Abstract
Cancer research has carved an astonishing trajectory giving rise to a multi billion euro global network covering most domains of science and including all manner of research funders from industry to government and philanthropic funders. We have estimated that in 2004/2005 the global spend on cancer research was 14,030 million euros, with the USA, dominated by the NCI (c. 83%) accounting for the largest absolute spend. This is between 2 and 3 times the level of per capita spend compared to EU‐15 and Europe, respectively. In Europe, the UK is at comparable levels of spend compared to the USA. Funding for cancer research in Europe is split almost 50:50 between philanthropic and governmental sources. Cancer research productivity in terms of outputs (publications) is slightly greater in Europe compared to the USA with an increasing trend towards more applied (clinical) outputs. Both the USA and Europe have equally strong industry‐supported output levels.
Keywords: Cancer, Policy, Funding, Activity, Pharmaceutical, Outputs
1. Introduction
“Public interest in the cancer problem is now at the highest point in history”, wrote James Ewing in 1938. Since then cancer research has carved an astonishing trajectory giving rise to a multi billion euro global network covering most domains of science and including all manner of research funders from industry to government and philanthropic funders. There is hardly any developed country that does not conduct cancer research at some level. Thus the nexus of cancer research policymaking is the interplay between funding and activity. However, little is known about the global funders, funding or indeed activity. In this policy research we present new data on the current state and trends of global cancer research funding and outputs. This data provide an evidence‐based platform for the development of new policies to control and cure cancer.
2. Methodology
2.1. Analysis of cancer research funding using a direct survey of organisations (industry, governmental and philanthropic) in Europe and the USA
Over a 12‐month period (2006–2007) the ECRM conducted a second review of funding organisations across Europe and the USA through direct contact with the funding organisation (governmental and non‐governmental) and a web‐based interrogation of their accounts (the first survey was conducted in 2005 so the data presented in this paper represent a two year follow up). At the beginning of the survey, guidelines were established to help with this quantification, and were followed through the entire data collection and data entry phases. If a funding organisation reported a spend between two amounts, the higher amount was always used. Annual direct cancer research spend does not include educational grants, non‐research staff salaries (other than research managers), physical plant improvements; spend on advocacy and service delivery, and the like. Any organisation reporting spend in currencies other than Euro had the reported amount of spend converted using the web site www.xe.com, all currencies were converted within two days of receipt of the information.
At the end of the data collection phase of the survey, 144 of 153 identified European funding organisations had reported back to the Secretariat, giving a 96% completion rate, we had a 100% response rate from USA funders. In addition to direct contact with cancer funders in the USA we also interrogated RaDiUS, the Rand Corporation's RaDiUS (Research and Development in the United States) database (https://radius.rand.org). This database identifies (by agency) all the intramural and extramural cancer projects. The abstracts of the projects were reviewed to extract those that were not focused on cancer research (such as when cancer was only listed as criteria for exclusion in the study). At the end of data collection, amounts were converted from USD to Euro using the average exchange rate for 2007.
2.2. Analysis of cancer research funding bibliometrics
This method of quantifying the cancer research spend of various organisations is based on a methodology developed by City University (London, UK). The work was carried out in two phases. In the first of these, files of the bibliographic data on cancer research papers, 1994–2003, were compiled from the Science Citation Index (SCI) (©Thomson Scientific) on CD‐ROM. These were then analysed to show the outputs of the world and 35 countries, and compared with their health research outputs overall so as to reveal their relative commitment to cancer research. The research levels of the papers were determined, on a scale from clinical to basic, to show whether this was changing with time, and how European countries compared in this respect with the USA.
In the second phase, the leading cancer researchers world‐wide were identified, together with their addresses and e‐mail addresses. They were sent a short questionnaire asking about their cancer research budgets. From their responses, the mean cost per paper for each of them was determined, and hence the total amount spent on public‐domain cancer research world‐wide and in selected countries by multiplication of their annual outputs by this mean cost, corrected to allow for varying health research costs in different countries. The contributions of the leading funding organisations were also determined from an examination of the funding acknowledgements on a large sample of 2003 cancer papers from different countries. This analysis also allows for the funding (usually from governmental sources) of university and hospital papers without funding acknowledgements. Account was also taken of the much higher expenditures of the pharmaceutical industry by fractionating their published R&D spends in recent years by the percentage of their published papers within the sub‐field of cancer research.
In addition to an analysis of cancer research spend by the public sector we also carried and used this method to estimate spend by the major pharmaceutical firms (direct estimates of industry spend are not publicly available). Since it has been estimated by the Global Forum for Health Research that world‐wide expenditure on health research was $106 billion in 2001 and of this total $51 billion was estimated to have come from industry, it is clear that a major fraction of all cancer‐related research will also have come from companies, particularly the large pharmaceutical companies (big pharma). (Some health research money comes from non‐pharma companies, e.g. those involved with medical devices and instrumentation for diagnosis.) Since almost all big pharma companies are publicly listed, there is a requirement that they disclose their annual R&D expenditures in their annual reports, and data from these for the last five years have been compiled by the UK Department of Trade and Industry in their annual R&D “Scoreboard” reports. For the pharmaceutical companies, over 160 are listed from 13 countries, but 32 of these are UK subsidiaries of foreign companies that have their own labs and research programmes. For the 129 Independent companies, their combined R&D expenditure for 2001 was about $45 billion: this is 88% of the estimated total commercial health research expenditure for that year given above. The remaining 12% will partly be accounted for by smaller pharma companies missing from the DTI list, and partly by non‐pharma companies. We may reasonably assume that the pharma company total would have been about $48 billion.
Of this total, the large majority (80%) was spent by the 24 largest companies whose combined R&D expenditure was $38.7 billion in 2001. All of these companies were represented among the addresses on cancer papers in the SCI files for the years 1999–2003. A search was also made in the SCI for all papers with an address from one or more of each of these companies in these same five years. The assumption was then made that the company's R&D expenditure was devoted to cancer research in the proportion that its cancer papers bore to its total output of papers (both on integer counts). For example, Pfizer had an annual average R&D expenditure from 1999 to 2003 of $4.05 billion and published an average of 477 papers per year, of which 23.2 were on cancer (5.2%). It was therefore estimated that its total cancer R&D spend would have averaged $209 M over the period. Of this, a small amount would have gone on the work (mainly in public‐domain labs) actually reported in SCI papers; much more would have been spent internally and in ways not leading to published outputs.
Bibliometrics were also used to determine overall spend on cancer research in Canada, Japan, Australia and the Rest of the World (mainly China and India). Identifying and quantifying the spend the Far East is particularly challenging as the names of many authors are homonyms and much of the output of this region is published in language and country specific journals which do not appear on main English‐language databases. This observation is also true for cancer research conducted in Latin America, and other regions such as Russia (FIS) and India. As such this approach will underestimate both the global spend and activity, although it will capture what is arguably of ‘global significance’, i.e. published research in the lingua franca of international science.
2.3. Analysis of the global activity in cancer research
Outputs (publications) are the most objective, unambiguous product of global cancer research activity. All research systems, irrespective of their location or type are primarily geared towards the production of knowledge through publication. As such publications in cancer research provide an objective surrogate for (a) overall activity, (b) type of research (this can be analysed in a variety of ways, basic to population, and by specific sub‐domains, e.g. industry‐sponsored), and (c) collaboration's, etc. In this study we have focused on overall activity (in absolute terms and relative to the country or regions GDP), including trends and the research level of the published cancer research papers.
The research level (RL) of the papers was calculated from the titles of the papers appearing in the given journal that had a biomedical word in their addresses (Lewison and Paraje, 2004). Over 100 “clinical” title words and a similar number of “basic” words were used to determine if a journal paper was clinical, basic or both: clinical papers were given an RL of unity, basic papers an RL of 4 and “both” papers an RL of 2.5. From these values, it was possible to calculate the mean RL for papers in the journal as a decimal number between 1 and 4.
3. Results
3.1. Funding cancer research in Europe
Since the first ECRM survey Europe has increased its funding of cancer research through both philanthropic and governmental funders, in the former case the average spend per annum has risen from €21.5 M to €27.5 M partly as a result of the creation of new charitable funders, e.g. Champalimaud Foundation in Portugal but also largely due to a huge increase in philanthropic funding in the UK (a rise from €232 M to €396 M). Governmental funding from Ministries and/or Research Councils (but not including infrastructure funding through healthcare and university systems) has also increased to an average of €31 M from €21.4 M in the last survey. This aggregate data, however, hide wide variation in the respective spend on cancer research by different organisations and European states. We have found that nine out of the 32 countries surveyed have an imbalance between charitable and governmental funding. In particular Sweden and Denmark had low governmental spend whereas the philanthropic funding was found to be relatively underdeveloped in Spain and Greece (see Figure 1).
Figure 1.
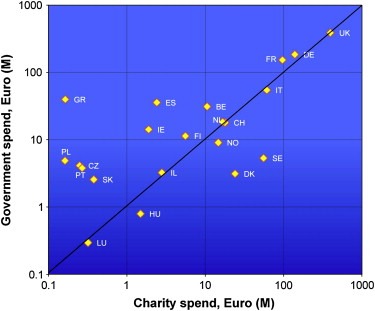
European cancer research spend by country and type of funding organisation (FY2004/5).
In Europe the majority of the spend is concentrated (90.9%) in the original 15 Member States, a situation that remains unchanged since the last survey. Contributing some €10 M to European spend were also trans‐European research funders such as the EORTC. The European Commission is also a contributor to European spend through the Framework programmes with just under €100 M per annum under the 6th Framework Programme (Jungbluth et al., 2007). However, this second survey was undertaken in the gap between the 6th and 7th Framework programmes. In the previous Programme the European Commission invested a total of €485 M between 2002 and 2006, inclusive. Notably such investment was heavily geared towards basic cancer research (causes and mechanisms in panel (a) of Figure 2), which absorbed over 49% of this spend compared with under 4% for research into cancer prevention.
Figure 2.
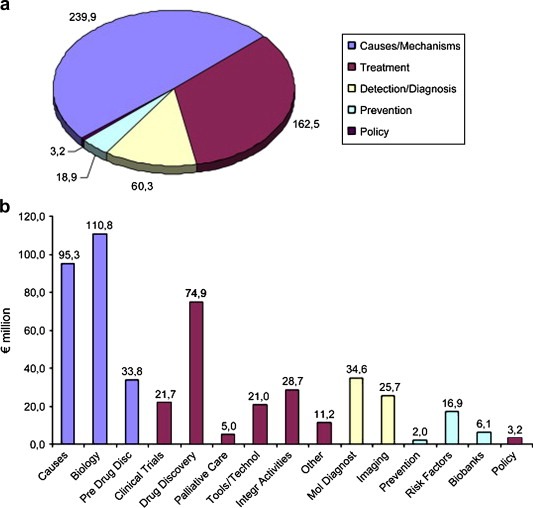
Total spend on cancer research by European Commission during Framework Programme 6 (2002–2006). (Taken from Jungbluth et al., 2007).
A similar allocation of funding has also been found with an assessment of categories of spend in Europe and the USA during the last ECRM survey and, more recently, by the Canadian Cancer Research Alliance in an assessment of its own portfolio (Canadian Cancer Research Alliance, 2007). In all three surveys basic cancer research dominated, accounting between 25% and 45% of total spend, compared to 2–9% for prevention (Figure 3).
Figure 3.
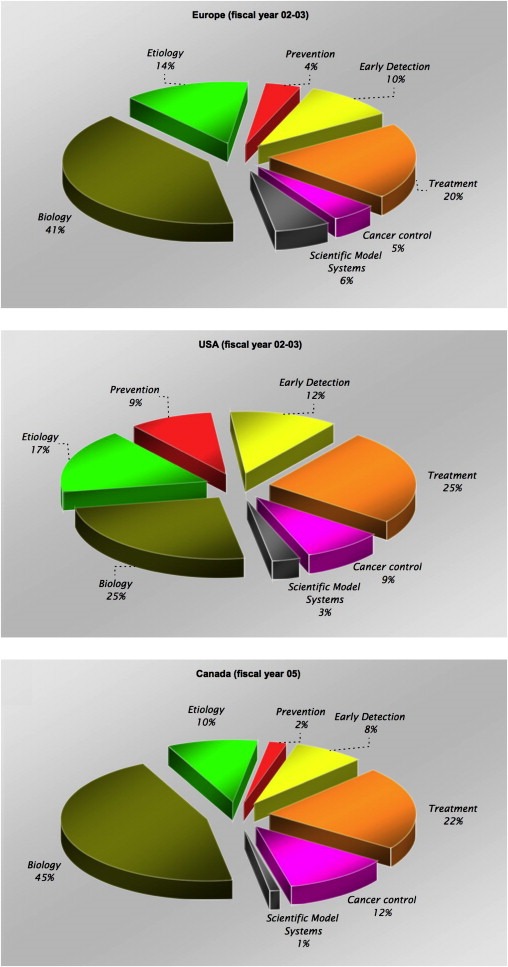
Spend by category of research (Common Scientific Outline) – note different fiscal year for Canada.
3.2. Funding cancer research in the USA
One of the unique features of Richard Nixon's National Cancer Act in 1971 was the creation of a hypothecated cancer research funding (National Cancer Institute) with bypass budget authority directly to the President without need for NIH or DHSS authorisation. Whilst the NCI has remained the core source of cancer research funding in the USA over the years many governmental and philanthropic funders have also joined.
In comparison to Europe, where the contribution of governmental and philanthropic funding is almost fifty–fifty, governmental funding in the USA (mostly through the NCI) accounts for 94% of the total spend with a contribution of €3.52 billion in FY2004/05 (Figure 4).
Figure 4.
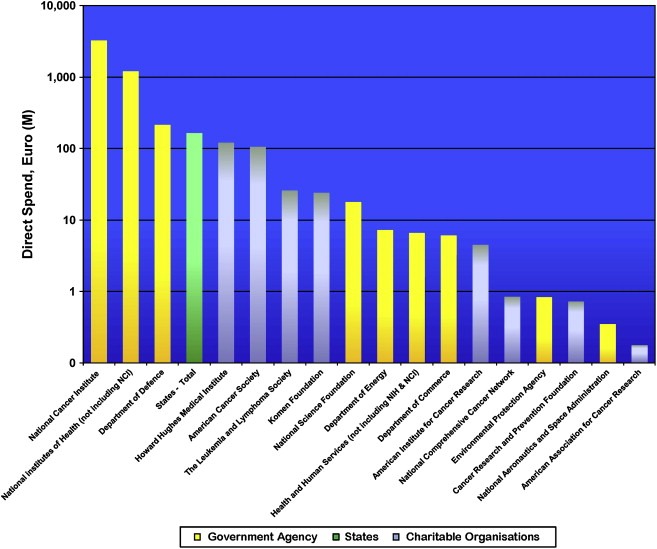
USA cancer research spend by type of funding organisation (FY2004/5).
In order to better understand the changes to the overall NCI budget and research spend we analysed data from the NCI over a 10‐year period (note: dollar data were converted to euro using that years average exchange rate from Fed Board) (Figure 5). Research spend was relatively constant until early 2000. By 2003 the NCI had seen an 80% increase in its congressional appropriations since 1998 (set against a doubling in NIH budget). However, the gap between actual research spend and budget allocation widened, representing an increased cost of conducting research (Figure 5).
Figure 5.
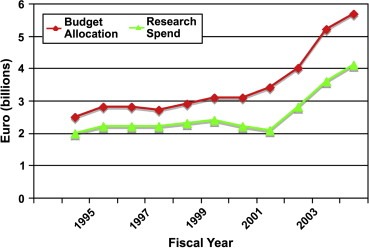
Ten‐year trend in the NCI's budget allocation and actual research spend (from NCI annual reports).
3.3. Global comparisons of cancer research funding
In the first comparative study of European and USA spend on cancer research we found the latter outspent the former 3 to 5 times as a % of GDP or per capita (Eckhouse and Sullivan, 2006). However, the regions have radically different systems and processes for disbursing funds with Europe in particular unusual for the high level of infrastructure funding distributed by governments into university and healthcare systems from general taxation. By using the bibliometric method we have been able to estimate this ‘indirect’ funding into university and/or healthcare systems. In addition this methodology can also be utilised to estimated cancer research spend in other regions that were not directly surveyed and 24 of the major cancer research active pharmaceutical companies.
Europe (in particular the original EU‐15 Member States) channels a substantial amount of funding for cancer research through it's university and/or healthcare systems (Figure 6). This accounts for between 21% and 44% of overall spend depending on Member State. In comparison cancer research funding in the USA is entirely through federal and other philanthropic organisations. The identification of this additional spend in Europe narrows the funding gap with the USA, although the USA retains its lead with over double the funding Europe enjoys.
Figure 6.
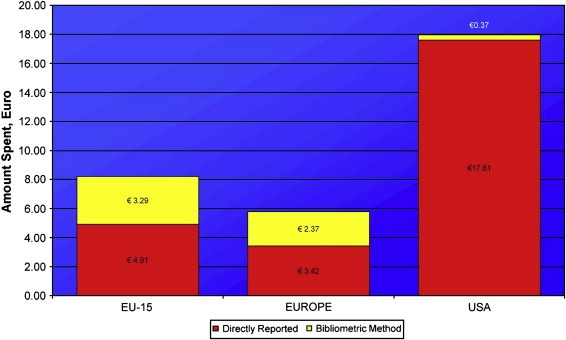
Comparison of spend per capita by funding organisations (directly reported) and through university/healthcare system infrastructure funding using bibliometric method.
Europe and USA account for the majority of global funding with a combined spend of over €8 billion per annum, compared to circa €3 billion for the rest of the world (Figure 7). The total global spend (excluding industry) is estimated at over €11 billion in 2004/5 fiscal year. In addition to this we have estimated that the top 24 pharmaceutical companies spent just over €3 billion in the same fiscal year on cancer research (this does not include development, registration clinical trials or marketing costs) (not shown). Despite different absolute spends Australia, Canada and Japan have broadly similar per capita spends (this similarity remains regardless of comparative denominator, e.g. GDP) at €7.93, €8.27 and €7.88, respectively. In comparison Europe as a whole only spends €5.79, however, when one views European cancer research spend as only those original EU‐15 Member States then this figure dramatically rises to €8.20. At €17.98 per capita USA funding is one of the highest in the world along with the UK which spends some €18.5 per capita (€13.18 of this comes directly from funding organisations and the remainder flows through infrastructure funding to the university and healthcare systems).
Figure 7.
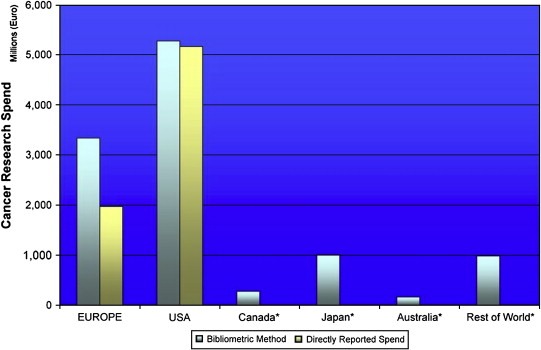
Global cancer research funding (FY2004/5) (excluding industry).
3.4. Global cancer research activity
Cancer research publications, one of the major outputs of funding, are an objective surrogate of overall cancer research activity. Europe and the USA were broadly comparable in terms of their overall cancer research productivity by volume of publications (see far right bars of Figure 8). Productivity across Europe varied widely from more than two cancer research papers per billion Euro of GDP to under 0.2. Most Member States were within their expected productivity range, with the suggestion of certain countries being low in terms of productivity (below 1.0) and others on the high productivity side (above 1.5).
Figure 8.
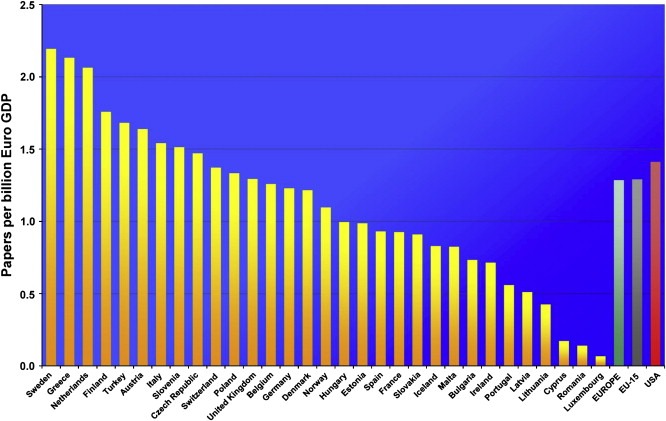
Cancer research productivity as measured by output (papers per billion €GDP).
Looking at overall trends in cancer research productivity Europe diverged from the USA around 1995 to a steady state of around 43% of global share in overall cancer research ouputs, whilst the USA has remained steady at around 38% (Figure 9). A similar pattern of change in total biomedical publications was also found.
Figure 9.
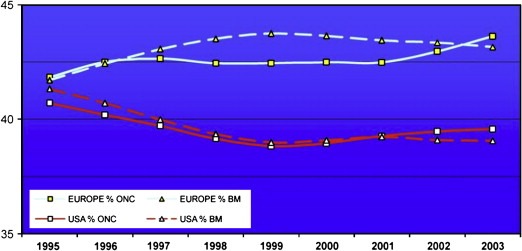
Trends in oncology (ONC) and biomedical (BM) research outputs (%).
We also examined the contribution of industry (top 24 companies) to cancer research outputs. We found that Europe (again predominantly but not exclusively EU‐15) and the USA dominated global industry‐sponsored cancer research with both regions broadly responsible for an equal share of industry‐sponsored putputs (see Figure 10).
Figure 10.
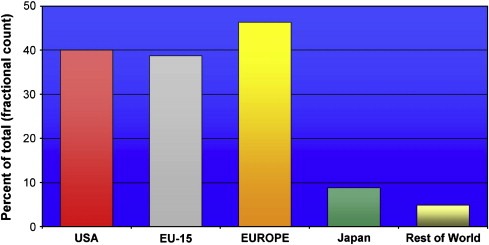
Cancer research outputs of top 24 pharmaceutical firms active in cancer research.
Finally one of the key strategic questions is the extent to which cancer research is becoming more or less applied, i.e. movement from basic science to clinical application. By reviewing the research levels of papers published in two cohorts we were able to determine whether a countries output was becoming more ‘basic’ or more applied (‘clinical’).
Most European Member States are becoming more clinical in their cancer research outputs, with some notable exceptions (Figure 11). In comparison to the USA (note: whose output is also becoming more clinical) with a research level of nearly 2.4 many European Member States are publishing much more clinical work. Indeed Europe as a whole has maintained since the mid 1990's a lead in publishing more clinical cancer research (data not shown).
Figure 11.
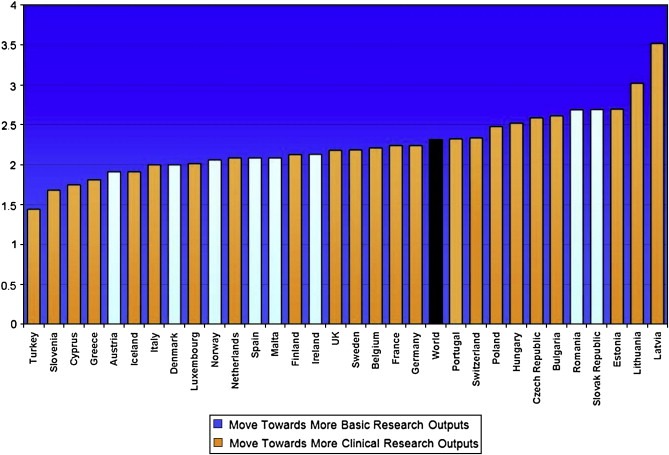
Countries Research Level (whether outputs are more ‘basic’ or ‘clinical’) in 2004/5 and trend since 1994.
4. Discussions
4.1. Trends in cancer research funding
In this second survey (the first ECRM survey was conducted two years ago (ECRM)) we have estimated cancer research funding that flows through European Member State healthcare and university systems. The figures for Europe are substantial and, at over a billion euro per annum pose a major challenge to designing policy tools for promoting coordinated cancer research with the European Research Area. Indeed despite the majority of public funding in cancer research being concentrated in a few major funding organisations across Europe (80% funds come from just 18 funders) the overall complexity of investment streams, particularly through so called infrastructure funds into healthcare systems and universities makes the development of cancer funding policies hugely difficult. Despite, or rather in spite of the rhetoric about uncoordinated and fragmented cancer research efforts one of the fundemental issues that has yet to be grasped in the search for European cohesion is the socio‐cultural levers within each of the these myriad and quiet separate funding streams. Altering behaviour to engender greater European ‘togetherness’ in cancer research, quiet irrespective of the very different and valid scientific divergences, simply cannot be achieved without this fundemental knowledge. Furthermore, such is the heterogeneity of funding streams that short‐term top down strategies for cohesion are doomed to failure from the outset. In the context of this funding pattern the last Framework Programme for cancer research funding suffered greatly from individual Member States failing to adequately support their national cancer research programmes; Commission funding became essentially substitutional rather than additional. Member States (57%) have increased their funding of cancer research in real terms since the last survey, over a third have not. Indeed the major policy issue is the real differences in cancer research investment between the Members States themselves, rather than the prevailing gaps in cancer research funding between Europe and the USA, which have been a driving force for EU policy making to date. If these are not corrected then Framework Programme funding will continue to suffer substitutional strategic failure.
A second strategic issue is the degree to which European creativity and productivity in cancer research can be raised by a more coherent pan‐European funding approach. With over 155 major cancer research funding organisations across Europe, in addition to European umbrella groups such as ECCO (European Cancer Organisations), cancer research policy initiatives (e.g. EURoCAN+), aim to improve the coordination of cancer research in Europe (www.eurocanplus.org), and patients groups (e.g. Europa Donna) that are involved in one way or another in cancer research the ‘fragmentation’ accusation is easy to level. However, the cancer research funding community across Europe has never been fragmented because it has never been together. We are therefore dealing with a unique set of players, most with competing agendas, to whom the benefits of co‐operation (access to complementary expertise, access to facilities or building critical mass through sharing) have to be woven in different ways for different goals. Indeed much the same has been said for the European Research Area as a whole (Rairio, 2007). Approaches to enhancing cohesion and co‐operation need to be far less ‘top‐down’ and far more sensitive to some basic evolutionary psychology. Individuals and groups are highly sensitive to altruism be it kin (Hamilton, 1964) or reciprocal (Trivers, 1971). With the former approach (kin altruism) Member State funders can view the funding of cancer research outside their national borders as favourable if it benefits directly researchers in that country. With may orphan cancers the numbers of national cases and simply too small to carry out any form of clinical or population based research and therefore this is a natural environment for trans‐country collaboration. Indeed this has already happened in many cases, e.g. Innovative Therapies for Children with Cancer network (ITCC). The same argument can also be used for large‐scale population studies. In terms of reciprocal altruism the model of directly funding cancer research outside the Member State without the involvement of any of its cancer research community is perhaps harder to sustain, irrespective of the downstream benefits. In one way this already happens with Member State taxation flowing through to the Framework Programme and European Research Council. The fundamental question remains though whether national governmental funders and philanthropic organisations are ready to change partisan policies. If more policy was focused on the real end result, namely the improvement of cancer control and cure for patients and families rather than the national economic advantage or fundraising strategies then perhaps that would be the case.
European funding policy has a major influence on the intrinsic creativity of European cancer research. However, it is debatable at current levels of spend whether cancer research funding at the EU level through the Framework Programmes and other streams will have a major impact on the rate and direction of European cancer research (European Cancer Research). With a budget for Framework Programme 7 (2007–2013) set at €5984 million (EC‐US), however, there is scope for the European Commission to have a major impact, in addition to its commitment for the European Research Council and Joint Technology Platforms (through which the Innovative Medicine Initiative will be funded). The EU research policy of specific research programmes and thematic calls has been questioned (Laredo, 1998), however, the suggested solutions – networks and delegation of research programmes to specific agencies should be in addition to ring‐fenced funding for cancer not as a substitute. Commission and national cancer research policy needs to recognise and fund core trans‐EU infrastructure such as phase IV clinical trials, paediatric research networks (e.g. Innovative Therapies for Children with Cancer) and Cancer Registries to name but a few (European Strategy Forum on Research Infrastructures, 2006). Where should this funding come from in the absence of a dramatically increased funding pot? Our data clearly show that basic research now dominates the majority of global research funding. Are the proportions right? Probably not and there is a strong case for more initiatives in translational/clinical cancer and prevention research. Furthermore even where more translational/clinical cancer research is being supported the dominance of drug development in capturing public funding streams has left many essential areas of cancer research, e.g. surgical oncology dangerously exposed.
Philanthropy plays a remarkable and essential role in supporting cancer research. Unsurprisingly given the fiscal dominance of the NCI Europe has a great portion of its funding through the philanthropic sector. The USA, however, dominates overall philanthropic giving with levels of nearly 2% of GDP (compared to 0.8% UK, 0.5% Netherlands and Sweden and 0.3% France (Comparative Nonprofit Sector Project)). In Europe the role of philanthropy has been belatedly recognised as an underexploited source of income for research (EUR 22005, 2006). However, charity is a complex phenomenon with different attitudes and giving patterns almost on a country by country basis (Wright, 2000). Furthermore our understanding of altruism as a sociobiological phenomenon when applied to today's philanthropy, particularly those around secular causes has not been studied in any depth beyond the theoretical (Humpries, 1997). What might work at one level in a one Member State may not work in another. Other health charities, overseas aid, human welfare and heritage preservation groups are also increasing the pressure on charitable funds. Because of these inherent uncertainties philanthropy in cancer research should in policy terms be seen as additional to the overall global effort, which is mainly funded through taxation and private enterprise (industry). However, much more could be achieved by the major European charities cooperating to fund trans‐European research, despite perceived issues around the partisan nature of fundraising a concerted effort to overcome nationalism in philanthropy could pay dividends. Indeed this has already been recognised by Commission sponsored initiatives (EUR, 2006). Other major issues around the future role and responsibilities of philanthropy in European and indeed USA cancer research funding remain. With the influx of single, wealthy donors to philanthropic causes the talk has been of a shift into philanthrocapatalism, essentially the ‘businessnification’ of charity (Economist, 2006). Whilst this might superficially appear to inject more rigour into philanthropy there is little evidence that it is the right path. Indeed becoming more like a business in the social sector is unlikely to be the right route for the simple reason that most businesses are mediocre.
USA funding of cancer research has taken a radically different direction from Europe. Bucking the clearly articulated US medical research policy view in the 1940's that funding for any type of medical research, “was not going to be established by hard concensus on a grand design. It would be fragmentary and incremental; in short evolutionary” (Greenberg, 1967), the USA embarked in 1971 on one of the most ambitious funding plans in research history, the NCI. The era of Big Biology under the umbrella of cancer had arrived. Since then the NCI and other funders have continued the trend of high levels of hypothecated funding for cancer research which has seen the budget allocation grow dramatically since 2000. In terms of per capita spend or as a proportion of GDP the USA enjoys one of the highest levels of funding in the world, only bettered by the UK which has seen astonishing levels of growth in funding (faster even than the USA). However, the gap between allocation and spend is growing representing a real downward pressure on available funds due to the increased cost per unit of research (Niederhuber, 2007). Why? Simply put the bureaucracy (secondary to regulations) and over‐management of cancer research is draining increasing sums away from front line research. The impact of regulatory policy on research funding and productivity remains, as it was for the first survey a critical issue for all countries. As Europe has recently discovered, changes to regulatory policy can have a dramatic effect on the cost of research (Hartmann and Hartmann‐Vareillas, 2006). Over the last decade the fashion for ever‐increasing regulation across all domains – clinical trials, healthcare data, human tissue – has led to an increase in the unit cost of research in the absence of any tangible social benefit of many of those regulations. Good research governance is essential but bureaucracy is absorbing too much of the global investment in cancer research (Hearn and Sullivan, 2007). There is in an urgent need to reconsider the regulatory paradigms that have been built into a thriving industry around cancer research, and reverse this trend. The data from the USA should act as a warning to all global funders of cancer research. Indeed much of the history of cancer research funding in the USA provides salient lessons for existing and new cancer funding organisations. As if to prove the point that there is nothing new under the sun a paper written in 1947 after the failure of the Neely‐Pepper Bill for a $100 million cancer research appropriation sets out in six objectives for properly controlling cancer research expenditure that remain as true today as there did back then (Cowdry, 1947).
Finally our research has only just begun to touch on the overall global funding trends. Clearly the rise of the Far East will lead to a greater expenditure on the global cancer research effort (Cheng, 2007). Whether this will be effective expenditure remains an open question. We have also attempted to estimate the global direct cancer research spend by top 24 pharmaceutical industries, which at around €3095 M represents some 22% of the estimated global spend on cancer research. Additional investment by other commercial sources is likely to increase this figure to at least a quarter of global spend. With most of this spend directed towards drug development the importance of public spend directed towards other essential areas of cancer research not within the sphere of industry becomes even more apparent.
4.2. Trends in cancer research activity
Unsurprisingly Member States outside EU‐15 also have very low levels of cancer research output. Over a 10‐year period Germany, UK, Italy and France dominated absolute European cancer research output, however, when analysed against GDP Sweden, Greece and Netherlands had the greatest output. What is the context for these underlying trends? Unsurprisingly Northern Europe remains, as it has for many areas in Science and Technology highly productive in cancer research terms. The relationships between a countries overall S&T commitment, GDP and other economic indicators is a complex one. For example Germany is currently enjoying a high spend on R&D with some 2.5% of its GDP compared with the UK which has seen spend on R&D fall from 1.87% in 2002 to 1.73% in 2004. However, the latter Member State has injected in real terms substantially more spend into cancer research since 2001 (although this has only just started to translate into an increase in the volume of cancer research publications).
Nearly all of Europe has seen a shift towards more applied, clinical research. There are some notable exceptions to this – Spain and Denmark, for example. In the latter case constriction of overall R&D/S&T budgets is probably the reason why research is moving towards ‘less’ expensive fundamental research that traditionally has a quicker return on investment (De Laine, 2008). This overall move towards more clinical outputs reflects a real advantage for Europe that is, at the moment ahead of the USA in terms of its overall translational/clinical activity (Mowery, 1998).Such research is seen as having high public value (Saunders et al., 2007) and a more concerted effort to support clinical cancer research at national and European level is certainly warranted. Indeed whilst the gap between cancer research funding in Europe and USA remains substantial cancer research outputs over a 10‐year period have been similar with Europe producing more cancer research publications by a steady 4–5% above USA since 1997. Indeed our data suggest that Europe is now increasing its share of global cancer research outputs with an upward trend that started in 2001, at the same time that the USA remains relatively flat. Interestingly a separate study has found that globally cancer research has changed from a bipolar allegiance to either clinical or laboratory styles in the 1980's to the creation of a ‘third’ style by 2000 where research activity is structured by a common orientation to a translational research domain (Cambrosio et al., 2006).
As the OECD noted industry is increasingly making use of public research through direct funding and more collaboration with public research institutions (Science and Innovation Policy, 2004). Although the USA is the dominant country for commercially sponsored phase III pivotal clinical trials (CMR International, 2006a) this survey has found substantial cancer research activity conducted by the pharmaceutical industry in both Europe and USA on the basis of the geographical origin of published cancer research papers. Much of this work (>50%) is the result of collaborations with the public sector. Our data (not presented in this report) show that this trend has been increasing. Our estimates of cancer research spend by the major pharmaceutical companies necessarily underestimate total global spend by omission of SME and biotech firms and current spend on pivotal phase III clinical trials. However, the gross figures of just over 3 billion € per annum helps place industries global contribution in perspective with other governmental and charitable funders. Industry is responsible for around a quarter of global investment in cancer research. To put the industry expenditure into perspective in 2004 global pharmaceutical R&D expenditures reached €41 billion (circa $56 billion) with, according to this survey, around 7% of this flowing into cancer research. Traditionally Europe has been considered relatively weak in attracting industry R&D funding, however, certainly when one considers the geographical origin of pharmaceutical industry publications Europe is very much an equal partner with the USA in cancer research. Indeed Europe attracts some 45.9% of total pharmaceutical R&D expenditure (CMR International, 2006b). Nearly all major recent policy cancer research funding and policy initiatives have emphasised the public–private partnership route(;) and whilst welcome EU money is being partnered with industry, there is a real danger that if all increases in EU cancer research funding go this way Europe's intrinsic creativity would be distorted by encouraging subsidy‐seeking behaviour and essential areas of cancer public health not amenable to business approach would remain orphans. Increasingly research policy has been directed to supporting the transfer of technology from knowledge‐generating organisations in the public sector (e.g. universities) to firms through the establishment of co‐operative links (Faulkner and Senker, 1995). In considering the global role of industry in cancer control it is truism as the WHO have articulated that any new treatment is unlikely to be a ‘magic bullet’ and that health promotion and cancer prevention must remain a very high priority for governmental and charitable funders (Kaplan and Laing, 2004). Indeed there is sound reason to believe that priority‐setting focused on predicted practical relevance, i.e. industrial utility should be avoided by Europe. Firstly most technology advances are derived from a broad base of scientific and technological fields and second, as Keith Pavitt describes, “our ability to understand the present and to predict successful future applications, is very limited. In detail, predictions will often be wrong, and in broad scope it will be obvious (Pavitt, 1998).”
Overall a number of key policy conclusions arise from the data presented in this paper. The first is the vast interconnected nature of the global cancer research effort, an effort that is growing all the time. Indeed whilst one region may suffer cutbacks to funding (now that is the USA) another enjoys a renaissance, e.g. the UK. The problem is one of interconnectedness and co‐operation. There are few high level links between the major Research Funding Organisations, and often little understanding of strategic agendas. This must be corrected with a Forum or some other ‘light‐touch’ engagement process. The aim must be to enhance creativity by better supporting the research community across national boundries. Enhancing creativity is not simply about jumping on whatever bandwagon happens to be there at the time (Fujimura, 1988), supporting complex interdisciplinary and orphan research areas in prevention and surgery, for example, is now more urgent than ever.
Bureaucracy and over‐management remain constant dangers to progress. The data from the USA on funding and productivity should act as a warning to Europe and beyond. Such is the problem of over‐regulation that we must now talk about de‐regulation. The research community is also being consumed by an equally pervasive culture of over‐management. Research Funding Organisations unthinkingly apply business processes where they are clearly inappropriate and the Healthcare‐University environment is subject to ever more Byzantine administration. The intellectual underpinnings of cancer research are radically different from the usual input–output model (Chubin and Studer, 1978) and, as John Cooper former President of the Association of American Medical Colleges put it, “In the Cancer Conquest program the targets are diffuse, unseen and largely unknown” (US Senate, 1971). In this vein the fall of cancer research into the pit of mediocre dogma is a real threat if over‐regulation and over‐management continue to grow.
Finally, never has there been a more urgent need for a ‘third culture’ to drive the engagement between research community and public (Snow, 1963). Taking public support for granted and allowing media hegemony to dominate the cancer agenda is a mistake. A new paradigm needs to be developed for the public understanding of cancer.
Eckhouse Seth, Lewison Grant, Sullivan Richard, (2008), Trends in the global funding and activity of cancer research, Molecular Oncology, 2, doi: 10.1016/j.molonc.2008.03.007.
This work was supported by European Cancer Research Managers Forum (ECRM) and a previous EU 5th Framework grant (QLG1‐CT‐2002‐30203/ECRM).
References
- Canadian Cancer Research Alliance, September 2007. Cancer Research Investment in Canada, 2005 Canadian Cancer Research Alliance; [Google Scholar]
- Cowdry, E.V. , 1947. Financing cancer research. Science 105, (2716) 53–57. [DOI] [PubMed] [Google Scholar]
- Cheng, M.H. , 2007. Cancer research funding in Asia. Molecular Oncology 1, 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambrosio, A. , 2006. Mapping the emergence and development of translational cancer research. European Journal of Cancer 42, 3140–3148. [DOI] [PubMed] [Google Scholar]
- CMR International, 2006. 2006/2007 Pharmaceutical R&D FactBook CMR; [Google Scholar]
- Centre for Medicines Research International, 2006. The 2005/2006 CMR International R&D Factbook CMR; Surrey: [Google Scholar]
- Chubin, D.E. , Studer, K.E. , 1978. The politics of cancer. Theory and Society 6, (1) 55–74. [Google Scholar]
- De Laine, M. , February 15, 2008. Cutbacks in budget for 2008 ‘damaging’ for research, Danish Universities say. Research Europe 14, [Google Scholar]
- Eckhouse, S. , Sullivan, R. , 2006. A Survey of Public Funding of Cancer Research in the European Union. PLoS Medicine 3, (7) e267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Strategy Forum on Research Infrastructures. European Roadmap for Research Infrastructures Report, 2006, Luxembourg.
- Economist. The business of giving. A survey of wealth and philanthropy. February 25th, 2006.
- Faulkner, W. , Senker, J. , 1995. An approach supported by numerous policy studies. Knowledge Frontiers: Public Sector Research and Industrial Innovation in Biotechnology Clarendon; Oxford: [Google Scholar]
- Full report at www.ecrmforum.org.
- Fujimura, J.H. , 1988. The molecular biological bandwagon in cancer research: where social worlds meet. Social Problems 35, (3) 261–283. [Google Scholar]
- Giving More for Research in Europe: Strengthening the Role of Philanthropy in the Financing of Research, Brussels, 27–28 March 2006, EUR 2261 EN.
- Greenberg, D.S. , 1967. The Politics of Pure Science New American Library; NY: 200 pp. [Google Scholar]
- Hamilton, W.D. , 1964. The genetical evolution of social behaviour I and II. Journal of Theoretical Biology 7, 1–52. [DOI] [PubMed] [Google Scholar]
- Humpries, N. , 1997. Varieties of altruism – and the common ground between them. Social Research 64, 199–209. [Google Scholar]
- Hartmann, M. , Hartmann-Vareillas, F. , 2006. The clinical trials Directive: how is it affecting Europe's non-commercial research?. PLoS Clinical Trials 1, (2) e13 10.1371/journal.pctr.0010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn, J. , Sullivan, R. , 2007. The impact of the ‘Clinical Trials’ directive on the cost and conduct of non-commercial cancer trials in the UK. European Journal of Cancer 43, 8–13. [DOI] [PubMed] [Google Scholar]
- Jungbluth, S. , 2007. Europe combating cancer: the European Union's commitment to cancer research in the 6th Framework Programme. Molecular Oncology 1, 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, W., Laing, R. November 2004. Priority Medicines for Europe and the World. WHO/EDM/PAR/2004.7.
- Lewison, G. , Paraje, G. , 2004. The classification of biomedical journals by research level. Scientometrics 60, 145–157. [Google Scholar]
- Laredo, P. , 1998. The networks promoted by the framework programme and the questions they raise about its formulation and implementation. Research Policy 27, 589–598. [Google Scholar]
- Mowery, D.C. , 1998. The changing structure of the US national innovation system: implications for international conflict and co-operation in R&D policy. Research Policy 27, 639–654. [Google Scholar]
- Niederhuber, J.E. , 2007. A look inside the National Cancer Institute budget process: implications for 2007 and beyond. Cancer Research 67, 3 [DOI] [PubMed] [Google Scholar]
- Presentation by Dr Octavi Quintana Trias (Director, DG Research) Biotechnology for Health Future Perspectives to EC-US Task Force for Biotechnology, 19 July 2006.
- Philanthropic giving as a % of GDP, 1995–2002. John Hopkins Comparative Nonprofit Sector Project.
- Science and Innovation Policy. Key Challenges and Opportunities, OECD. Meeting of the Committee for Scientific and Technological Policy at Ministerial Level 29–30 January 2004 (Report).
- Pavitt, K. , 1998. The inevitable limits of EU R&D funding. Research Policy 27, 559–568. [Google Scholar]
- Rairio, K. ERA – From Casualty Ward to Intensive Care, Research Eur 20007, 20 September, 6–8.
- Report of the Independent Expert Group on R&D and the Innovation Appointed Following the Hampton Court Summit and Chaired by Mr Esko Aho, Creating an Innovative Europe, January 2006, EUR 22005.
- See http://cordis.europa.eu/lifescihealth/cancer/cancer-pro-calls.htm for the cancer proposals funded under FP6.
- Saunders, C. , 2007. Beyond scientific rigour: funding cancer research of public value. Health Policy 84, 234–242. [DOI] [PubMed] [Google Scholar]
- US Senate. Conquest of Cancer Act, 1971. Hearings before the Subcommittee on Health of the Committee on Labor and Public Welfare, United States Senate, 92nd Congress, Washington, DC 1971.
- Snow, C.P. , 1963. The Two Cultures and the Scientific Revolution. New York:, second ed. Cambridge University Press; [Google Scholar]
- Trivers, R.L. , 1971. The evolution of reciprocal altruism. Quarterly Review of Biology 46, (1) 35–57. [Google Scholar]
- Wright, K. , 2000. Charitable change – creating a new culture of giving for Britain. LSE Magazine Winter 19–21. [Google Scholar]


