Abstract
A young man suffered cardiac arrests with polymorphic ventricular tachycardia (PVT) and ventricular fibrillation (VF) triggered by ventricular premature contractions (PVCs). The arrhythmia was resistant to anti-arrhythmics, so after ICD implantation he underwent successful ablation of the triggering VE beat, which was pace-mapped to the left posterior hemi-fascicle. We review the evidence for the role of the Purkinje network in the initiation and maintenance of PVT and VF, postulating a channelopathy as a possible underlying cause, and provide recommendations for PVC ablation.
Keywords: Ventricular fibrillation, Sudden cardiac death, Purkinje fibres, Channelopathy
1. Case report
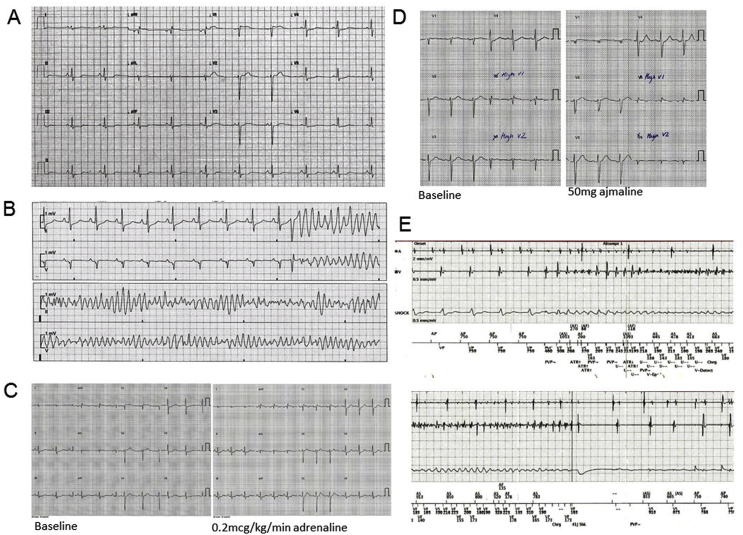
A 32 year old man was admitted to hospital following a syncopal episode. He was in sinus rhythm on admission (Fig. 1A), but then had several arrests where cardiac monitoring demonstrated polymorphic ventricular tachycardia (PVT) and ventricular fibrillation (VF) triggered by short coupled ventricular ectopic (VE) beats on the T-wave (Fig. 1B). Coronary angiography and echocardiography were normal.
Fig. 1.
A Admission ECG; B Episode of polymorphic VT; C Negative adrenaline challenge; D Negative ajmaline challenge; E ICD electrograms at time of shock.
An inherited arrhythmogenic condition was suspected and further tests requested. Corrected QT intervals remained within normal limits. Cardiac MRI was normal and specifically did not show any evidence of arrhythmic right ventricular cardiomyopathy or false tendon. Adrenaline (Fig. 1C) and ajmaline challenge tests (Fig. 1D) were negative for Brugada and Long QT syndrome. However, family screening demonstrated QT prolongation in his mother, suggesting a possible familial channelopathy.
Beta-blockade proved ineffective. He was therefore started on amiodarone and referred for ICD implantation. Whilst a single chamber ICD, and indeed even a subcutaneous ICD, would be adequate to provide therapy for PVT and VF, a dual chamber device was implanted to program atrial pacing to suppress ectopy. However, he then had two shocks from his ICD for PVT (Fig. 1E).
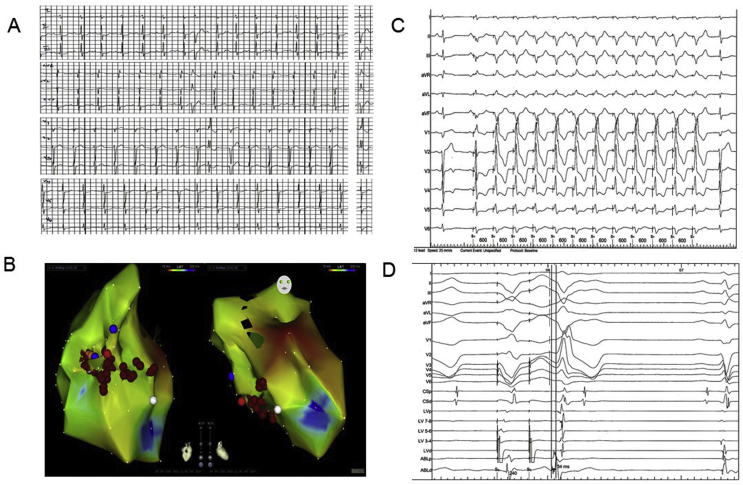
12-lead Holter recording demonstrated a predominant PVC morphology with a relatively narrow QRS, right bundle branch block (RBBB) pattern and left axis deviation, suggesting a focus from the left posterior hemi-fascicle (Fig. 2A). The patient was referred for ablation of the ectopic focus, likely to be occurring through a mechanism of abnormal automaticity or triggered activity. Fast anatomic mapping using the CARTO (Biosense Webster) electroanatomic (EAM) system was used to provide geometry of the LV. An activation mapping approach of the PVC beats was undertaken (Fig. 2B), with a decapolar catheter placed along the left ventricular septum via a retrograde aortic approach. There was unfortunately insufficient clinical ectopy to allow a full activation map using the EAM system; however limited activation mapping suggested the earliest signal lay at an anatomical position consistent with the left posterior hemi-fascicle, and pace mapping in this area gave an 11/12 match (Fig. 2C). During programmed stimulation, a spontaneous clinical ectopic occurred and presystolic Purkinje potentials 34 ms before QRS onset were identified (Fig. 2D). With radiofrequency ablation in the posterior hemi-fascicle there was a transient run of clinical PVCs, after which PVCs were no longer inducible. The patient was maintained on mexelitine and bisoprolol and over a 2 year follow-up has had a single shock for VF triggered by a short coupled VE.
Fig. 2.
A ECG showing predominant PVC morphology; B CARTO electroanatomical map in sinus rhythm showing location of ablation lesions in red; C Pace mapping from the posterior hemi-fascicle showing 11/12 match; D Electrograms from decapolar catheter during programmed stimulation; a spontaneous clinical ectopic occurred and presystolic Purkinje potentials 34 ms before QRS onset are seen on the distal pole of the ablation catheter placed at the posterior hemi-fascicle.
2. Discussion
The Purkinje system is responsible for the mechanisms of a variety of ventricular arrhythmias, which may manifest as monomorphic VT through either verapamil-sensitive left fascicular VT, Purkinje fibre-mediated VT post infarction, bundle branch re-entry or interfascicular re-entry VTs. There is also growing evidence that the Purkinje network plays an important role in the initiation and maintenance of PVT and VF through abnormal automaticity or triggered activity. Specifically, a mechanism of Ca2+ overload in the Purkinje tissue resulting in delayed after-depolarisations leading to re-entry has been proposed [1]. Experimental models have also suggested a role for heterogeneities in upstroke velocity, intracellular coupling and action potential duration [2].
Mutations in several genes encoding ion channels have been identified as the basis for a variety of inherited arrhythmias. For example, an SCN5A-related channelopathy has recently been described with families affected by multifocal ectopic Purkinje-related premature contractions, associated with sudden death [3]. It is possible that a pathogenic channel mutation may provide a link between the patient's PVCs and his mother's prolonged QT.
Our patient was treated with amiodarone or beta-blockers; however, we could also have tried verapamil in an attempt to reduced calcium overload in the Purkinje fibres. This has been used empirically to treat the short-coupled variant of torsade de pointes [4], but in general there is a high recurrence rate of cardiac arrest with drug therapy alone, whether that be with amiodarone, betablockers or verapamil, although there is evidence that in some cohorts, quinidine may be effective [5]. Amiodarone has the additional effect of reducing the probability of degeneration into VF by increasing tissue refractoriness.
Advances in mapping techniques have allowed the use of catheter ablation as a treatment option. Several groups have reported successful ablation of Purkinje-related VF post myocardial infarction, as well as in amyloidosis, chronic myocarditis and non-ischemic cardiomyopathy. Unlike cases of structural heart disease in which there is a clear substrate, the role of ablation in idiopathic VF, in which the underlying abnormality may be a genetic condition with more widespread electrical instability, remains undefined [6]. Recurrence rates are reported at around 18% at 2 years [7] and therefore ablation cannot be thought as a cure or substitute for an ICD, although it plays a useful role in controlling electrical storm.
Clinical learning points include the consideration of a Purkinje focus for triggering VPCs in cases of PVT/VF and referral for ablation in cases refractory to anti-arrhythmics. We demonstrate the importance of recording 12 lead ECGs of the PVCs to allow pace mapping. Due to the unpredictable nature of PVCs, the optimal time for ablation is often immediately following an electrical storm when PVCs are most frequent and may be captured during electrophysiological study.
Pace mapping to reproduce a similar activation pattern and QRS morphology is frequently used as a surrogate for the target site. A potential limitation in the case of any Purkinje or fascicle-related arrhythmia, whether automatic or reentrant, is that it may be difficult to capture the specialized conduction fibres without also capturing surrounding myocardium or other nearby Purkinje fibres with disparate exits. Thus, a similar pace map may be seen but because of a large virtual electrode capturing critical circuit elements that are farther away, ablation locally might not necessarily be successful. Alternatively, a significantly different pace map may be seen despite being at the site of a critical circuit component because of simultaneous capture of local ventricular myocardium or differences in ventricular activation through distal components of the fascicular–Purkinje system during pacing versus tachycardia.
Family screening and genetic testing should be considered to investigate for underlying inherited channelopathies. With further improvements in our understanding of the mechanisms responsible for VF, we should aim to predict which patients with PVCs are at greatest risk of VF or recurrence, and define what represents the most effective ablation strategy.
Acknowledgements
PL was supported by University College of London Hospitals Biomedicine Research Centre, a Partnership between University College of London and University College of London Hospitals NHS Trust, funded by the National Institute for Health Research (NIHR).
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Myerburg R.J., Nilsson K., Gelband H. Physiology of canine intraventricular conduction and endocardial excitation. Circ Res. 1972;30:217–243. doi: 10.1161/01.res.30.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Berenfeld O., Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ Res. 1998;82:1063–1077. doi: 10.1161/01.res.82.10.1063. [DOI] [PubMed] [Google Scholar]
- 3.Laurent G., Saal S., Amarouch M.Y. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60:144–156. doi: 10.1016/j.jacc.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Leenhardt A., Glaser E., Burguera M., Nürnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S., Belhassen B. Idiopathic ventricular fibrillation. Am Heart J. 1990;120:661–671. doi: 10.1016/0002-8703(90)90025-s. [DOI] [PubMed] [Google Scholar]
- 6.Tan V.H., Yap J., Li-Fern Hsu, Liew R. Catheter ablation of ventricular fibrillation triggers and electrical storm. Europace. 2012;14:1687–1695. doi: 10.1093/europace/eus050. [DOI] [PubMed] [Google Scholar]
- 7.Knecht S., Sacher F., Wright M. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]