Abstract
Oncoproteomics is playing an increasingly important role in the diagnosis and management of cancer as well as in the development of personalized treatment of cancer. Innovative proteomic technologies relevant to cancer are described briefly, which are helping in the understanding of mechanism of drug resistance in cancer and will provide some leads to improve the management. Most important of these are nanoproteomics, i.e. application of nanobiotechnology to proteomics is playing an important role in nanooncology. Examples of some cancers will be given to point out the challenges and future prospects of oncoproteomics including those involving translation of technologies from the bench to the bedside.
Keywords: Cancer biomarkers, Nanobiotechnology, Nanooncology, Oncoproteomics, Personalized medicine, Proteomics
1. Introduction
Oncoproteomics refers to the application of proteomic technologies in oncology and parallels the related field of oncogenomics (Jain, 2002). Considerable progress has been made during the past few years and oncoproteomics is playing an increasingly important role in the diagnosis and management of cancer (Jain, 2007a; Cho and Cheng, 2007). Use of proteomic technologies for study of cancer is well established. This article focuses on some innovative technologies used for study of molecular biology of cancer, detection of cancer biomarkers and their application in molecular diagnosis of cancer as well as anticancer drug discovery. Some of the challenges will be identified and finally the role of oncoproteomics in the development of personalized cancer therapy will be discussed.
2. Innovations in proteomic technologies used in study of cancer
Proteins may be actively secreted or released by the tumor cells as a result of necrosis or apoptosis and released into the circulation, thereby changing the serum protein profile. The difference in signal intensities may be detected by comparison with sera from normal individuals. A large number of technologies are used in proteomics and have been described in detail elsewhere (Jain, 2008a). Classical technologies such as 2D gel electrophoresis are still being used and several innovations of mass spectrometry have been introduced. Some proteomic technologies relevant to cancer, particularly for detection of biomarkers, are
-
■
nanoproteomics,
-
■
antibody microarrays,
-
■
aptamer‐based molecular probes,
-
■
cancer immunomics to identify
-
■
autoantibody signatures,
-
■
tissue microarrays.
2.1. Nanoproteomics
Nanoproteomics – application of nanobiotechnology to proteomics – improves on most current protocols including protein purification/display and automated identification schemes that yield unacceptably low recoveries with reduced sensitivity and speed while requiring more starting material. Low abundant proteins and proteins that can only be isolated from limited source material (e.g. biopsies) can be subjected to nanoscale protein analysis – nano‐capture of specific proteins and complexes, and optimization of all subsequent sample handling steps leading to mass analysis of peptide fragments. This is a focused approach, also termed targeted proteomics, and involves examination of subsets of the proteome, e.g. those proteins that are either specifically modified, or bind to a particular DNA sequence, or exist as members of higher order complexes, or any combination thereof.
Nanooncology, application of nanobiotechnology to cancer, is playing an important role in advances in oncology (Jain, 2008b). Nanobiotechnologies have refined molecular diagnostics and proteomics thus enabling discovery of biomarkers of cancer as well as early detection of tumors. Nanomaterials provide a solution to many of the technical challenges in proteomics and protein based molecular diagnostics (Johnson et al., 2008). Use of nanoscale devices for proteomics, e.g. nanofluidics and nanoarrays will improve the study of oncoproteomics. Low abundant proteins and proteins that can only be isolated from limited source material (e.g. biopsies) can be subjected to nanoscale protein analysis – nano‐capture of specific proteins and complexes, and optimization of all subsequent sample handling steps leading to mass analysis of peptide fragments with individual protein identification sensitivity at the low zeptomole level. Nanoproteomics can be used to identify how genetic determinants of cancer alter cellular physiology and response to agonists. It will thus facilitate the development of personalized therapy for cancer.
2.2. Antibody microarrays
The use of antibody microarrays continues to grow rapidly due to the recent advances in proteomics and automation, and the opportunity this combination creates for high‐throughput multiplexed analysis of protein biomarkers. Antibody arrays enable simultaneous measurement in a single sample of many proteins that function in pathways and networks in cancer (Kopf and Zharhary, 2007). However, a primary limitation of this technology is the lack of PCR‐like amplification methods for proteins. Therefore, to realize the full potential of array‐based protein biomarker screening it is necessary to construct assays that can detect and quantify protein biomarkers with very high sensitivity, in the femtomolar range, and from limited sample quantities. Scientists at BioForce Nanosciences Inc have described the construction of ultramicroarrays, combining the advantages of microarraying including multiplexing capabilities, higher throughput and cost savings, with the ability to screen very small sample volumes (Nettikadan et al., 2006). Antibody ultramicroarrays for the detection of IL‐6 and PSA, a widely used biomarker for prostate cancer screening, were constructed. These ultramicroarrays were found to have a high specificity and sensitivity with detection levels using purified proteins in the attomole range.
2.3. Aptamer‐based molecular probes for cancer proteins
Aptamers can bind to a given ligand with high affinity and specificity due to their particular 3D structure and thereby antagonize the biological function of the ligand. Aptamers, because of the tendency of short DNA to fold into shapes that bind to specific proteins, are used to detect protein signatures of cells. The technology can be used with biochips and may provide a method for monitoring protein changes in the blood as an indication of development of carcinogenesis, for example in women with genetic risk of breast cancer associated with BRCA1 dysfunction.
Use of a two‐step strategy, aptamer selection and biomarker discovery, combined with mass spectrometry, has enabled identification of protein tyrosine kinase 7 (PTK7) as a biomarker for T‐cell acute lymphoblastic leukemia (Shangguan et al., 2008). Aptamers for leukemia cells were selected using the cell‐SELEX (Systematic Evolution of Ligands by Exponential Enrichment) process, without any prior knowledge of the cell biomarker population, conjugated with magnetic beads and then used to capture and purify their binding targets on the leukemia cell surface. This two‐step strategy thus substantially improves the effectiveness of biomarker discovery and will facilitate the development of diagnostic tools and therapeutic approaches to cancer.
2.4. Cancer immunomics to identify autoantibody signatures
The increased incidence of autoantibodies in cancer is well known. Cancer immunomics has been used to identify autoantibody signatures produced in response to the presence of either breast or colorectal cancer. SERological proteome analysis (SERPA) is performed by 2D GE, immunoblotting, image analysis, and MS (Hardouin et al., 2007). Alternatively, to identify the antigens recognized by the autoantibodies of cancer patients, an approach has been developed that combines 2D immunoaffinity chromatography, enzymatic digestion of the isolated antigens, nanoflow separation of the resulting peptides, and identification: MAPPing (multiple affinity protein profiling). By these approaches both proteins recognized by autoantibodies independently of a cancer status are identified as well as a limited number of proteins reacting preferentially with cancer sera.
2.5. Tissue microarrays
A tissue microarray (TMA) is a high‐throughput technology for the analysis of molecular biomarkers in oncology. Tissue microarrays enable analysis of up to 1000 tissue samples in a single microscopic slide. TMAs are very useful for the study of tumor biology, development of diagnostic tests, and detection of cancer biomarkers (Voduc et al., 2008). TMAs are a useful tool for oncoproteomics. Tissue proteomics can be combined with laser capture microdissection. Direct tissue proteomics can be used to identify proteins directly from formalin‐fixed paraffin‐embedded prostate cancer tissue samples (Hwang et al., 2007). TMA technology has been applied for testing of breast carcinoma for human epidermal growth factor receptor 2 (HER2) status and compares favorably with immunohistochemical and fluorescent in situ hybridization (Drev et al., 2008).
3. Proteomic biomarkers of cancer
Biomarkers are characteristic that can be objectively measured and evaluated as an indicator to the pathological process or response to a therapeutic intervention. A cancer biomarker is defined as any measurable specific molecular alteration of a cancer cell either on DNA, RNA, protein, or metabolite level. Basic characteristics of biomarkers and technologies used for their discovery are described elsewhere (Jain, 2007b). Biomarkers form the basis of several molecular diagnostics of cancer as well as targets for drug discovery. The main clinical applications of cancer biomarkers are for classification of tumors, determination of prognosis and prediction as well as monitoring of response to therapy. Expression of a distinct gene can enable its identification in a tissue with none of the surrounding cells expressing the specific marker. In the past decade, molecular dissection of the cancer cells by means of mRNA expression profiling enabled detailed classification according to tumor subtypes. Proteomic technologies are playing an important role in the discovery of biomarkers of cancer.
4. Role of proteomics in molecular diagnosis of cancer
Proteomic technologies have emerged as an important addition to the genomic and antibody‐based technologies for the diagnosis of cancer. Many of these assays are based on discovery of cancer biomarkers and study of protein patterns. Nanobiotechnology is enabling further refinements of these tests, which will play an important role in the integration of diagnostics with therapeutics for development of personalized treatment of cancer.
4.1. Serum proteome analysis for early detection of cancer
Proteome analysis has been used for the identification of biomarkers or biomarker patterns that may allow for the early diagnosis of cancer. This tool is of special interest, since it allows for the identification of tumor‐derived secretory products in serum or other body fluids. In addition, it may be used to detect reduced levels or loss of proteins in the serum of cancer patients that are present in noncancer individuals. These changes in the serum proteome may result from cancer‐specific metabolic or immunological alterations, which are, at least partly, independent of tumor size or mass, thereby facilitating early discovery (Ebert et al., 2006).
SELDI‐TOF‐MS of platelet extracts for proteomic profiling shows increased amounts of angiogenic regulatory proteins such as VEGF and endostatin in platelet but not in plasma. This is a selective sequestration process and not a simple association with the platelet surface. This novel property of platelets detects human cancers of a microscopic size undetectable by any presently available diagnostic method. This is more inclusive than a single biomarker because it can detect a wide range of tumor types and tumor sizes. Relative changes in the platelet angiogenic profile permit the tracking of a tumor throughout its development, beginning from an early in situ cancer.
4.2. Protein patterns for diagnosis of cancer
Protein patterns offer better diagnostic possibilities than a single biomarker and use of proteomic patterns is well established for diagnosis of several cancers. By this approach, the pattern itself, independent of the identity of the proteins or peptides, is the discriminator, and might represent a new diagnostic paradigm. The use of machine learning techniques such as decision trees, neural networks, and various algorithms has been the basis for pattern determination. Cancer is known to involve signaling pathways that are regulated through post‐translational modification of proteins, which are detectable with high confidence by high‐resolution MS. Data generated by use of a prOTOF MS on samples from patients with ovarian cancer and cutaneous T‐cell lymphoma were used to build models for comparison of peak pairs with a conventional individual peak technique (Liu et al., 2007). The results showed that the peak pairs gave classification equal to or better than the conventional technique that used multiple individual peaks and could be used for identification of important peak pairs involved in the disease process.
Proteomic profiling has potential for diagnosis of early‐stage ovarian cancer. Combining the newly discovered biomarkers of ovarian cancer progression with CA125 has resulted in a clear increase of the sensitivity and should be validated in large ovarian cancer and control groups (Helleman et al., 2007). Such a multimarker assay could be suitable for disease monitoring during and after therapy and might also be useful for ovarian cancer screening.
4.3. HER‐2/neu oncoprotein as biomarkers for cancer
HER‐2/neu oncoprotein has been widely studied for many years and has been shown to play a pivotal role in the development and progression of breast cancer. HER‐2/neu has been shown to be an indicator of poor prognosis with patients exhibiting aggressive disease, decreased overall survival and a higher probability of recurrence of disease. As evidenced by numerous published studies, elevated levels of HER‐2/neu (also referred to as overexpression) are found in about 30% of women with breast cancer. Determination of a patient's HER‐2/neu status may be valuable in identifying whether that patient has a more aggressive disease and would, thus, derive substantial benefit from more intensive or alternative therapy regimens. Elevated levels of HER‐2/neu are found not only in breast cancer but also in several other tumor types including prostate, lung, pancreatic, colon and ovarian cancers.
Traditional HER‐2/neu testing is generally limited to tissue from primary breast cancer and does not provide information regarding the HER‐2/neu status in women with recurrent, metastatic breast cancer. The introduction of microtiter plate ELISA HER‐2/neu testing using a serum sample offers a less invasive diagnostic tool and provides a current assessment of a woman's HER‐2/neu status over the course of disease. Prechemotherapy serum HER‐2/neu is a significant predictor of response to neoadjuvant anthracycline‐based chemotherapy for breast cancer (Schippinger et al., 2007).
4.4. Proteomic analysis of cancer cell mitochondria
Mutations in mitochondrial DNA have been frequently reported in cancer cells. Significance of gene expression patterns is not established yet. Study of mitochondrial proteome can advance our knowledge of cancer by:
Identification of abnormally expressed mitochondrial proteins in cancer cells is possible by mitochondrial functional proteomics.
Proteomics can identify new biomarkers for early detection and risk assessment, as well as targets for therapeutic intervention.
2D gel electrophoresis examination of the mitochondria‐enriched fraction has revealed high expression of four mitochondrial proteins in a human gastric cancer cell line: ubiquinol‐cytochrome c reductase, mitochondrial short‐chain enoyl‐coenzyme A hydratase‐1, heat shock protein 60, and mitochondrial elongation factor Tu (Kim et al., 2007). These findings provide clues to the mechanism of the mitochondrial changes in cancer at the protein level and may serve as potential cancer biomarkers in mitochondria.
5. Role of proteomics in drug discovery for cancer
Proteomic technologies are being used in an effort to correct some of the deficiencies in traditional drug discovery. Proteins are important targets for drug discovery, particularly for cancer as well because there is a defect in the protein machinery of the cell in malignancy. Because proteome analysis can produce comprehensive molecular description of the differences between normal and diseased states, it can be used to compare the effect of candidate drugs on the disease process. The trend now is to integrate oncoproteomics with oncogenomics for drug discovery and target validation in oncology (Jain, 2005). Human saliva proteomics may be useful for anticancer drug discovery as it contributes to the target discovery/validation, assessment of efficacy as well as toxicity of candidate drugs, identification of disease subgroups, and prediction of responses of individual patients (Hu et al., 2007).
Among the large number of proteomic technologies available for anticancer drug discovery, the most important ones are 3D protein structure determination, protein biochips, laser capture microdissection and study of protein–protein and protein–drug interactions. Cancer biomarkers and signaling pathways involved in malignancy are important drug targets. The wealth of new information in proteomics databases, along with microarrays and bioinformatics, provides unlimited possibilities for designing new therapeutic agents for cancer.
6. Proteomics and drug resistance in cancer
One of the major problems in chemotherapy of cancer is the development of resistance to anticancer drugs. In multidrug resistance (MDR), treatment with one anticancer drug leads to cross‐resistance with a wide range of other drugs. These MDR cells express frequently plasma transport proteins like P‐glycoprotein. But cellular resistance to chemotherapy is multifactorial and may be affected by the cell cycle stage and proliferation status, biochemical mechanisms such as detoxification, cellular drug transport, or DNA replication and repair mechanisms. Several laboratory techniques, such as polymerase chain reaction, immunocytochemistry, flow cytometry, blotting, and fluorescent microscopy have been used for the identification of MDR markers and mechanisms.
Advances in proteomic technologies have enabled the identification of multiple proteins involved in drug‐resistant cancers. 2D GE, followed by image analysis and MS can lead to the identification of proteins differentially expressed between resistant versus sensitive cells. This might lead to the development of various strategies to modify the action of such proteins when their inappropriate structure or expression is contributing to drug‐resistant disease.
Mutations in epithelial growth factor receptor (EGFR) have been identified as a clinical correlation to objective response to gefitinib in patients with lung cancer but it does not indicate resistance to gefitinib. Epithelial membrane protein‐1, an adhesion molecule, is a surface biomarker whose expression correlates with acquisition of gefitinib resistance. Combined analyses of multiple proteomic studies of various drug‐resistant cancer cell lines have revealed that many mechanisms of resistance likely exist in any given drug‐selected cancer cell line and that common mechanisms of resistance may be selected in a spectrum of cancer cell lines (Zhang and Liu, 2007). These observations suggest that combination therapies targeting multiple mechanisms to sensitize drug‐resistant cancers may be necessary to eradicate cancers in the future.
7. Oncoproteomics and personalized management of cancer
An emerging concept for improving healthcare is personalized or individualized medicine, which simply means the prescription of specific treatments and therapeutics best suited for an individual's genotype but can be based on various other factors that influence the disease outcome and response to treatment. Developments in oncoproteomics will enable development of personalized therapy of cancer (Jain, 2008c). The impact of proteomics on cancer will not be limited to the identification of new biomarkers for early detection and new targets. Proteomic technologies will be used to design rational drugs according to the molecular profile of the cancer cell and thus facilitate the development of personalized cancer therapy. Relation of oncoproteomics to personalized medicine is shown schematically in Figure 1. Nanobiotechnology will have an impact on the development of personalized therapy of cancer. Examples of proteomic technologies used for personalizing cancer therapy are shown in Table 1.
Figure 1.
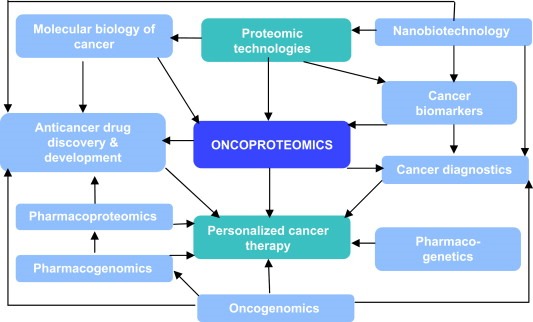
This figure shows the interrelationships of oncoproteomics with other technologies used. Oncogenomics plays an important role by diagnostics as well as drug discovery, as both are components of personalized medicine. Technologies such as nanobiotechnology can also enhance both diagnostics and drug development for cancer.
Table 1.
Examples of proteomic technologies used for personalizing cancer therapy
| Technologies | Applications |
|---|---|
| Glycoproteomics: the biochemical signature of cancer on cell membrane in the form of glycoproteins | Targeted cancer therapy against molecular targets expressed in tumors |
| Proteomic approaches plus biochemical assays to identify the target antigen | Antibody‐based therapies for cancer |
| Mass spectrometry‐based fingerprints of peptides/proteins plus laser capture microdissection and high density protein arrays | Proteomic profiling/molecular classification of human tumors |
| Determination of structure of protein kinases: important drug targets in cancer | Screening for personalized anticancer drugs |
| Reverse phase protein microarrays provide a functional map of known cell‐signaling networks or pathways (Espina et al., 2008) | Correlation of pathways with biological and clinical information for an individual patient obtained directly from a cancer biopsy specimen for personalization of therapy |
©Jain PharmaBiotech.
8. Role of proteomics in cancers of various organs
The role of proteomics in diagnosis, therapy as well as combination of both and in development of personalized approaches will be described with examples of breast cancer, colorectal cancer, leukemia, lung cancer and prostate cancer.
8.1. Breast cancer and proteomics
An example of combination of diagnostics and therapeutics in management of breast cancer is the human epidermal growth factor receptor‐2 (HER2) gene, which is amplified in 20–30% of breast cancers. HER2 overexpression is associated with response to Herceptin (Genentech) and its lack with resistance to therapy. HER‐2/neu status of the primary breast cancer is determined by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH). HercepTest (DakoCytomation), an approved IHC test, is used to identify patients with breast cancer whose tumor tissue overexpresses the HER2 protein. HER2 FISH pharmDx™ Kit is designed to quantitatively determine interphase HER2/neu gene amplification. Mammary5 trial by the National Cancer Institute of Canada showed that amplification of HER2 in breast cancer cells is associated with better clinical responsiveness to anthracycline‐containing chemotherapy regimen when compared with the regimen of cyclophosphamide, methotrexate, and fluorouracil (Pritchard et al., 2006). However, due to technical factors, tests used for primary breast cancer may not accurately reflect the metastatic tumor in terms of HER‐2/neu status. Guidelines recommend that tumors should be defined as HER‐2/neu positive if 30% or more of the cells are 3+. Circulating levels of the HER‐2 extracellular domain can be measured in serum using an approved test and increased serum HER‐2/neu levels to above 15ng/ml reflect breast cancer progression (Carney et al., 2007).
8.2. Colorectal cancer and proteomics
Current screening methods for colorectal cancer (CRC) such as fecal occult blood test and colonoscopy have contributed to early detection and reduction of mortality but most of the carcinomas are still detected at a later stage when prognosis is poor. Proteomic technologies enable a distinction between the healthy patient and the cancer patient with high sensitivity and specificity and could greatly improve early detection and classification systems for CRC (Habermann et al., 2008). A suitable method for detecting new serum biomarkers of CRC by serum protein profiling using SELDI‐TOF MS followed by classification of tree pattern analysis has been described (Engwegen et al., 2006). These biomarkers have a potential role in monitoring the disease as well as the treatment. However, there is still a need to identify panels of predictive markers to improve response rates and decrease toxicity with the ultimate aim of tailoring treatment to an individual patient and tumor profile. Use of proteomic technologies for CRC remains to be transferred from the bench to the bedside.
Metastasis is a common phenomenon and the major lethal cause of CRC. Differential proteome analysis of two CRC cell lines was conducted using 2D GE combined with matrix‐assisted laser desorption/time of flight (MALDI‐TOF) MS (Zhao et al., 2007). Obvious differences were detected between the protein profiles of colorectal cell lines with varying potential for metastasis. Overexpression of heat shock protein (HSP) 27 was shown to play an important role in metastasis and progression of CRC.
8.3. Leukemia and proteomics
Proteomics is being used to subclassify leukemia, because cytogenetic analysis is costly and time‐consuming. Several useful protein biomarkers have been discovered that can rapidly identify different types of leukemia: alpha‐enolase, RhoGDI2, annexin A10, catalase, peroxiredoxin 2, tromomyosin 3, and annexin 1. These are differentially expressed in acute myeloid leukemia (AML) and can be used as biomarkers for disease prognosis and outcome (Lopez‐Pedrera et al., 2006). They provide potential new targets for rational pathogenesis‐based therapies of AML.
Despite enormous therapeutic efforts that range from various cytotoxic agents to allogeneic stem cell transplantation, overall survival of patients with acute myeloid leukemia (AML) remains unsatisfactory. There is hope that elucidation of the AML‐specific proteome will prompt the discovery of novel therapeutic targets and biomarkers in AML, which will enable the development of personalized treatment of AML. One major challenge is limitations in protein detection sensitivity, which presents a problem as many of the regulatory proteins that are pivotal in response to therapy occur in low abundance (Gjertsen and Sjoholt, 2008). Ongoing developments in proteomic technologies are expected to open new avenues for personalized molecular therapy of AML and improve efficacy and reduce the toxicity of current treatment.
8.4. Lung cancer and proteomics
Currently, clinical and pathologic staging of lung cancer is suboptimal for achieving the goals of assessing prognosis and selecting therapy. Major progress in understanding the molecular basis of lung cancer has been made due to technical developments in proteomic and genomic analyses and their application to diagnosis and prognosis of lung cancer. Exhaled breath condensate collection is a simple and noninvasive technique, which enables the study of a wide variety of biomarkers including proteins by sampling respiratory tract fluid and may be applied to the detection of lung cancer (Conrad et al., 2008). A panel of four serum proteins (carcinoembryonic antigen, retinol binding protein, alpha1‐antitrypsin, and squamous cell carcinoma antigen) is valuable in suggesting the diagnosis of lung cancer may be useful for detecting individuals at high risk for lung cancer (Patz et al., 2007). If a reliable protein profile can be identified that is associated with poor prognosis, it may provide potential therapeutic targets. The development of a simple serum test for diagnosis of lung cancer before clinical manifestation is feasible and may simultaneously identify the chemotherapeutic agents to which the tumor is sensitive, enabling personalized treatment (D'Amico, 2008). There is a need for further improvements in sample preparation techniques and translating the technologies from the laboratory to the bedside before the impact on improvement of care of patients with lung cancer that can be assessed properly.
8.5. Prostate cancer and proteomics
With the advent of molecular targeted therapeutics, biomarkers for identification, characterization and monitoring of the signaling events within actual human biopsies will be critical for patient‐tailored therapy. Although several non‐2D gel electrophoresis technologies are used for research in proteomics of prostate cancer, 2D gel electrophoresis remains the most powerful method for analysis of cellular protein phenotype and may reveal gene regulations that cannot be detected on a genetic level.
Gene expression studies of prostate cancer have shown androgen‐regulated genes such as prostate‐specific antigen (PSA) and several novel complementary DNAs. Protein expression profiles of androgen‐stimulated prostate cancer cells have been generated by 2D gel electrophoresis. Quantification of the number of PSA molecules/cell has been conducted on human prostate tissue cells by coupling of LCM with sensitive quantitative chemiluminescent immunoassays. A proteomics‐based approach is useful for developing a more complete picture of the protein profile of prostate needle biopsy specimens, and changes in FLNA(7‐15), FKBP4, and PRDX4 expression have been confirmed by immunoblot analyses (Lin et al., 2007). This approach may provide useful molecular targets for diagnosis and treatment of prostate cancer.
9. Integration of oncoproteomics with other technologies
Proteomic technologies are used in cancer in conjunction with many other technologies including oncogenomics. Proteomics is facilitating the integration of diagnostics and therapeutics and fulfilling many of the requirements for personalized therapy of cancer. Nanobiotechnology is contributing to the development of nanoproteomics, which has important applications in nanooncology and will facilitate the development of personalized management of cancer, which has already started. Nanoparticles can be used for diagnostics as well as therapeutics.
10. Challenges and future prospects
The next 5 years will see important developments in cancer biomarkers and their validation. Several proteomics‐based tests for cancer detection, prognosis and for guidance of anticancer therapy are expected to become available. An important challenge will be validation of cancer biomarkers and to sort out from the plethora of biomarkers being discovered. Several anticancer drug development projects are anticipated based on cancer biomarker discovery. Many promising discoveries in the laboratory have not yet been translated to the bedside of the patient. Personalized management of cancer faces some challenges but will be established within the next decade.
Financial disclosure/Acknowledgements
The author has no financial involvement in any of the technologies, products or companies mentioned in this publication. There is no conflict of interest.
Jain Kewal K., (2008), Innovations, challenges and future prospects of oncoproteomics, Molecular Oncology, 2, doi: 10.1016/j.molonc.2008.05.003.
References
- Carney, W.P. , Leitzel, K. , Ali, S. , Neumann, R. , Lipton, A. , 2007. HER-2/neu diagnostics in breast cancer. Breast Cancer Res. 9, 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, W.C. , Cheng, C.H. , 2007. Oncoproteomics: current trends and future perspectives. Expert Rev. Proteomics 4, 401–410. [DOI] [PubMed] [Google Scholar]
- Conrad, D.H. , Goyette, J. , Thomas, P.S. , 2008. Proteomics as a method for early detection of cancer: a review of proteomics, exhaled breath condensate, and lung cancer screening. J. Gen. Intern. Med. 23, (Suppl. 1) 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico, T.A. , 2008. Molecular biologic staging of lung cancer. Ann. Thorac. Surg. 85, S737–S742. [DOI] [PubMed] [Google Scholar]
- Drev, P. , Grazio, S.F. , Bracko, M. , 2008. Tissue microarrays for routine diagnostic assessment of HER2 status in breast carcinoma. Appl. Immunohistochem. Mol. Morphol. 16, 179–184. [DOI] [PubMed] [Google Scholar]
- Ebert, M.P. , Korc, M. , Malfertheiner, P. , Rocken, C. , 2006. Advances, challenges, and limitations in serum-proteome-based cancer diagnosis. J. Proteome. Res. 5, 19–25. [DOI] [PubMed] [Google Scholar]
- Engwegen, J.Y. , Helgason, H.H. , Cats, A. , Harris, N. , Bonfrer, J.M. , Schellens, J.H. , Beijnen, J.H. , 2006. Identification of serum proteins discriminating colorectal cancer patients and healthy controls using surface-enhanced laser desorption ionisation-time of flight mass spectrometry. World J. Gastroenterol. 12, 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina, V. , Wulfkuhle, J. , Calvert, V.S. , Liotta, L.A. , Petricoin, E.F. , 2008. Reverse phase protein microarrays for theranostics and patient-tailored therapy. Methods Mol. Biol. 441, 113–128. [DOI] [PubMed] [Google Scholar]
- Gjertsen, B.T. , Sjoholt, G. , 2008. Proteomic strategies of therapeutic individualization and target discovery in acute leukemia. In Daoud S.S., Cancer Proteomics Humana Press; Totowa, New Jersey: 161–187. [Google Scholar]
- Habermann, J.K. , Bader, F.G. , Franke, C. , Zimmermann, K. , Gemoll, T. , Fritzsche, B. , Ried, T. , Auer, G. , Bruch, H.P. , Roblick, U.J. , 2008. From the genome to the proteome-biomarkers in colorectal cancer. Langenbecks Arch. Surg. 393, 93–104. [DOI] [PubMed] [Google Scholar]
- Hardouin, J. , Lasserre, J.P. , Sylvius, L. , Joubert-Caron, R. , Caron, M. , 2007. Cancer immunomics: from serological proteome analysis to multiple affinity protein profiling. Ann. N Y Acad. Sci. USA 1107, 223–230. [DOI] [PubMed] [Google Scholar]
- Helleman, J. , van der Vlies, D. , Jansen, M.P. , Luider, T.M. , van der Burg, M.E. , Stoter, G. , Berns, E.M. , 2007. Serum proteomic patterns for ovarian cancer monitoring. Int. J. Gynecol. Cancer 10.1111/j.1525-1438.2007.01139.x [DOI] [PubMed] [Google Scholar]
- Hu, S. , Yen, Y. , Ann, D. , Wong, D.T. , 2007. Implications of salivary proteomics in drug discovery and development: a focus on cancer drug discovery. Drug Discov. Today 12, 911–916. [DOI] [PubMed] [Google Scholar]
- Hwang, S.I. , Thumar, J. , Lundgren, D.H. , Rezaul, K. , Mayya, V. , Wu, L. , Eng, J. , Wright, M.E. , Han, D.K. , 2007. Direct cancer tissue proteomics: a method to identify candidate cancer biomarkers from formalin-fixed paraffin-embedded archival tissues. Oncogene 26, 65–76. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2002. Recent advances in oncoproteomics. Curr. Opin. Mol. Ther. 4, 203–209. [PubMed] [Google Scholar]
- Jain, K.K. , 2005. Proteomics-based anticancer drug discovery. In LaRochelle W.J., The Oncogenomics Handbook The Humana Press; Totowa, New Jersey: 123–134. [Google Scholar]
- Jain, K.K. , 2007. Recent advances in clinical oncoproteomics. J. BUON 12, (Suppl. 1) S31–S38. [PubMed] [Google Scholar]
- Jain, K.K. , 2007. Cancer biomarkers: current issues and future directions. Curr. Opin. Mol. Ther. 9, 563–571. [PubMed] [Google Scholar]
- Jain, K.K. , 2008. Proteomics: Technologies, Markets and Companies Jain PharmaBiotech Publications; Basel, Switzerland: pp. 1–570 [Google Scholar]
- Jain, K.K. , 2008. Recent advances in nanooncology. Technol. Cancer Res. Treat. 7, 1–13. [DOI] [PubMed] [Google Scholar]
- Jain, K.K. , 2008. Oncoproteomics for personalized management of cancer. In Daoud S.S., Cancer Proteomics Humana Press; Totowa, New Jersey: 81–99. [Google Scholar]
- Johnson, C.J. , Zhukovsky, N. , Cass, A.E. , Nagy, J.M. , 2008. Proteomics, nanotechnology and molecular diagnostics. Proteomics 8, 715–730. [DOI] [PubMed] [Google Scholar]
- Kim, H.K. , Park, W.S. , Kang, S.H. , Warda, M. , Kim, N. , Ko, J.H. , Ael-B, Prince , Han, J. , 2007. Mitochondrial alterations in human gastric carcinoma cell line. Am. J. Physiol. Cell Physiol. 293, C761–C771. [DOI] [PubMed] [Google Scholar]
- Kopf, E. , Zharhary, D. , 2007. Antibody arrays – an emerging tool in cancer proteomics. Int. J. Biochem. Cell Biol. 39, 1305–1317. [DOI] [PubMed] [Google Scholar]
- Lin, J.F. , Xu, J. , Tian, H.Y. , Ga, X. , Chen, Q.X. , Gu, Q. , Xu, G.J. , Song, J.D. , Zhao, F.K. , 2007. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int. J. Cancer 121, 2596–2605. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Shea, N. , Rucker, S. , Harvey, L. , Russo, P. , Saul, R. , Lopez, M.F. , Mikulskis, A. , Kuzdzal, S. , Golenko, E. , 2007. Proteomic patterns for classification of ovarian cancer and CTCL serum samples utilizing peak pairs indicative of post-translational modifications. Proteomics 7, 4045–4052. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrera, C. , Villalba, J.M. , Siendones, E. , Siendones, E. , Barbarroja, N. , Gomez-Diaz, C. , Rodriguez-Ariza, A. , Buendia, P. , Torres, A. , Velasco, F. , 2006. Proteomic analysis of acute myeloid leukemia: identification of potential early biomarkers and therapeutic targets. Proteomics 6, (Suppl. 1) S293–S299. [DOI] [PubMed] [Google Scholar]
- Nettikadan, S. , Radke, K. , Johnson, J. , Xu, J. , Lynch, M. , Mosher, C. , Henderson, E. , 2006. Detection and quantification of protein biomarkers from fewer than ten cells. Mol. Cell. Proteomics 5, 895–901. [DOI] [PubMed] [Google Scholar]
- Patz, E.F. , Campa, M.J. , Gottlin, E.B. , Kusmartseva, I. , Guan, X.R. , 2007. Herndon JE 2nd. Panel of serum biomarkers for the diagnosis of lung cancer. J. Clin. Oncol. 25, 5578–5583. [DOI] [PubMed] [Google Scholar]
- Pritchard, K.I. , Shepherd, L.E. , O'Malley, F.P. , Andrulis, I.L. , Tu, D. , Bramwell, V.H. , Levine, M.N. , National Cancer Institute of Canada Clinical Trials Group, 2006. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N. Engl. J. Med. 354, 2103–2111. [DOI] [PubMed] [Google Scholar]
- Shangguan, D. , Cao, Z. , Meng, L. , Mallikaratchy, P. , Sefah, K. , Wang, H. , Li, Y. , Tan, W. , 2008. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 10.1021/pr700894d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippinger, W. , Dandachi, N. , Regitnig, P. , Hofmann, G. , Balic, M. , Neumann, R. , Samonigg, H. , Bauernhofer, T. , 2007. The predictive value of EGFR and HER-2/neu in tumor tissue and serum for response to anthracycline-based neoadjuvant chemotherapy of breast cancer. Am. J. Clin. Pathol. 128, 630–637. [DOI] [PubMed] [Google Scholar]
- Voduc, D. , Kenney, C. , Nielsen, T.O. , 2008. Tissue microarrays in clinical oncology. Semin. Radiat. Oncol. 18, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J.T. , Liu, Y. , 2007. Use of comparative proteomics to identify potential resistance mechanisms in cancer treatment. Cancer. Treat. Rev. 33, 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Liu, L. , Wang, S. , Zhang, Y.F. , Yu, L. , Ding, Y.Q. , 2007. Differential proteomic analysis of human colorectal carcinoma cell lines metastasis-associated proteins. J. Cancer Res. Clin. Oncol. 133, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]


