stroma was used as the residual tumor size. The measurement excluded the area of fibrotic tumor bed beyond the area containing viable tumor cells (Figure 1B). For the cases which had microscopic viable residual PDAC invading the pancreas and/or soft tissue beyond the tumor bed area, the largest dimension of the entire area involved by all islands of viable residual PDAC with the intervening stroma, pancreas and/or soft tissue was used as the final tumor size (Figure 1C). All the cases in this study were reviewed by a gastrointestinal pathologist (D.C.) who was blinded about the clinical and follow up data. For cases with a diagnosis of complete pathologic response (after submission of the entire pancreatic tissue for histologic evaluation), review of the original pathology specimens (histology and cytology) were done to confirm the original diagnosis.
Figure 1.
Schematic drawing to illustrate the method for tumor size measurement. For cases which had only single microscopic focus of viable residual tumor, the largest linear dimension of the viable tumor focus on H & E slide was used as the final tumor size (A). For cases which had more than one microscopic foci (multifocal) of viable residual tumor, the largest linear dimension of the area involved by all islands of viable residual tumor and the intervening fibrotic stroma was used as the residual tumor size (B). For cases which had microscopic viable residual tumor invading the pancreas and/or soft tissue beyond the tumor bed area, the largest dimension of the entire area involved by all islands of viable residual tumor with the intervening stroma, pancreas and/or soft tissue was used as the final tumor size (C).
Statistical analysis
Chi-square analyses were used to compare categorical data, and analysis of variance (ANOVA) was used to compare continuous variables. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. Disease-free survival (DFS) was calculated from the date of surgery to the date of first recurrence after surgery in patients with recurrence or to the date of last follow-up in patients without recurrence. Overall survival (OS) was calculated from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. The prognostic significance of patients’ clinical and pathologic characteristics was determined using a univariate Cox regression analysis. Cox proportional hazards models were fitted for multivariate analysis. After the interactions between the variables had been examined, a backward stepwise procedure was used to derive the best-fitting model. A statistical analysis was performed using Statistical Package for Social Sciences software for Windows (Version 22, SPSS, Inc., Chicago, IL). A 2-sided significance level of 0.05 was used for all statistical analyses.
Results
Pathologic evaluation and staging
Treated tumor size ranged from 0 to 8.5 cm, with a median of 2.5 cm. Among the 398 cases in our study, 9 (2.3%) were ypT0 (pathologic complete response with no evidence of tumor), 23 (5.8%) were ypT1, 8 (2%) were ypT2, and 358 (89.9%) were ypT3 based on the AJCC Staging Manual, 7th edition. According to the new pT staging system (AJCC Staging Manual, 8th edition), 9 cases (2.3%) were ypT0, 16 (4%) ypT1a, 14 (3.5%) ypT1b, 122 (30.7%) ypT1c, 203 (51%) ypT2, and 34 (8.5%) ypT3. Of the 358 cases that had been classified as ypT3 using the AJCC 7th edition, 129 (36.0%) were ypT1, 195 (54.5%) were ypT2, and 34 (9.5%) were ypT3 respectively under the new pT staging system. In the 9 cases that were ypT0, pre-treatment scanning revealed radiologic evidence of a mass and a diagnosis of PDAC had been confirmed by cytologic examination in all cases. There were no ypT4 patients in our study population.
According to the WHO classification standards, of the 389 cases with residual tumor, 243 (62.5%) were well to moderately differentiated and 146 (37.5%) were poorly differentiated. An R0 resection was achieved in 367 of the 398 cases (92.2%). Thirty-one cases (7.8%) had microscopic evidence of tumor involvement of one or more surgical margins (R1 resection). There were no R2 resections. According to our proposed modified CAP grading scheme for residual carcinoma (4, 5), 9 cases (2.3%) had modified CAP grade 0 response; 54 cases (13.6%) had modified CAP grade 1 response, and 335 cases (84.2%) modified CAP grade 2 response. The total number of lymph nodes examined ranged from 12 to 68, with a median of 22. Lymph nodes that were positive for metastatic disease were present in 215 cases (54%). Among the lymph node positive group, the number of positive lymph nodes ranged from 1 to 25, with a median of 2. One hundred forty-two cases (66%) had 1–3 positive lymph nodes (ypN1), and 73 cases (34%) had ≥ 4 positive lymph nodes (ypN2), based on the AJCC Staging Manual, 8th edition. The new ypT stage correlated with the modified CAP tumor response grades (p<0.001), current ypT stage (p<0.001), ypN stage based on AJCC 7th edition (p<0.001) and new ypN stage (p=0.001, Table 1). There were no significant correlations between the new ypT stage and age, gender, different treatment groups, differentiation or margin status (p>0.05).
Survival analysis
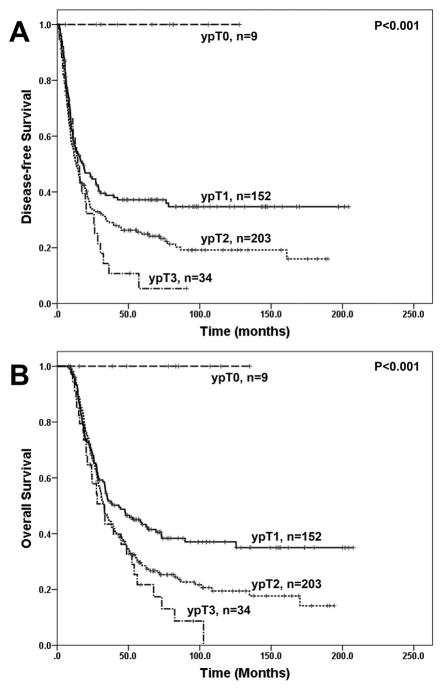
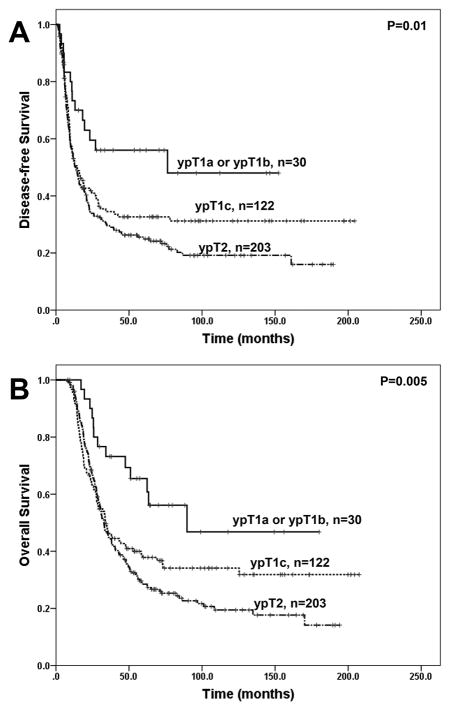
The follow up time ranged from 7.6 to 177.5 months with a median of 32.0 months in the overall study group and ranged from 8.2 to 177.5 months with median of 63.0 months among the 140 patients who did not die from the disease. During follow up, none of the nine patients who had pathologic complete response (ypT0) developed recurrence or died from disease. Patients with new ypT0 and new ypT1 disease had better disease-free survival (DFS) and overall survival (OS) than those with new ypT2 and new ypT3 disease (Figure 2A and 2B, p<0.0001). The five-year OS rates for patients with new ypT1, ypT2, and ypT3 were 41.1%, 29.3%, and 17.4%, respectively. However, there was no significant difference between new ypT2 group and new ypT3 group in either DFS or OS (p>0.05, Figure 2A, 2B, and Table 2). Among the patients with new ypT1 disease, there was no significant difference in either DFS or OS between patients with ypT1a and those with ypT1b disease (p>0.05, Table 2). Patients with ypT1c disease had shorter DFS (p=0.046) and OS (p=0.03) than those with ypT1a and ypT1b disease, but had no significant difference in either DFS or OS when compared to those with new ypT2 disease significant (p>0.05, Fig. 3A, 3B and Table 2).
Figure 2.
Kaplan-Meier curves for disease-free survival (A) and overall survival (B) in patients with pancreatic ductal adenocarcinoma who underwent PD after neoadjuvant therapy, stratified by ypT stage using the new AJCC Staging Manual (8th edition).
Table 2.
Univariate Cox Regression Analysis of Disease-free Survival and Overall Survival
| Characteristic | No. of patients | Disease-free survival | Overall survival | ||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | 389 | 0.98 (0.97–0.997) | 0.01 | 0.99 (0.98–1.01) | 0.21 |
| Sex | |||||
| Female (reference) | 170 | ||||
| Male | 219 | 0.94 (0.84–1.06) | 0.33 | 0.92 (0.81–1.03) | 0.15 |
| Tumor differentiation | |||||
| Well-moderate (reference) | 243 | ||||
| Poor | 146 | 1.29 (1.01–1.64) | 0.04 | 1.36 (1.07–1.73) | 0.01 |
| Margin status | |||||
| Negative (reference) | 358 | ||||
| Positive | 31 | 1.47 (0.97–2.22) | 0.07 | 1.71 (1.13–2.59) | 0.01 |
| Treatment responseCAP score | |||||
| 1 (reference) | 54 | ||||
| 2 and 3 | 335 | 1.69 (1.15–2.47) | 0.007 | 1.78 (1.22–2.61) | 0.003 |
| ypT stage by AJCC 8th edition* | 0.01 | 0.006 | |||
| ypT1 (reference) | 152 | ||||
| ypT2 | 203 | 1.37 (1.06–1.77) | 0.02 | 1.40 (1.08–1.82) | 0.01 |
| ypT3 | 34 | 1.71 (1.12–2.62) | 0.01 | 1.83 (1.19–2.80) | 0.006 |
| ypT1b vs ypT1a | 0.73 (0.25–2.12) | 0.56 | 0.44 (0.13–1.48) | 0.18 | |
| ypT1c vs ypT1a or ypT1b | 1.71 (0.97–3.02) | 0.07 | 1.99 (1.08–3.67) | 0.03 | |
| ypT2 vs ypT1c | 1.21 (0.92–1.59) | 0.17 | 1.15 (0.87–1.52) | 0.34 | |
| ypT3 vs ypT2 | 1.36 (0.88–1.98) | 0.18 | 1.36 (0.90–205) | 0.15 | |
| ypT stage by AJCC 7th edition* | 0.01 | 0.01 | |||
| ypT1 (reference) | 23 | ||||
| ypT2 | 8 | 3.06 (1.09–8.59) | 0.03 | 2.71 (0.97–7.63) | 0.06 |
| ypT3 | 358 | 2.74 (1.41–5.32) | 0.003 | 2.80 (1.44–5.44) | 0.002 |
| ypN stage by AJCC 8th edition | 0.00 | 0.00 | |||
| Negative (ypN0, reference) | 175 | ||||
| 1–3 positive nodes (ypN1) | 141 | 1.60 (1.22–2.09) | 0.001 | 1.54 (1.17–2.03) | 0.002 |
| ≥ 4 positive nodes (ypN2) | 73 | 2.65 (1.94–3.62) | 0.00 | 2.67 (1.95–3.66) | 0.00 |
Nine patients with ypT0 stage were not included in the analyses as the number of patients in this category was too small.
Abbreviations: HR, Hazard ratio; CI, confidence interval; AJCC, American Joint Committee for Cancer
Figure 3.
Kaplan-Meier curves for disease-free survival (A) and overall survival (B) in patients with ypT1a or ypT1b, ypT1c and ypT2 pancreatic ductal adenocarcinoma who underwent PD after neoadjuvant therapy using the new AJCC Staging Manual (8th edition). Patients with ypT1a or ypT1b disease have significantly longer disease-free survival and overall survival than those with ypT1c and those with ypT2 disease (p<0.05). However there is no significant difference in either disease-free survival or overall survival between the patients with ypT1c disease and those with ypT2 disease (p>0.05).
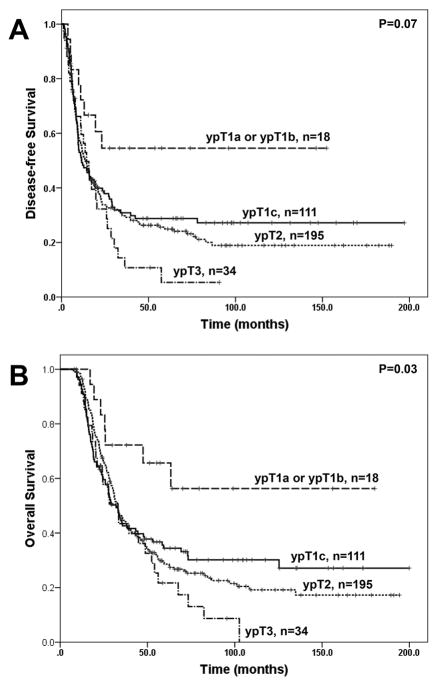
Similar results were obtained among patients whose tumors were classified as ypT3 based on AJCC Staging Manual, 7th edition. Among these patients, the group with ypT1a and ypT1b disease has longer OS than the group with ypT1c disease (p=0.03), although the difference in DFS between these two groups was not significant (p=0.08). Similar to the results from the overall study population, no significant difference in either DFS or OS was observed among the patients whose tumors were reclassified as ypT1c, new ypT2 and new ypT3 (Figure 4A and 4B). Our data suggest that tumor size cut off of 1.0 cm work better for ypT2 than the proposed tumor size cut off of 2.0 cm in in PDAC patients who underwent PD after receiving neoadjuvant therapy.
Figure 4.
Prognostic significance of new ypT stage grouping in patients whose tumors were classified as ypT3 using the 7th edition AJCC Staging Manual. Kaplan-Meier curves for disease-free survival (A) and overall survival (B) using the new AJCC Staging Manual (8th edition) are shown. Patients with ypT1a or ypT1b disease have significantly longer disease-free survival and overall survival than those with ypT1c, ypT2 or ypT3 disease (p<0.05). However there is no significant difference in either disease-free survival or overall survival between the patients with ypT1c disease and those with ypT2 or ypT3 disease (p>0.05).
The correlations of DFS and OS with clinicopathologic parameters by univariate analyses are shown in Table 2. There was a significant association between DFS and age at diagnosis (p=0.01), tumor differentiation (p=0.04), modified CAP tumor response grade (p=0.007), new ypT stage (p=0.01), and new ypN stage (p<0.001). The OS correlated significantly with differentiation (p=0.01), modified CAP tumor response grade (p=0.003), margin status (p=0.01), new ypT stage (p=0.006) and new ypN stage (p<0.001). On multivariate analysis, tumor differentiation, modified CAP tumor response grade, and new ypN stage are independent prognostic factor for both DFS and OS (Table 3). The new ypT3 and positive resection margins were significant prognostic factor for shorter OS. The new ypT stage is not a significant prognosticator for DFS (p>0.05).
Table 3.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival
| Characteristic | No. of patients | Disease-free survival | Overall survival | ||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Tumor differentiation | |||||
| Well-moderate (reference) | 243 | ||||
| Poor | 146 | 1.29 (1.01–1.65) | 0.04 | 1.41 (1.10–1.80) | 0.006 |
| Margin status | |||||
| Negative (reference) | 358 | ||||
| Positive | 31 | 1.28 (0.84–1.95) | 0.25 | 1.52 (1.00–2.32) | 0.05 |
| Treatment response CAP score | |||||
| 1 (reference) | 54 | ||||
| 2 and 3 | 335 | 1.48 (1.01–2.16) | 0.046 | 1.52 (1.03–2.24) | 0.03 |
| ypT stage by AJCC 8th edition* | 0.16 | 0.11 | |||
| ypT1 (reference) | 152 | ||||
| ypT2 | 203 | 1.21 (0.93–1.57) | 0.15 | 1.21 (0.93–1.58) | 0.16 |
| ypT3 | 34 | 1.46 (0.95–2.25) | 0.08 | 1.56 (1.01–2.41) | 0.04 |
| ypN stage by AJCC 8th edition | 0.00 | 0.00 | |||
| Negative (ypN0, reference) | 175 | ||||
| 1–3 positive nodes (ypN1) | 141 | 1.54 (1.17–2.04) | 0.002 | 1.47 (1.12–1.93) | 0.006 |
| ≥ 4 positive nodes (ypN2) | 73 | 2.58 (1.88–3.53) | 0.00 | 2.56 (1.86–3.52) | 0.00 |
Nine patients with ypT0 stage were not included in the analyses as the number of patients in this category was too small.
Abbreviations: HR, Hazard ratio; CI, confidence interval; AJCC, American Joint Committee for Cancer
Discussion
Pathologic staging is the most significant prognostic parameter in patients with malignant tumors and provides the basis for management decisions to a great extent. Tumor size has long been recognized as a prognostic parameter for patients with PDAC and has been included in the TNM staging system (25–27). The AJCC 7th edition pT1 and pT2 are tumors limited to the pancreas, and pT3 is defined as a tumor of any size with extrapancreatic extension or involvement of adjacent structures. Since the pancreas does not have a true capsule, the extension into the peripancreatic soft tissue is loosely defined and shows significant inter-observer variability which has been reflected in the frequency of tumor invasion into peripancreatic adipose tissue of the resected PDAC cases in previous publications (20, 28). Tumor invasion into peripancreatic adipose tissue was reported in 37% (51/138) cases in a study by Jamieson et al (28). In another study, Saka et al. examined 223 consecutive PD specimens with PDAC processed by a uniform grossing protocol and reported tumor invasion into peripancreatic adipose tissue in 91% of their cases (20).
In our previous study of neoadjuvant treated PDAC, 91% of cases was classified as ypT3 using AJCC Staging Manual, 7th edition (4). The identification of tumor extension into peripancreatic soft tissue, in the absence of a true capsule, is arbitrarily based on the histologic finding of the absence of non-neoplastic pancreatic parenchyma at the tumor edge. This scenario could even be greater in the setting of postneoadjuvant resections, where there is often an associated atrophy of the non-neoplastic pancreatic parenchyma. Since vast majority of resected PDAC cases are classified as pT3/ypT3 based on AJCC Staging Manual, 7th edition, the utility of the current T-stage grouping system is greatly reduced and does not stratify the patients effectively. A few studies have attempted to further classify the current stage T3 tumors into more meaningful groups; Park et al (21) showed that patients with tumors ≤ 2 cm, with extrapancreatic extension, had a significant survival benefit over those with tumors that were > 2 cm, with extrapancreatic extension. In a study by Saka et al (4), a size-based T-stage protocol was devised that defined pT1 as ≤ 2 cm, pT2 as > 2–4 cm, and pT3 as > 4 cm. This revised protocol was tested in a large cohort and showed a very good relationship with survival (20, 21). The upcoming AJCC 8th edition pT staging system is size-based and is proposed to be an improvement over the 7th edition system, in terms of its prognostic significance, on the basis of studies of PDAC patients who underwent upfront PD, without neoadjuvant therapy. In this study, we examined the prognostic significance of ypT staging system based on 7th edition and the new ypT staging system (8th edition) in a large cohort of 398 PDAC patients who underwent PD after receiving neoadjuvant therapy. Using the new size-based criteria for PDAC, more than 90% of the tumors that were classified as ypT3 using the 7th edition AJCC Staging Manual were reclassified as ypT1 (36.0%) and ypT2 (54.5%). Only 9.5% of our cases were ypT3 based on the new tumor stage grouping. The new ypT stage performed better than the current ypT stage grouping in predicting both DFS and OS in our patient population in that the patients with new ypT1 disease had significantly better DFS and OS than those with new ypT2 and new ypT3 disease. By multivariate analysis, new ypT3 was a significant predictor for poor OS (p=0.04). Our results were similar to the previous studies which showed that the new pT stage grouping was a significant prognostic factor for survival in patients who underwent upfront resection for PDAC (20, 21, 29). In addition, the new ypT-stage correlated with lymph node metastasis (p < 0.001) and tumor regression grade (p < 0.001) among all study patients.
In our study population, all 9 cases with a complete pathologic response (ypT0) had no recurrence of disease or any mortality due to disease. Cases with tumors < 1 cm (ypT1a and ypT1b combined) showed a significant longer OS (p = 0.03) and longer DFS (p = 0.046) compared to those ≥ 1 cm. However, there was no statistically significant difference between ypT1c and ypT2 or between ypT2 and ypT3. Therefore, the results of our study suggest that a tumor size cut-off of 1.0 cm for ypT2 is more useful in predicting survival than is 2.0 cm for PDAC patients who received PD after neoadjuvant therapy. This could be in part due to the fact that neoadjuvant therapy may reduce the tumor size and the difference in tumor biology after neoadjuvant treatment. In our study, we could not establish any difference between ypT1a and ypT1b. This could be due to the small sample size in these groups (16 cases [4%] for pT1a and 14 cases [3.5%] for pT1b, respectively).
While the aim of introducing the new tumor staging system (AJCC 8th edition) was to achieve better reproducibility, the major challenge to apply this new tumor stage system to the cohort of neoadjuvant treated cases is the difficulty to assess the gross tumor size accurately, since neoadjuvant therapy often leads to marked fibrosis which typically involves both the tumor and the adjacent non-neoplastic parenchyma. The assessment of tumor size is sometimes arbitrary with the inherent problems of gross tumor identification. In some cases, there is no grossly identifiable tumor after neoadjuvant therapy. Therefore systematic approach to generously sample the possible tumor area, the adjacent pancreas/soft tissue and/or adjacent organ(s) is critical to accurately measure and to validate the tumor size based on histologic examination. In our study, we used this approach to measure the tumor size.
In conclusion, our study is the first in applying the new AJCC 8th edition tumor stage in PDAC to a large cohort of patients who received neoadjuvant therapy. We found that new ypT stage grouping performed better than the AJCC 7th edition ypT stage grouping in predicting the prognosis and correlated with lymph node metastasis and tumor regression grade. Our study suggest that a tumor size cut-off of 1.0 cm for ypT2 is a more accurate prognostic indicator than is 2.0 cm as proposed in AJCC Staging Manual (8th edition) for PDAC patients who underwent PD after neoadjuvant therapy.
Acknowledgments
Supported by the National Institutes of Health grant (1R01 CA196941-01A1) and Khalifa Bin Zayed Al Nahyan Foundation Institute for Pancreatic Cancer Research at The University of Texas M. D. Anderson Cancer Center
The authors would like to thank Eric M. Wang for proof reading the manuscript.
Footnotes
Disclosure: The authors have no conflicts of interest or funding to disclose
References
- 1.Are C, Chowdhury S, Ahmad H, et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol. 114:736–742. doi: 10.1002/jso.24410. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Mirkin KA, Hollenbeak CS, Wong J. Survival impact of neoadjuvant therapy in resected pancreatic cancer: A Prospective Cohort Study involving 18,332 patients from the National Cancer Data Base. Int J Surg. 34:96–102. doi: 10.1016/j.ijsu.2016.08.523. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 118:3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SM, Katz MH, Liu L, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40:1653–1660. doi: 10.1097/PAS.0000000000000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. doi: 10.1016/j.anndiagpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee D, Rashid A, Wang H, et al. Tumor invasion of muscular vessels predicts poor prognosis in patients with pancreatic ductal adenocarcinoma who have received neoadjuvant therapy and pancreaticoduodenectomy. Am J Surg Pathol. 2012;36:552–559. doi: 10.1097/PAS.0b013e318240c1c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–277. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 10.Fischer LK, Katz MH, Lee SM, et al. The number and ratio of positive lymph nodes affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology. 2016;68:210–220. doi: 10.1111/his.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Katz MH, Lee SM, et al. Superior Mesenteric Artery Margin of Posttherapy Pancreaticoduodenectomy and Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Am J Surg Pathol. 2015;39:1395–1403. doi: 10.1097/PAS.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 12.Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:1168–1175. doi: 10.1245/s10434-014-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:3801–3811. doi: 10.1002/cncr.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge S, Byrd D, Compton C, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 15.Merkel S, Mansmann U, Meyer T, et al. Confusion by frequent changes in staging of exocrine pancreatic carcinoma. Pancreas. 2004;29:171–178. doi: 10.1097/00006676-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Jouffret L, Turrini O, Ewald J, et al. Long-term survivors after pancreatectomy for cancer: the TNM classification is outdated. ANZ J Surg. 85:860–864. doi: 10.1111/ans.13277. [DOI] [PubMed] [Google Scholar]
- 17.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 29:127–141. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 265:185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saka B, Balci S, Basturk O, et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: </=2, pT2: >2–</=4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol. 2016;23:2010–2018. doi: 10.1245/s10434-016-5093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H, An S, Eo SH, et al. Survival effect of tumor size and extrapancreatic extension in surgically resected pancreatic cancer: proposal for improved T classification. Hum Pathol. 45:2341–2346. doi: 10.1016/j.humpath.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Edge S, Greene F, et al., editors. AJCC Cancer Staging Manual. New York: Springer International Publishing; 2017. [Google Scholar]
- 23.Chatterjee D, Katz MH, Rashid A, et al. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63:841–851. doi: 10.1111/his.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 28:1185–1201. doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 25.Bittner R, Roscher R, Safi F, et al. Effect of tumor size and lymph node status on the prognosis of pancreatic cancer. Chirurg. 1989;60:240–245. [PubMed] [Google Scholar]
- 26.Hermanek P. Staging of exocrine pancreatic carcinoma. Eur J Surg Oncol. 1991;17:167–172. [PubMed] [Google Scholar]
- 27.Hermanek P. Pathology and biology of pancreatic ductal adenocarcinoma. Langenbecks Arch Surg. 1998;383:116–120. doi: 10.1007/s004230050102. [DOI] [PubMed] [Google Scholar]
- 28.Jamieson NB, Foulis AK, Oien KA, et al. Peripancreatic fat invasion is an independent predictor of poor outcome following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg. 15:512–524. doi: 10.1007/s11605-010-1395-4. [DOI] [PubMed] [Google Scholar]
- 29.Marchegiani G, Andrianello S, Malleo G, et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann Surg. doi: 10.1097/SLA.0000000000001837. [DOI] [PubMed] [Google Scholar]