Abstract
Growth of multiple myeloma cells is controlled by various factors derived from host bone marrow microenvironments. Interaction between multiple myeloma cells and bone marrow stromal cells (BMSCs) plays an important role in the expression of adhesive molecules and secretion of growth factors involved in multiple myeloma (MM) cell growth, survival, and resistance to anticancer drugs. Recently, the possibility of developing novel anti‐cancer therapeutic strategies targeting both MM cells and MM cell–BMSC interactions has been discussed. Here we present data showing that curcumin, a major constituent of turmeric compounds extracted from the rhizomes of the plant Curcuma longa, effectively reduced the growth of MM cells and BMSCs. Upon treatment with curcumin, IL‐6/sIL‐6R‐induced STAT3 and Erk phosphorylation was dramatically reduced in the co‐cultured cells. In addition, curcumin inhibited the production of pro‐inflammatory cytokines and VEGF, factors that are associated with the progression of multiple myeloma, from both MM cells and BMSCs. In a combination treatment with curcumin and bortezomib, IL‐6/sIL‐6R‐induced STAT3 and Erk phosphorylation was effectively inhibited. Moreover, this combination treatment synergistically inhibited the growth of MM cells co‐cultured with BMSCs as compared to controls. Taken together, these results indicate that curcumin potentiates the therapeutic efficacy of bortezomib in MM suggesting this combination therapy to be of value in the clinical management of MM.
Keywords: Bortezomib, Curcumin, Multiple myeloma, Bone marrow stromal cell, Interleukin-6
1. Introduction
Multiple myeloma (MM) is a malignant B‐cell neoplasm that is characterized by the accumulation of malignant plasma cells in the bone marrow (BM) (Sirohi and Powles, 2004; Kyle and Rajkumar, 2008). Growth and survival of MM cells are affected by BM microenvironments, in which bone marrow stromal cells (BMSCs) interact with MM cells (Pagnucco et al., 2004; Podar et al., 2007). Even though conventional therapeutic agents including thalidomide, lenalidomide and dexamethasone can achieve high response rates in patients, these conventional therapeutic agents had little effect on relapsed patients. Recently, bortezomib (Velcade™) was developed as an anticancer drug that is a selective, reversible inhibitor of the catalytic site of the 20S proteasome. Bortezomib affects numerous important cellular signaling pathways in MM (Blade et al., 2005; Popat et al., 2006). To achieve high response rates in relapsed MM patients, various combinations of bortezomib plus conventional agents with additive or synergistic activity have been used successfully in clinical trials (Yang et al., 2003). In order to further improve the outcome of relapsed MM patients, it is essential to investigate novel agents that may potentiate the clinical efficacy of bortezomib.
The search for effective chemopreventive agents derived from natural food substances has identified several plant‐derived compounds with anti‐tumor activity against many different cancers (Landis‐Piwowar et al., 2006; Sarkar and Li, 2006). Curcumin [1,7‐bis‐(4‐hydroxy‐3‐methoxyphenyl‐1,6‐hepta‐diene‐3,5‐dione)] is the major constituent of turmeric compounds extracted from the rhizomes of the plant Curcuma longa found in south and southeast tropical Asia. The biological effect of curcumin has been well characterized in several types of cancers, and in MM, curcumin has been shown to inhibit MM cell proliferation through the inhibition of growth factor receptor signaling pathways and NF‐κB activation (Bharti et al., 2003; Hatcher et al., 2008). However, the effects of curcumin on bone marrow stromal cells (BMSCs) interacting with MM cells in bone marrow microenvironments have not been investigated. In this study, we demonstrated that curcumin is able to induce apoptosis in MM cells accompanied by the activation of apoptosis related proteins via inhibition of cell signaling pathways in MM cells co‐cultured with BMSCs.
2. Results
2.1. Induction of apoptosis in U266 cells by curcumin
To study the apoptotic effect of curcumin on MM cells, we treated U266 cells with different concentrations of this compound (10, 25, 50μM). The results showed that curcumin induced apoptosis by stimulating the cleavage of PARP, and decreasing pro‐caspase 3 levels (Figure 1). Also, curcumin inhibited the expression of the cell cycle related proteins, cyclin D1 and CDK4. In addition, curcumin increased p21 expression, suggesting induction of cell cycle arrest (Figure 1). Taken together, these data indicated that curcumin induced apoptosis in U266 cells via increasing apoptotic protein expression and inhibiting G1‐S cell cycle phase regulated proteins.
Figure 1.
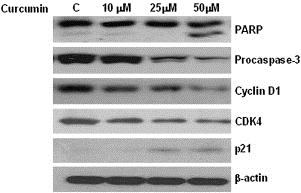
Induction of apoptosis in U266 cells by curcumin. U266 cells were treated with indicated concentrations of curcumin for 24h and whole‐cell extracts were prepared. Then, 30μg of extracts were analyzed by Western blot for PARP, pro‐caspase 3, cyclin D1, CDK4, p21 and β‐actin.
2.2. Effect of curcumin on growth inhibition of MM cells alone or co‐cultured with BMSCs
As curcumin induced apoptosis in MM cells, we further examined its effect on MM cells alone or co‐cultured with BMSCs. As shown in Figure 2, curcumin did not inhibit the proliferation of co‐cultured MM cells when compared to MM cells alone in the first 24h. However, after exposure to curcumin for 72h, the proliferation of MM cells alone or co‐cultured was inhibited in a dose‐dependent manner. RPMI 8226 cells, on the other hand, alone or co‐cultured with BMSCs, were more sensitive to curcumin even at lower doses (10μM) than U266 cells (Figure 2). These findings indicate that curcumin inhibited MM cell growth independently of the presence of BMSC, although there was some protective effect conferred by BMSCs in both cell lines.
Figure 2.
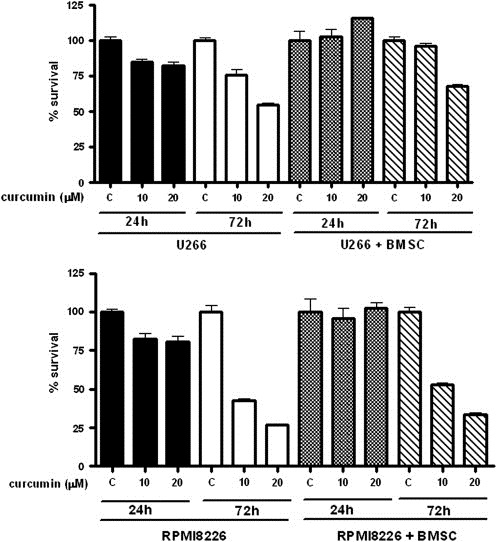
Effect of curcumin on the growth of MM cells with or without the presence of BMSCs. MM cell lines (U266 and RPMI 8226; 5×104/mL) and BMSCs (1×104/mL) were treated with indicated concentrations of curcumin for 24h and 72h and cell proliferation was measured using CCK‐8 cell proliferation assay kit. Data shown are the means±SEs of 3 independent experiments.
2.3. Curcumin inhibited the activation of the JAK/STAT and MAPK pathways through the release of factors by MM patients' BMSCs
To address whether BMSCs interact with MM cells to prolong survival, BMSCs derived from three MM patients' bone marrow were incubated in serum‐free culture media for 96h and the cell culture supernatants (CCSs) were subsequently collected. U266 cells were treated with serially increased volumes of CCSs. As shown in Figure 3A, we observed an enhancement in STAT3 and Erk phosphorylation with increasing volumes of CCSs. Also, we examined whether curcumin inhibited the activation of JAK/STAT and MAPK pathways in the presence of CCSs. We found that curcumin inhibited STAT3 and Erk phosphorylation as compared to controls (CCSs without curcumin) (Figure 3B). These results suggest to us that BMSCs produce various factors that promote MM cell growth; these factors are involved in JAK/STAT and MAPK‐mediated signaling and their biological action can be inhibited by curcumin.
Figure 3.
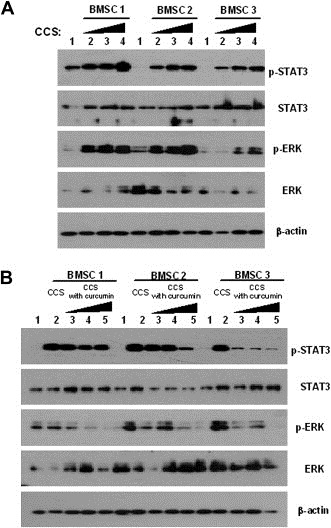
Conditioned media obtained from primary cultured MM patients' BMSCs activated JAK/STAT and MAPK pathways in U266 cells and those effects were blocked by curcumin. (A) Conditioned culture supernatants (CCSs) were collected from BMSCs from three different MM patients after incubation with serum free culture media for 96h. After serum starvation, U266 cells were treated with CCSs for 10min. Lane: 1. control; 2. 0.5mL CCS; 3. 1mL CCS; 4. 2mL CCS. (B) BMSCs were incubated with CCSs and different concentrations of curcumin in serum free culture media for 96h. Lane: 1. control; 2. 1mL CCS; 3. 1mL CCS treated with 5μM curcumin; 4. 1mL CCS treated with 10μM curcumin; 5. 1mL CCS treated with 20μM curcumin.
2.4. Regulation of IL‐6/sIL‐6R‐induced JAK/STAT and MAPK pathway signaling in U266 cells
To investigate the effect of cell signal transduction inhibitors on the IL‐6/sIL‐6R‐induced JAK/STAT and MAPK pathway signaling in MM cells, we pre‐treated U266 cells with various inhibitors, including curcumin and bortezomib, for 1h followed by treatment with either IL‐6 or IL‐6/sIL‐6R. Even though sIL‐6R alone was unable to induce STAT3 and Erk phosphorylation, the combination of IL‐6 and sIL‐6R was more potent in inducing STAT3 and Erk phosphorylation in U266 cells than IL‐6 alone (Figure 4A). Among the agents tested, 6‐amino‐4‐quinazoline and curcumin effectively inhibited IL‐6‐induced STAT3 and Erk phosphorylation. However, curcumin was a more potent inhibitor of STAT3 and Erk phosphorylation than 6‐amino‐4‐quinazoline (Figure 4B and C). These results indicate that sIL‐6R potentiates IL‐6‐induced pathways and curcumin is a more effective inhibitor against IL‐6/sIL‐6R‐induced STAT3 and Erk phosphorylation than the other inhibitors tested.
Figure 4.
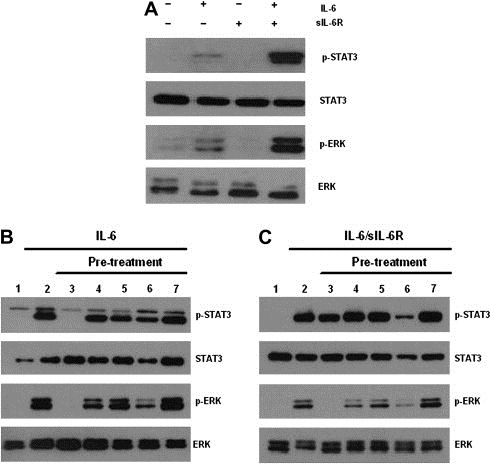
Inhibition of IL‐6/sIL‐6R‐induced JAK/STAT and MAPK pathways by various signal transduction inhibitors, including curcumin and bortezomib in U266 cells. (A) sIL‐6R potentiated IL‐6‐induced JAK/STAT and MAPK pathways. After serum starvation, U266 cells were treated with either IL‐6 or sIL‐6R alone or combined IL‐6 and sIL‐6R for 15min. Lane: 1. control; 2. 5ng/mL IL‐6; 3. 25ng/mL sIL‐6R; 4. 5ng/mL IL‐6 and 25ng/mL sIL‐6R. (B–C) After serum starvation, U266 cells were pretreated with various signal transduction inhibitors for 1h and then stimulated with IL‐6 (5ng/mL) or IL‐6 (5ng/mL) and sIL‐6R (25ng/mL) for 15min and 30μg of whole‐cell extracts were analyzed by Western blot for phosphorylated STAT3, total STAT3, phosphorylated Erk, and total Erk. (B) Lane: 1. control; 2. 5ng/mL IL‐6; 3. 2μM 6‐amino‐4‐quinazoline and 5ng/mL IL‐6; 4. 20μM PD98059 and 5ng/mL IL‐6; 5. 20μM AG490 and 5ng/mL IL‐6; 6. 5μM curcumin and 5ng/mL IL‐6; 7. 1nM bortezomib and 5ng/mL IL‐6. (C) Lane: 1. control; 2. 5ng/mL IL‐6 and 25 ng/mL sIL‐6R; 3. 2μM 6‐amino‐4‐quinazoline, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 4. 20μM PD98059, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 5. 20μM AG490, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 6. 5μM curcumin, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 7. 1nM bortezomib, 5ng/mL IL‐6 and 25ng/mL sIL‐6R.
2.5. Inhibitory effect of curcumin and bortezomib on IL‐6 and sIL‐6R secretion in U266 cells
We also determined the inhibitory effect of curcumin and bortezomib on IL‐6 and sIL‐6R secretion in MM cells. After serum starvation, U266 cells were treated with varying concentrations of bortezomib and curcumin for different periods of time. Release of IL‐6 and sIL‐6R was inhibited by bortezomib, especially at 24h (Figure 5A and B). Curcumin also inhibited the release of IL‐6 and sIL‐6R, but the dose‐dependent inhibition was more notable in the case of sIL‐6R (Figure 5C and D).
Figure 5.
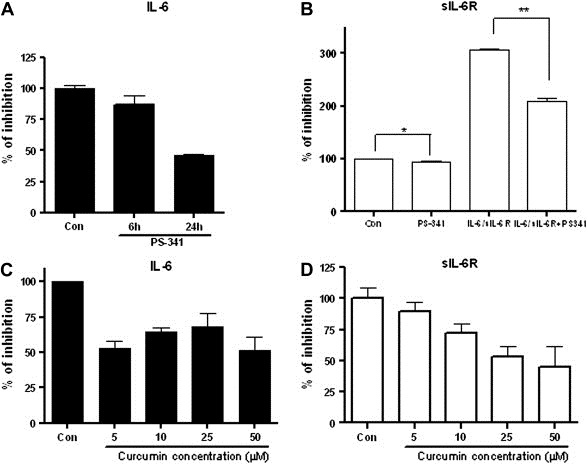
Effect of curcumin and bortezomib on IL‐6 and sIL‐6R secretion in U266 cells. After serum starvation, U266 cells were treated with indicated concentrations of bortezomib and curcumin for indicated time periods. Culture media supernatants were collected and IL‐6 and sIL‐6R expressions were measured using ELISA. (A) IL‐6 secretion was inhibited by bortezomib. After serum starvation, U266 cells were treated with 5nM bortezomib for 6 and 24h. (B) Autocrine sIL‐6R secretion was inhibited by bortezomib. After serum starvation, U266 cells were pretreated with 5nM bortezomib for 6h and then treated with IL‐6 (5ng/mL) and sIL‐6R (25ng/mL) for 24h. (C–D) IL‐6 and sIL‐6R secretion was inhibited by curcumin in a dose dependent manner. After serum starvation, U266 cells were treated with indicated concentrations of curcumin for 24h.
2.6. Effect of curcumin on IL‐6, sIL‐6R, and VEGF secretion in BMSCs from three different sources
As secretion of IL‐6 and sIL‐6R was decreased by curcumin in U266 cells, we next sought to determine the effect of curcumin on IL‐6, sIL‐6R and VEGF secretion in BMSCs from three different sources. Following serum starvation, BMSCs were treated with curcumin for 24h and the expression levels of IL‐6, sIL‐6R and VEGF were determined. Even though their expression levels varied among the three different BMSCs, secretion of both IL‐6 and VEGF could be inhibited by exposure to 25 and 50μM curcumin. Levels of sIL‐6R, on the other hand, were only slightly inhibited by curcumin (Figure 6). These results indicate that curcumin inhibits the release of pro‐inflammatory cytokines from either MM cells or BMSCs.
Figure 6.
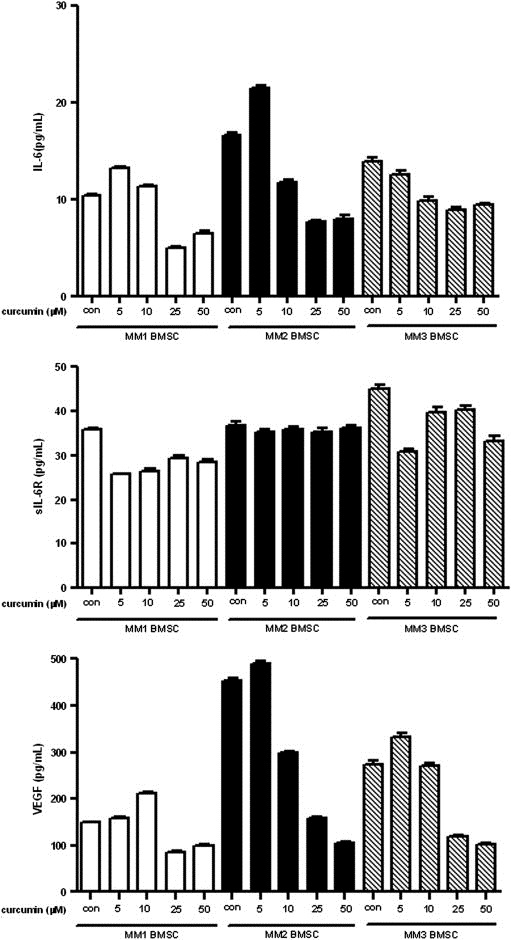
Effect of curcumin on IL‐6, sIL‐6R, and VEGF secretion in BMSCs from three different sources. After serum starvation, BMSCs from three different sources were treated with indicated concentrations of curcumin for 24h. (A) IL‐6 secretion in three BMSCs. (B) sIL‐6R secretion in three BMSCs. (C) VEGF secretion in three BMSCs.
2.7. Growth inhibitory effect of combined treatment of bortezomib and curcumin in U266 cells
In the present study, bortezomib had little effect on IL‐6 and IL‐6/sIL‐6R‐induced STAT3 and Erk phosphorylation, but secretion of IL‐6 and sIL‐6R was effectively inhibited (Figures 4B and C, 5A and B). Curcumin was also able to inhibit secretion of IL‐6 and sIL‐6. Therefore, we investigated the cell growth inhibitory effect of the combined treatment of bortezomib and curcumin in U266 cells. First, we determined whether a combination of bortezomib and curcumin synergistically inhibits the growth of U266 cells exposed to various concentrations of these compounds for 96h. As presented in Figure 7, the CIs indicated that certain combinations of bortezomib and curcumin (i.e. 4nM bortezomib/4μM curcumin and 0.5nM bortezomib/8μM curcumin) synergistically inhibited U266 cell growth. As MM cell growth was inhibited by the combination of bortezomib and curcumin, we further examined the effect of bortezomib and curcumin on the IL‐6/sIL‐6R‐induced signaling pathways in U266 cells. Exposure of cells for 6h to the combination of bortezomib and curcumin or to curcumin alone effectively inhibited IL‐6‐induced STAT3 and Erk phosphorylation (Figure 8). The bortezomib/curcumin combination was more effective in enhancing the cleavage of PARP and decreasing the levels of pro‐caspase 3 than either curcumin or bortezomib alone. Also, p‐IκBα expression was induced by the combined treatment of bortezomib and curcumin (Figure 9). These results show that combined treatment with bortezomib and curcumin increased apoptosis in U266 cells as compared with either compound alone.
Figure 7.
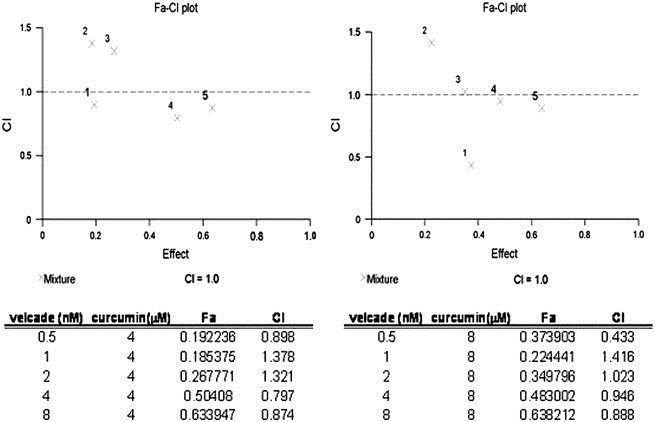
Growth inhibitory effect of the combined treatment of bortezomib and curcumin on U266 cells. U266 cells were treated with indicated concentrations of combined bortezomib and curcumin for 96h and CI was calculated using Calcusyn Software.
Figure 8.
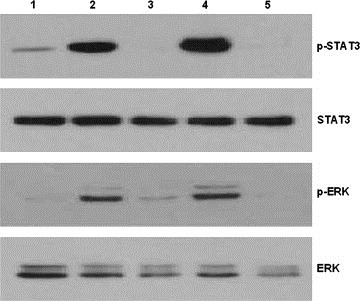
Effect of the combination of bortezomib and curcumin on IL‐6/sIL‐6R‐induced JAK/STAT and MAPK pathways in U266 cells. U266 cells were pretreated with either bortezomib (1nM) or curcumin (5μM) or combined bortezomib and curcumin for 1h and then treated with IL‐6 (5ng/mL) and sIL‐6R (25ng/mL) for 4h. Whole‐cell extracts (30μg) were analyzed by Western blot for phosphorylated STAT3, total STAT3, phosphorylated Erk, and total Erk. Lane: 1. control; 2. 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 3. 5μM curcumin, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 4. 1nM bortezomib, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 5. 5μM curcumin, 1nM bortezomib, 5ng/mL IL‐6 and 25ng/mL sIL‐6R.
Figure 9.
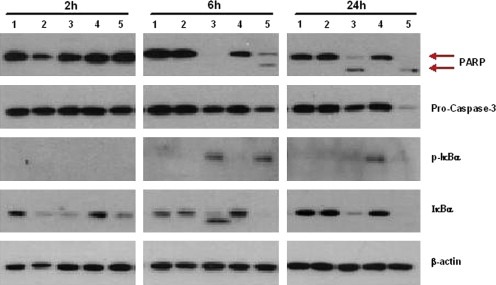
Synergistic effect of bortezomib and curcumin on apoptosis induction in U266 cells. U266 cells were pretreated with either bortezomib (1nM) or curcumin (5μM) or combined bortezomib and curcumin for 1h and then treated with IL‐6 (5ng/mL) and sIL‐6R (25ng/mL). Cultures were incubated at indicated time periods and then 30μg of whole‐cell extracts were analyzed by Western blot for PARP, pro‐caspase 3, phosphorylated IκBα, total IκBα and β‐actin. Lane: 1. control; 2. 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 3. 5μM curcumin, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 4. 1nM bortezomib, 5ng/mL IL‐6 and 25ng/mL sIL‐6R; 5. 5μM curcumin, 1nM bortezomib, 5ng/mL IL‐6 and 25ng/mL sIL‐6R.
3. Discussion
Interaction between MM cells and BMSCs triggers pro‐inflammatory cytokines including IL‐6, VEGF, and SDF‐1α which are involved in MM cell proliferation, survival, and drug resistance (Anderson, 2001). In spite of recent therapeutic advances and development of novel drugs, MM is still an incurable disease. Therefore, recent studies have focused on developing novel anti‐cancer therapeutic strategies targeting both MM cells and MM cells–BMSCs interactions (Podar et al., 2007; Hideshima and Anderson, 2002; Caers et al., 2008). The results presented here showed that MM cells co‐cultured with BMSCs grew faster at 24h, but curcumin inhibited MM cell growth, with or without the presence of co‐cultured BMSCs at 72h. Interestingly, cell viability of the IL‐6 dependent cell line, U266, was enhanced by BMSCs, indicating that survival of U266 cells was considerably influenced by the interaction with BMSCs, most likely due to the release by BMSCs of several growth factors that promote MM cell growth. Indeed, when MM cells were treated with conditioned culture media of BMSCs, STAT3 and Erk phosphorylation was increased commensurate with increasing volumes of conditioned media. However, curcumin decreased STAT3 and Erk phosphorylation in MM cells treated with conditioned culture media in a dose dependent manner suggesting that curcumin inhibits the release of growth stimulating factors from BMSCs that activate JAK/STAT and MAPK cell signal pathways which promote MM cell growth.
IL‐6/sIL‐6R has been shown to potentiate IL‐6‐induced JAK/STAT and MAPK pathways (Barille et al., 2000). Among the various inhibitors with different IC50 values used in these experiments, curcumin was the most effective in inhibiting IL‐6‐induced STAT3 and Erk phosphorylation as compared with other protein kinase inhibitors. Surprisingly, when U266 cells were treated with combined IL‐6 and sIL‐6R, curcumin was the most effective compound, among the cell signal inhibitors tested, in inhibiting IL‐6 mediated cell signaling. These data suggest that curcumin could be a promising candidate as a novel agent for MM treatment.
Bortezomib (Velcade™) was developed as an anticancer drug that is a selective, reversible inhibitor of the catalytic site of the 20S proteasome. Several clinical reports have demonstrated that bortezomib or bortezomib containing combination regimens are very effective in the clinical management of MM patients (Blade et al., 2005; Popat et al., 2006). In our study the combination of curcumin and bortezomib was more effective than either agent alone and the CI showed that synergism between curcumin and bortezomib can be achieved at low concentrations of bortezomib (0.5nM) combined with 8μM curcumin. These findings suggest that curcumin can potentiate the therapeutic efficacy of low dose bortezomib, thus reducing toxicity issues associated with the use of high‐dose bortezomib. To further investigate the molecular mechanisms by which the combined treatment of curcumin and bortezomib inhibited MM cell growth, we examined the activation of protein kinases in U266 cells treated with a combination of 1nM bortezomib and 5μM curcumin. Since sIL‐6R significantly affects MM cell survival (Barille et al., 2000; Kallen, 2002), phosphorylation patterns of STAT3 and Erk were examined in U266 cells pretreated with IL‐6 and sIL‐6R. In the combined treatment, both p‐Erk and p‐STAT3 were dramatically decreased. An increase in cleaved PARP and decrease in pro‐caspase 3 were also observed in the combined treatment. Between bortezomib and curcumin, only curcumin dramatically blocked the phosphorylation of both STAT3 and Erk. Phosphorylation of STAT3 and Erk protects tumor cells from undergoing apoptosis when cancer cells are exposed to anti‐cancer drugs. Bortezomib, through its inhibition of the proteasome, elevates IκB, which, in turn, inactivates NF‐κB, promoting tumor cell apoptosis (Popat et al., 2006; Ma et al., 2003). In addition, bortezomib blocks IL‐6 production, a key growth factor in myeloma proliferation (Hideshima et al., 2003). In the present study, we could show that 1nM bortezomib did not suppress the phosphorylation of STAT3 and Erk. However, the reduction of phosphorylation of STAT3 and Erk by curcumin promoted the proapoptotic effect of bortezomib. In summary, we showed that curcumin suppressed production of IL‐6 and sIL‐6R. It also blocked phosphorylation of STAT3 and Erk induced by IL‐6 and sIL‐6R. Combined treatment of curcumin and bortezomib effectively inhibited the growth of U266 cells via induction of apoptotic signals including inactivation of NF‐κB. Thus, the combination of curcumin and bortezomib can be utilized as a novel MM treatment regimen.
4. Experimental procedures
4.1. Cell line and cell culture
Human MM cell lines, U266 and RPMI 8226 were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in RPMI 1640 medium (Gibco‐BRL, Gaithersburg, MD, USA) supplemented with 10% heat‐inactivated fetal bovine serum, penicillin (100U/mL), and streptomycin (100μg/mL) (GIBCO, Grand Island, NY, USA). They were cultured in a highly humidified atmosphere of 5% CO2 and 95% air at 37°C. All experiments were conducted using cells in the logarithmic growth phase. Bone marrow specimens were obtained from MM patients under a protocol approved by the Seoul National University Hospital Institutional Review Board for the use of samples for research, and mononuclear cells separated by Ficoll‐Hipaque density sedimentation to establish long‐term BMSC cultures as previously described (Uchiyama et al., 1993). When an adherent cell monolayer had developed, BMSCs were harvested in Hank's Buffered Saline Solution containing 0.25% trypsin and 0.02% EDTA, washed, and collected by centrifugation.
4.2. Reagents
Recombinant human IL‐6 and sIL‐6R were purchased from R&D Systems (Minneapolis, MN, USA), rehydrated in phosphate‐buffered saline (PBS) containing 0.1% bovine serum albumin, and stored as a stock solution at −20°C. The specific proteasome inhibitor, bortezomib (Velcade™; formerly known as PS‐341), was generously provided by Janssen Korea, Ltd. (Seoul, Korea). Curcumin was purchased from Sigma Co., (St Louis, MO, USA). PD98059, AG490, and 6‐amino‐4‐(4‐phenoxyphenylethylamino) quinazoline (NF‐κB activation inhibitor) were purchased from Calbiochem Corp. (San Diego, CA, USA). These agents were dissolved in DMSO as a stock solution, stored at −20°C, and subsequently diluted with serum‐free RPMI 1640 prior to use.
4.3. Cell proliferation assay
Cell proliferation assay was performed using Cell Counting Kit‐8 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions (downloadable at http://www.dojindo.com/newimages/CCK‐8TechnicalInformation.pdf). Briefly, 100μL of cell suspension was incubated in 96‐well culture plates at 37°C. Then, indicated reagents were added into culture media in the plate and cells were incubated for the indicated time period. Then, 10μL of CCK‐8 solution was added to each well for 4h. The absorbance of each well was measured in a microplate reader (Becton Dickinson Labware, Le Pont de Claix, France) at 450nm (reference 650nm) after shaking as stated above. Means and standard deviations were generated from three independent experiments. Absorbance values were normalized to the values obtained from control group to determine the value for % of survival. Values are the mean±S.D.
Combination index (CI) values for each combination treatment were calculated using Calcusyn Software (Biosoft, Ferguson, MO, USA). CI value >1.2 was defined as antagonism, 0.8–1.2 as additive and <0.8 as synergistic effect.
4.4. Western blot analysis
The cells were treated with the indicated reagents for the indicated time periods, washed once in ice‐cold phosphate buffered saline (PBS), and resuspended in lysis buffer (50mM Tris–HCl (pH 7.4), 150mM NaCl, 1% NP‐40, Na‐deoxycholate 0.25%, 1mM EDTA, 1mM NaF, 1mM Na3VO4, 1mM PMSF, aprotinin, leupeptine, and pepstatin 1μg/mL). The protein concentration of lysate was measured, 30μg of cytoplasmic protein extracts were boiled for 5min and the proteins were resolved in 10% SDS‐polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked in Tris‐buffered saline containing 0.05% Tween 20 and 5% nonfat dry milk for 1h at room temperature and incubated with the appropriate primary antibody for 2h. Immunoreactive proteins were detected using horseradish peroxidase‐conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., PA, USA) and an enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Antibodies for the following proteins were utilized in this study; phospho‐STAT3, phospho‐Erk, phospho‐IκBα, STAT3, Erk and IκBα (Cell Signaling Technology, Beverly, MA, USA), PARP, caspase 3, cyclin D1, CDK4 (Santa Cruz, CA, USA), p21, and β‐actin (Sigma, MI, USA).
4.5. ELISA
Enzyme‐linked immunosorbent assays were conducted with IL‐6, a soluble IL‐6 receptor (sIL‐6R), and VEGF kit (R&D Systems, Minneapolis, MN, USA). The cells were treated with the indicated reagents for the indicated time period, and the cell‐free supernatants were harvested. These samples were measured for IL‐6, soluble IL‐6R, and VEGF levels in accordance with the manufacturer's instructions.
4.6. Statistic analysis
The statistical significance of differences observed in experimental versus control cells was determined via the Student's t test. The minimal level of significance was p<0.05.
Acknowledgments
This work was supported by a grant no. 21‐2004‐025‐0 and a grant no. 03‐2008‐026 from the S.N.U.H Research Fund.
Park Juwon, Ayyappan Vasudevan, Bae Eun-Kyung, Lee Chansu, Kim Byung-Su, Kim Byoung Kook, Lee Young-Yiul, Ahn Kwang-Sung, Yoon Sung-Soo, (2008), Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells, Molecular Oncology, 2, doi: 10.1016/j.molonc.2008.09.006.
Contributor Information
Kwang-Sung Ahn, Email: kwangsung.ahn@gmail.com.
Sung-Soo Yoon, Email: ssysmc@snu.ac.kr.
References
- Anderson, K.C. , 2001. Multiple myeloma. advances in disease biology: therapeutic implications. Semin. Hematol. 38, 6–10. [DOI] [PubMed] [Google Scholar]
- Barille, S. , Bataille, R. , Amiot, M. , 2000. The role of interleukin-6 and interleukin-6/interleukin-6 receptor-alpha complex in the pathogenesis of multiple myeloma. Eur. Cytokine Netw. 11, 546–551. [PubMed] [Google Scholar]
- Bharti, A.C. , Donato, N. , Singh, S. , Aggarwal, B.B. , 2003. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 101, 1053–1062. [DOI] [PubMed] [Google Scholar]
- Blade, J. , Cibeira, M.T. , Rosinol, L. , 2005. Bortezomib: a valuable new antineoplastic strategy in multiple myeloma. Acta Oncol. 44, 440–448. [DOI] [PubMed] [Google Scholar]
- Caers, J. , Van Valckenborgh, E. , Menu, E. , Van Camp, B. , Vanderkerken, K. , 2008. Unraveling the biology of multiple myeloma disease: cancer stem cells, acquired intracellular changes and interactions with the surrounding micro-environment. Bull. Cancer 95, 301–313. [DOI] [PubMed] [Google Scholar]
- Hatcher, H. , Planalp, R. , Cho, J. , Torti, F.M. , Torti, S.V. , 2008. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 65, 1631–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima, T. , Anderson, K.C. , 2002. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat. Rev. Cancer 2, 927–937. [DOI] [PubMed] [Google Scholar]
- Hideshima, T. , Chauhan, D. , Hayashi, T. , Akiyama, M. , Mitsiades, N. , Mitsiades, C. , Podar, K. , Munshi, N.C. , Richardson, P.G. , Anderson, K.C. , 2003. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene 22, 8386–8393. [DOI] [PubMed] [Google Scholar]
- Kallen, K.J. , 2002. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta 1592, 323–343. [DOI] [PubMed] [Google Scholar]
- Kyle, R.A. , Rajkumar, S.V. , 2008. Multiple myeloma. Blood 111, 2962–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis-Piwowar, K.R. , Milacic, V. , Chen, D. , Yang, H. , Zhao, Y. , Chan, T.H. , Yan, B. , Dou, Q.P. , 2006. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist. Updat. 9, 263–273. [DOI] [PubMed] [Google Scholar]
- Ma, M.H. , Yang, H.H. , Parker, K. , Manyak, S. , Friedman, J.M. , Altamirano, C. , Wu, Z.Q. , Borad, M.J. , Frantzen, M. , Roussos, E. , Neeser, J. , Mikail, A. , Adams, J. , Sjak-Shie, N. , Vescio, R.A. , Berenson, J.R. , 2003. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin. Cancer Res. 9, 1136–1144. [PubMed] [Google Scholar]
- Pagnucco, G. , Cardinale, G. , Gervasi, F. , 2004. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann. N. Y. Acad. Sci. 1028, 390–399. [DOI] [PubMed] [Google Scholar]
- Podar, K. , Richardson, P.G. , Hideshima, T. , Chauhan, D. , Anderson, K.C. , 2007. The malignant clone and the bone-marrow environment. Best Pract. Res. Clin. Haematol. 20, 597–612. [DOI] [PubMed] [Google Scholar]
- Popat, R. , Joel, S. , Oakervee, H. , Cavenagh, J. , 2006. Bortezomib for multiple myeloma. Expert Opin. Pharmacother. 7, 1337–1346. [DOI] [PubMed] [Google Scholar]
- Sarkar, F.H. , Li, Y. , 2006. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 66, 3347–3350. [DOI] [PubMed] [Google Scholar]
- Sirohi, B. , Powles, R. , 2004. Multiple myeloma. Lancet 363, 875–887. [DOI] [PubMed] [Google Scholar]
- Uchiyama, H. , Barut, B.A. , Mohrbacher, A.F. , Chauhan, D. , Anderson, K.C. , 1993. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood 82, 3712–3720. [PubMed] [Google Scholar]
- Yang, H.H. , Vescio, R. , Schenkein, D. , Berenson, J.R. , 2003. A prospective, open-label safety and efficacy study of combination treatment with bortezomib (PS-341, velcade and melphalan in patients with relapsed or refractory multiple myeloma). Clin. Lymphoma 4, 119–122. [DOI] [PubMed] [Google Scholar]


