Abstract
Objective
To evaluate the relationship between RAS mutation and resection margin status in patients undergoing resection of colorectal liver metastases (CLM).
Background
In patients undergoing resection of CLM, resection margin status is a significant predictor of survival, particularly in patients with suboptimal response to preoperative therapy. RAS mutations have been linked to more invasive and migratory tumor biology and poor response to modern chemotherapy.
Methods
Patients who underwent curative resection of CLM from 2005 through 2013 with known RAS mutation status were identified from a prospectively maintained database. A positive margin was defined as tumor cells less than 1 mm from the parenchymal transection line.
Results
The study included 633 patients, of whom 229 (36.2%) had mutant RAS. The positive margin rate was 11.4% (26/229) for mutant RAS and 5.4% (22/404) for wild-type RAS (P = 0.007). In multivariate analysis, the only factors associated with a positive margin were RAS mutation (hazard ratio [HR], 2.439; P = 0.005) and carcinoembryonic antigen level 4.5 ng/mL or greater (HR, 2.060; P = 0.026). Among patients presenting with liver-first recurrence during follow-up, those with mutant RAS had narrower margins at initial CLM resection (median, 4 mm vs. 7 mm; P = 0.031). A positive margin (HR, 3.360; P < 0.001) and RAS mutation (HR, 1.629; P = 0.044) were independently associated with worse overall survival.
Conclusion
RAS mutations are associated with positive margins in patients undergoing resection of CLM. Tumors with RAS mutation should prompt careful efforts to achieve negative resection margins.
Keywords: RAS, mutation, resection, margin, colorectal liver metastases
INTRODUCTION
Historically, the finding of viable tumor cells at the resection margin after resection of colorectal liver metastases (CLM) has been associated with reduced overall and recurrence-free survival.1–10 However, reports based on more recent patient series have called into question the impact of positive resection margins on survival.11–13 This discrepancy has been attributed to more effective modern chemotherapy and targeted therapies administered preoperatively and/or postoperatively.13 We recently showed that a positive resection margin remained significantly associated with worse prognosis even in the era of modern preoperative chemotherapy.14 In a recent report of a detailed pathologic analysis of resection margins in patients undergoing resection of CLM, improved survival was reported in patients with negative margins smaller than 1 mm.15 However, the biologic factor(s) driving these margin-based differences in prognosis remain unclear.
Rat sarcoma viral oncogene homolog (RAS) mutations are found in 15% to 35% of patients with resectable CLM and have been associated with reduced overall and recurrence-free survival after hepatectomy.16–18 Furthermore, RAS mutations have been found to predict worse morphologic and pathologic response to chemotherapy and not only to monoclonal antibodies targeting the epidermal growth factor receptor.19–22 Other reports have suggested that RAS mutation indicates a more migratory and invasive tumor biology.23–25 Taken together, these findings indicate that RAS mutation reflects a more aggressive tumor phenotype and may have implications regarding the optimization of local therapy in patients with resectable CLM.
Previously, investigators hypothesized that a positive resection margin is a surrogate marker of worse tumor biology irrespective of the apparent correlation between a positive resection margin and poor surgical technique.13, 26 Based on this hypothesis, and in support of findings indicating that RAS mutation represents a more aggressive tumor phenotype, the aim of the current study was to evaluate the relationship between RAS mutation and resection margin status in patients undergoing resection of CLM.
METHODS
Study Population
This study was approved by the Institutional Review Board (IRB) of The University of Texas MD Anderson Cancer Center (IRB protocol PA13-0795). The prospective institutional liver database was searched to identify patients who underwent curative resection of CLM with known RAS mutation status at MD Anderson from 2005 through 2013 without concomitant radiofrequency ablation. For each patient, the following data were extracted from the prospective institutional liver database or updated by journal review if missing: sex, age, location of primary cancer, lymph node status of primary cancer, disease-free interval between resection of the primary cancer and presentation with liver metastases, number of CLM, diameter of the largest CLM, RAS mutation status, preoperative chemotherapy, number of cycles of preoperative chemotherapy, type of preoperative chemotherapy, type of liver resection, pathologic response to preoperative chemotherapy, site of any recurrence, and overall survival.
Disease Management
Helical computed tomography of the chest, abdomen, and pelvis with a triphasic liver protocol was used in all patients to assess resectability and extrahepatic disease. Resection of CLM in the presence of extrahepatic disease was only performed if the extrahepatic disease was judged to be completely resectable. Two-stage hepatectomy and portal vein embolization were used to extend resectability in patients with insufficient standardized future liver remnant volume.27, 28 Intraoperative ultrasonography was used in all patients to assess the vascular anatomy of the portal pedicles and the hepatic veins and to assess previously known and undetected lesions. The parenchymal transection was performed using a two-surgeon technique with the Cavitron Ultrasonic Surgical Aspirator (Valleylab, Boulder, CO) and saline-linked cautery (Dissecting Sealer DS 3.0, Tissue Link Medical, Inc., Dover, NH) under total or selective hepatic inflow.29 Preoperative oxaliplatin- or irinotecan-based chemotherapy including bevacizumab (6 cycles as a standard) was used in the majority of patients. In most patients, chemotherapy was reintroduced after surgery to complete a total of 12 cycles. Radiological follow-ups were performed every 4 months after surgery to assess for recurrence.
Histological Examination and RAS Mutation Profiling
Upon histological examination of the resected specimen, the pathologist verified the presence of CLM and assessed the width of the margin and the percentage of viable tumor cells. A positive resection margin was defined as viable tumor cells less than 1 mm from the resection margin as previously described.5 Complete or major pathologic response to preoperative chemotherapy was defined as 49% or fewer viable tumor cells.30 DNA from CLM was used to determine RAS mutation status: routine polymerase chain reaction-based primer extension assay was performed to screen for mutations in KRAS codons 12 and 13 in all patients and for mutations in KRAS codons 64 and 161 and NRAS codons 12, 13, and 62 in the majority of patients in the most recent years of the study period. The lower limit of detection of this assay was approximately one mutant allele in the background of nine wild-type alleles. Single mutations in the various codons of KRAS and NRAS were analyzed together and reported as RAS mutations.
Statistical Analysis
The Shapiro-Wilk test was used to assess whether continuous data were normally distributed and could thus be summarized in terms of mean with standard deviation and compared with independent t tests. Non-normally distributed continuous data were summarized in terms of median with range and compared with the Mann-Whitney U test. Categorical data were compared by Pearson chi-squared tests. A P value of less than 0.05 was considered statistically significant. When the continuous variables carcinoembryonic antigen level and diameter of the largest CLM were converted into binary categories, the cutoff was set between the median/mean values of the groups to be compared. Factors with P value of less than 0.1 from univariate analyses were entered into multivariate analyses. Binary logistic regression with enter method for the covariates was used to perform a multivariate analysis to assess predictors of a positive margin. Cox regression survival analyses with enter method for the covariates were conducted to determine factors associated with overall survival. Only factors with P value of less than 0.1 in multivariate analyses were reported. Kaplan-Meier method was used to estimate survival rates, and survival curves were compared using the log-rank test. The statistical analyses were performed with SPSS version 19.0 (SPSS Inc., IBM, Chicago, IL).
RESULTS
Patient Characteristics According to the Status of the Resection Margin
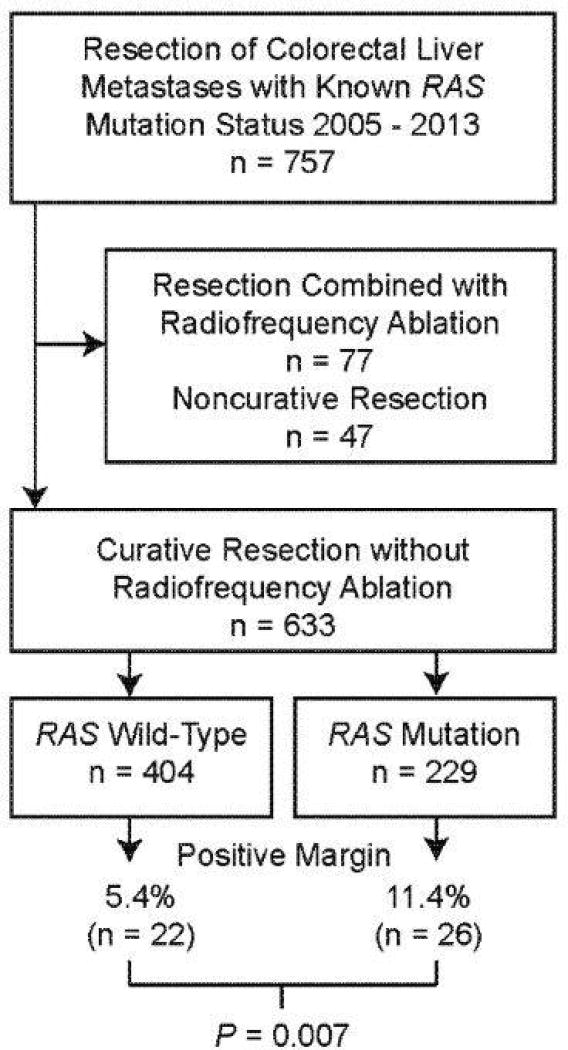
RAS mutation status was available in 757 patients who underwent resection of CLM from 2005 through 2013. Of these 757 patients, 633 underwent curative resection without the concomitant use of radiofrequency ablation and were thus eligible for analyses (Figure 1). Patient characteristics, characteristics of the CLM, use of preoperative chemotherapy and targeted therapies, types of resection (only resection characteristics that differed significantly by margin status are shown), and pathologic response according to resection margin status are listed in Table 1. The mean age was 55.8 years (range, 23–84 years), and there were 371 men (58.6%) and 262 women (41.4%). The primary cancer was located in the colon in 484 patients (76.5%) and in the rectum in 149 patients (23.5%). Positive lymph nodes in relation to the primary cancer were found in 403 patients (63.7%). There were no associations between a positive resection margin and sex, age, location of the primary cancer, or lymph node status of the primary cancer.
Figure 1.
Flow chart of study population according to RAS mutation status and resection margin positivity.
Table 1.
Patient, Disease, and Treatment Characteristics and Pathologic Response According to Resection Margin Status
| Resection margin status | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | All patients | Positive | Negative | P |
| Total number of patients, n (%) | 633 | 48 (7.6) | 585 (92.4) | |
| Sex, n (%) | ||||
| Female | 262 | 23 (8.8) | 239 (91.2) | 0.340 |
| Male | 371 | 25 (6.7) | 346 (93.3) | |
| Age, years, mean (SD) | 55.8 | 56.6 (12.1) | 55.8 (10.7) | 0.626 |
| RAS mutation status of CLM, n (%) | ||||
| Wild-type | 404 | 22 (5.4) | 382 (94.6) | 0.007 |
| Mutant | 229 | 26 (11.4) | 203 (88.6) | |
| Disease-free interval a, n (%) | ||||
| ≤ 12 months (metachronous CLM) | 187 | 11 (5.9) | 176 (94.1) | 0.295 |
| > 12 months (synchronous CLM) | 446 | 37 (8.3) | 409 (91.7) | |
| Number of CLM, n (%) | ||||
| Single | 296 | 22 (7.4) | 274 (92.6) | 0.851 |
| Multiple | 332 | 26 (7.8) | 306 (92.2) | |
| Diameter of largest CLM, mm, mean (SD) | 28 | 35 (30) | 27 (22) | 0.024 |
| CEA, ng/mL, median (range) | 3 (0–2915) | 6 (1–1993) | 3 (0–2915) | 0.031 |
| Preop chemo, n (%) | ||||
| Yes | 545 | 41 (7.5) | 504 (92.5) | 0.887 |
| No | 88 | 7 (8.0) | 81 (92.0) | |
| Cycles of preop chemo, n (%) | ||||
| ≤ 6 | 374 | 24 (6.4) | 350 (93.6) | 0.216 |
| > 6 | 182 | 17 (9.3) | 165 (90.7) | |
| Preop bevacizumab, n (%) | ||||
| Yes | 420 | 33 (7.9) | 387 (92.1) | 0.743 |
| No | 197 | 14 (7.1) | 183 (92.9) | |
| Preop cetuximab/panitumumab, n (%) | ||||
| Yes | 31 | 2 (6.5) | 29 (93.5) | 0.807 |
| No | 602 | 46 (7.6) | 556 (92.4) | |
| Extended liver resection, (%) | ||||
| Yes | 144 | 18 (12.5) | 126 (87.5) | 0.011 |
| No | 489 | 30 (6.1) | 459 (93.9) | |
| Left hepatectomy, n (%) | ||||
| Yes | 43 | 7 (16.3) | 36 (83.7) | 0.023 |
| No | 586 | 40 (6.8) | 546 (93.2) | |
| Partial (including wedge) resection, n (%) | ||||
| Yes | 461 | 28 (6.1) | 433 (93.9) | 0.019 |
| No | 172 | 20 (11.6) | 152 (88.4) | |
| Pathologic response, n (%) | ||||
| Complete or major (0–49% VTC) | 255 | 19 (7.5) | 236 (92.5) | 0.222 |
| Minor (50–100% VTC) | 175 | 19 (10.9) | 156 (89.1) | |
SD: standard deviation; CEA: carcinoembryonic antigen level at resection of CLM; Preop: preoperative; chemo: chemotherapy; VTC: viable tumor cells
Interval between resection of the primary colorectal cancer and diagnosis of CLM.
A RAS mutation was found in 229 patients (36.2%), and RAS mutation was associated with a positive resection margin in univariate analysis (rate of positive margins, 11.4% in patients with mutant RAS vs. 5.4% in patients with wild-type RAS; P = 0.007; Table 1; Figure 1).
Thirty-one patients (4.9%) received cetuximab or panitumumab perioperatively, and the rate of a positive resection margin was the same in these patients as in those who did not receive these treatments.
Factors Associated with a Positive Resection Margin
In univariate analyses (Table 2), factors associated with a positive resection margin were diameter of the largest CLM 30 mm or more, carcinoembryonic antigen level 4.5 ng/mL or more, RAS mutation, extended liver resection, left hepatectomy, and non-partial hepatectomy. Major hepatectomy (> 3 segments), right hepatectomy, the second stage of two-stage hepatectomy, and bilateral resection were not associated with a positive resection margin. The only independent factors predicting a positive resection margin were carcinoembryonic antigen level 4.5 ng/mL or more (HR, 2.060; 95% CI, 1.090–3.893; P = 0.026) and RAS mutation (HR, 2.439; 95% CI, 1.300–4.575; P = 0.005).
Table 2.
Logistic Regression Analyses of Factors Associated With a Positive Resection Margin
| Univariate analyses | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factor | HR | 95% CI | P | HR | 95% CI | P |
| Diameter of largest CLM ≥ 30 mm | 1.897 | 1.030 – 3.495 | 0.040 | |||
| CEA ≥ 4.5 ng/mL | 2.283 | 1.256 – 4.150 | 0.007 | 2.060 | 1.090 – 3.893 | 0.026 |
| RAS mutation | 2.224 | 1.229 – 4.023 | 0.008 | 2.439 | 1.300 – 4.575 | 0.005 |
| Extended resection | 2.186 | 1.180 – 4.050 | 0.013 | |||
| Left hepatectomy | 2.654 | 1.111 – 6.341 | 0.028 | |||
| Non-partial hepatectomy | 2.035 | 1.114 – 3.718 | 0.021 | |||
Width of the Resection Margin in Patients with RAS Mutant and RAS Wild-Type CLM
Among all patients, the median width of the resection margin was similar between patients with RAS mutant CLM (5 mm [range, 0–80]) and patients with RAS wild-type CLM (6 mm [range, 0–90]) (P = 0.131). However, in the group of patients with resection margins of 10 mm or less (n = 448), the median width of the resection margin was significantly smaller in patients with RAS mutant CLM: 3 mm (range, 0–10) versus 4 mm (range, 0–10) (P = 0.045).
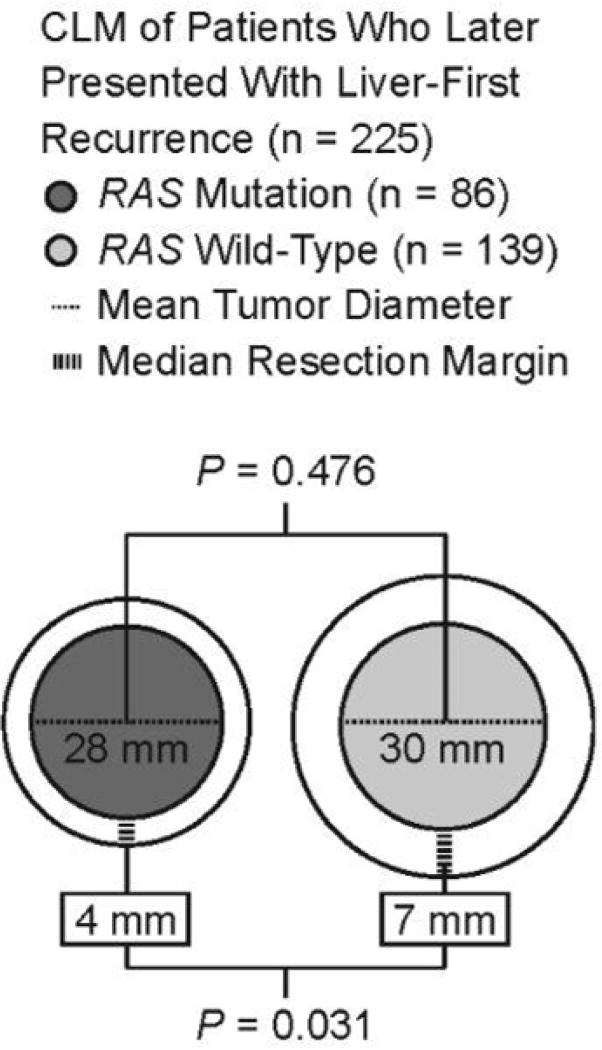
The mean follow-up time for the entire cohort was 26 months, during which 407 patients (64.3%) developed recurrence. Of the 407 patients with recurrence, 225 (55.3%) developed liver-first recurrence (Figure 2). Among these patients with liver-first recurrence, the median width of the resection margin at initial resection of CLM was smaller in patients with RAS mutant CLM than in patients with RAS wild-type CLM: 4 mm (range, 0–70) versus 7 mm (range, 0–67) (P = 0.031). Among the same patients, the mean diameter of the largest metastasis at initial resection of CLM (RAS mutant, 28 mm; RAS wild-type, 30 mm; P = 0.476) and the mean number of metastases at initial resection of CLM (RAS mutant, 2.6; RAS wild-type, 2.5 mm; P = 0.825) were similar between the patients with RAS mutant and RAS wild-type CLM.
Figure 2.
Mean diameter of the largest tumor and median width of the resection margin according to RAS mutation status in patients who presented with liver-first recurrence (n = 225) after resection of CLM.
Impact of Resection Margin Status and RAS Mutation Status on Overall Survival
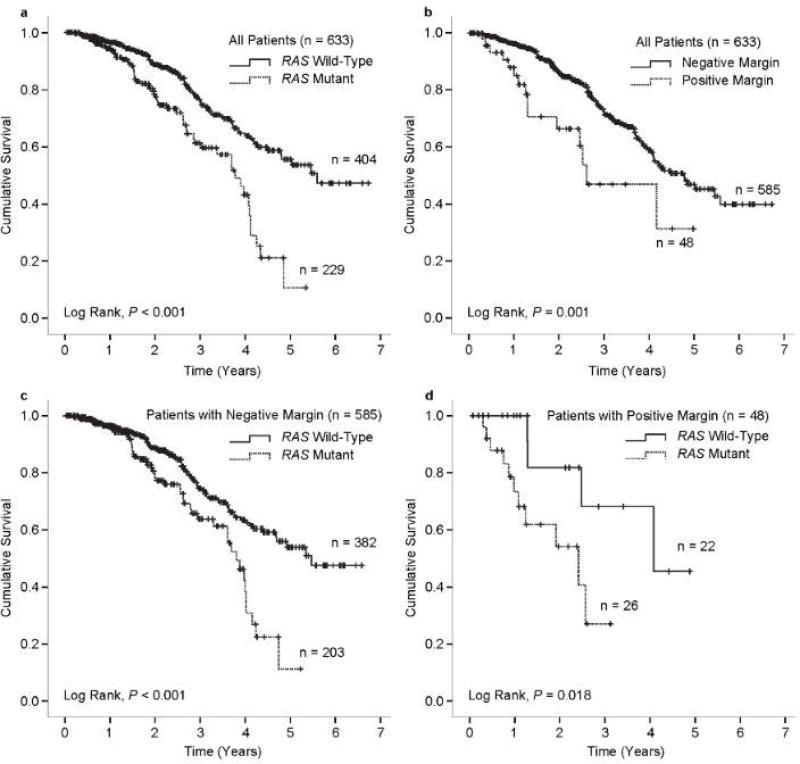
Factors potentially associated with overall survival after resection of CLM were analyzed (Table 3). Factors independently associated with reduced overall survival in multivariate analysis were a positive resection margin (HR, 3.360; 95% CI, 1.741–6.485; P < 0.001) and RAS mutation (HR, 1.629; 95% CI, 1.013–2.620; P = 0.044). Kaplan Meier plots of overall survival by RAS mutation status and margin status are shown in Figure 3.
Table 3.
Cox Regression Analyses of Factors Associated With Overall Survival
| Univariate analyses | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factor | HR | 95% CI | P | HR | 95% CI | P |
| Positive resection margin | 2.423 | 1.414 – 4.153 | 0.001 | 3.360 | 1.741 – 6.485 | < 0.001 |
| Positive lymph nodes in relation to primary cancer | 1.539 | 1.053 – 2.247 | 0.026 | |||
| Disease-free interval > 12 months | 1.293 | 0.891 – 1.877 | 0.176 | |||
| Diameter of largest CLM ≥ 30 mm | 1.564 | 1.106 – 2.214 | 0.012 | |||
| CEA ≥ 4.5 ng/mL | 1.288 | 0.919 – 1.804 | 0.141 | |||
| RAS mutation | 2.181 | 1.547 – 3.077 | < 0.001 | 1.629 | 1.013 – 2.620 | 0.044 |
| Minor pathologic response (50–100% viable tumor cells) | 1.728 | 1.095 – 2.727 | 0.019 | 1.562 | 0.973 – 2.506 | 0.065 |
Figure 3.
Kaplan-Meier plots of overall survival in all patients according to RAS mutation status (a) and resection margin status (b) and in patients with negative resection margins (c) and positive resection margins (d) stratified by RAS mutation status.
DISCUSSION
Several studies have recently reported worse overall and recurrence-free survival in patients with RAS mutation after resection of CLM, independent of perioperative chemotherapy or targeted therapy.16–18, 31, 32. In the current study, the resections were performed without knowledge of RAS status, and RAS mutations were associated with double the positive margin rate (11.4% vs. 5.4%), suggesting phenotypic differences associated with the mutational status of the tumor. In multivariate analysis, both RAS mutation and a positive margin independently predicted worse survival, confirming the importance of adequate local surgical therapy for curative treatment of CLM.14, 15, 33 To our knowledge, this is the first study reporting association of a higher positive margin rate with a feature indicating worse tumor biology.
Two types of tumor growth have been described in CLM, infiltrating growth pattern and pushing growth pattern. Tumors with infiltrating growth have been associated with worse survival and increased risk of liver recurrence after resection of CLM.34–37 Mentha et al. investigated the halo surrounding CLM further and described a dangerous halo of viable tumor cells that infiltrated the surrounding liver parenchyma. In contrast, the good halo had the appearance of a physiological pseudocapsule where the viable tumor cells were contained within a fibroinflammatory reaction and did not penetrate the surrounding liver parenchyma.38 RAS mutations were not assessed in these studies, but other studies have indicated an association between RAS mutations and a more migratory and invasive tumor biology.23–25 As such, the higher rate of positive margins among patients with RAS mutant CLM in the current study may indicate that RAS mutations are associated with a more infiltrating and/or migratory tumor phenotype.
Several studies have reported the presence of microscopic tumor deposits separate from CLM and investigators have aimed to identify the optimal tumor-free resection margin width to clear all viable tumor cells. Kokudo et al. investigated the normal liver parenchyma surrounding CLM and demonstrated the presence of KRAS mutant tumor DNA outside the measured tumor margin in patients with KRAS mutant metastases.39 Similarly, Holdhoff et al. reported detection of mutant tumor-specific DNA 4 mm beyond the visible tumor margin.40 Wakai et al. identified micrometastases, defined as satellites of tumor tissue undetectable on imaging and spatially separated from the gross CLM by normal liver tissue, upon histological analysis of the resected CLM specimens.41 In the current study, the median tumor-free margin was 3 mm narrower among patients with RAS mutant CLM than among patients with RAS wild-type CLM who later presented with liver recurrence. This finding indicates that the optimal tumor-free margin width may be inappropriate to investigate without considering differences in the underlying tumor biology. As such for now, the ideal tumor-free margin width remains unknown, and further studies evaluating tumor growth pattern, micrometastases, and RAS mutations are warranted.
We recently reported that a positive resection margin did not worsen survival in patients with a major pathologic response to preoperative chemotherapy.14 However, a positive resection margin was significantly associated with reduced survival in patients with suboptimal or poor response to preoperative chemotherapy, who represented the majority of patients.14 Mise et al. investigated RAS mutations in the context of pathologic and radiologic response and found a strong correlation between RAS mutation rate and the proportion of viable tumor cells in the specimen.19 In the current study, administration of preoperative chemotherapy, the number of preoperative chemotherapy cycles, and the administration of bevacizumab were not associated with a positive resection margin, indicating that the association between RAS mutation and a positive resection margin is independent and unaffected by the association between RAS mutation and response to preoperative chemotherapy.
The current study had the following limitations. First, this study does not provide data on margin recurrence. A previous study showed a low but definite increase in margin recurrence in patients with positive margins of resection of CLM.5 However, given the low incidence of margin recurrence reported in that study (3.8%),5 an analysis of margin recurrence in the current study would not have had sufficient power to detect a difference even if the recurrence data had been available. Second, mutations in KRAS codons 64 and 161 and NRAS mutations were not part of the standard set of mutations analyzed at the beginning of the study period, and some patients may have been misclassified with respect to RAS mutation status as a result. However, given the low rate of mutations at these codons (< 20%),22, 42, 43 this would represent less than 10% of the patients in the current study. Further, if analysis of mutations in KRAS codons 64 and 161 and NRAS had been performed in all patients, the difference between patients with RAS mutant and patients with RAS wild-type CLM would most likely have been even greater as the oncologic function of the different RAS mutations is similar.
The ideal width of the margin for RAS mutant CLM remains unknown. Therefore, in patients with RAS mutant CLM, we recommend the cautious approach proposed by Are et al.8 of obtaining a 10-mm margin if the margin is not limited by anatomical relationships, even though narrower margins have been proposed by other authors.39, 40, 44
CONCLUSION
RAS mutations are associated with a higher rate of positive margins after resection of CLM. No specific recommendations can be made as to the optimal width of margins in the subset of patients with RAS mutation, but future studies regarding tumor growth pattern, micrometastases, and local recurrence may contribute to optimization of local therapy for such patients. However, in the meantime, we do recommend careful intraoperative assessment of the resection margins in patients with known RAS mutations with the goal of achieving a 1-cm margin unless the margin is limited by anatomical relationships. Inversely, these findings support the use of aggressive surgery with margins smaller than 1 cm in patients with RAS wild-type tumors.
Acknowledgments
The authors thank Stephanie Deming, an employee of the Department of Scientific Publications at MD Anderson Cancer Center, for copyediting the manuscript and Ruth J. Haynes, an employee of the Department of Surgical Oncology at MD Anderson Cancer Center, for secretarial assistance in the preparation of the manuscript.
Source of Funding: This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672. Dr. Brudvik is supported by the Department of Hepato-Pancreato-Biliary Surgery, Oslo University Hospital, Norway, and was awarded the Unger-Vetlesen Medical Fund for 2014. Dr Passot is supported by the French Association of Surgery (AFC).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest relevant to this article.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–35. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 3.Bradpiece HA, Benjamin IS, Halevy A, Blumgart LH. Major hepatic resection for colorectal liver metastases. Br J Surg. 1987;74:324–6. doi: 10.1002/bjs.1800740434. [DOI] [PubMed] [Google Scholar]
- 4.Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–31. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–22. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–92. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 7.Figueras J, Burdio F, Ramos E, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–5. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 8.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–84. [PubMed] [Google Scholar]
- 10.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 11.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–37. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 12.Ayez N, Lalmahomed ZS, Eggermont AM, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2012;19:1618–27. doi: 10.1245/s10434-011-2114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truant S, Sequier C, Leteurtre E, et al. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB (Oxford) 2015;17:176–84. doi: 10.1111/hpb.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–88. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadot E, Groot Koerkamp B, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–85. doi: 10.1097/SLA.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–26. doi: 10.1097/SLA.0b013e3182a5025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stremitzer S, Stift J, Gruenberger B, et al. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99:1575–82. doi: 10.1002/bjs.8909. [DOI] [PubMed] [Google Scholar]
- 18.Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137–44. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mise Y, Zimmitti G, Shindoh J, et al. RAS Mutations Predict Radiologic and Pathologic Response in Patients Treated with Chemotherapy Before Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 21.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 22.Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. New England Journal of Medicine. 2013;369:1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 23.Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res. 2005;65:1244–50. doi: 10.1158/0008-5472.CAN-04-1911. [DOI] [PubMed] [Google Scholar]
- 24.Schramm K, Krause K, Bittroff-Leben A, Goldin-Lang P, Thiel E, Kreuser ED. Activated K-ras is involved in regulation of integrin expression in human colon carcinoma cells. Int J Cancer. 2000;87:155–64. doi: 10.1002/1097-0215(20000715)87:2<155::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Serova M, Astorgues-Xerri L, Bieche I, et al. Epithelial-to-mesenchymal transition and oncogenic Ras expression in resistance to the protein kinase Cbeta inhibitor enzastaurin in colon cancer cells. Mol Cancer Ther. 2010;9:1308–17. doi: 10.1158/1535-7163.MCT-10-0167. [DOI] [PubMed] [Google Scholar]
- 26.Mbah NA, Scoggins C, McMasters K, Martin R. Impact of hepatectomy margin on survival following resection of colorectal metastasis: the role of adjuvant therapy and its effects. Eur J Surg Oncol. 2013;39:1394–9. doi: 10.1016/j.ejso.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–9. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 28.Shindoh J, Tzeng CW, Aloia TA, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg. 2013;100:1777–83. doi: 10.1002/bjs.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–7. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–51. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 31.Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–8. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 32.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases British. Journal of Surgery. 2015 doi: 10.1002/bjs.9870. In Press. [DOI] [PubMed] [Google Scholar]
- 33.Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259:543–8. doi: 10.1097/SLA.0b013e3182902b6e. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi J, Komuta K, Matsuzaki S, Okudaira S, Fujioka H, Kanematsu T. Mode of infiltrative growth of colorectal liver metastases is a useful predictor of recurrence after hepatic resection. World J Surg. 2002;26:1122–5. doi: 10.1007/s00268-002-6267-y. [DOI] [PubMed] [Google Scholar]
- 35.Pinheiro RS, Herman P, Lupinacci RM, et al. Tumor growth pattern as predictor of colorectal liver metastasis recurrence. Am J Surg. 2014;207:493–8. doi: 10.1016/j.amjsurg.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen K, Rolff HC, Eefsen RL, Vainer B. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.4. [DOI] [PubMed] [Google Scholar]
- 37.Nagashima I, Oka T, Hamada C, Naruse K, Osada T, Muto T. Histopathological prognostic factors influencing long-term prognosis after surgical resection for hepatic metastases from colorectal cancer. Am J Gastroenterol. 1999;94:739–43. doi: 10.1111/j.1572-0241.1999.00945.x. [DOI] [PubMed] [Google Scholar]
- 38.Mentha G, Terraz S, Morel P, et al. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96:95–103. doi: 10.1002/bjs.6436. [DOI] [PubMed] [Google Scholar]
- 39.Kokudo N, Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–40. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- 40.Holdhoff M, Schmidt K, Diehl F, et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin Cancer Res. 2011;17:3551–7. doi: 10.1158/1078-0432.CCR-10-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakai T, Shirai Y, Sakata J, et al. Histologic evaluation of intrahepatic micrometastases in patients treated with or without neoadjuvant chemotherapy for colorectal carcinoma liver metastasis. Int J Clin Exp Pathol. 2012;5:308–14. [PMC free article] [PubMed] [Google Scholar]
- 42.Netzel BC, Grebe SK. Companion-diagnostic testing limited to KRAS codons 12 and 13 misses 17% of potentially relevant RAS mutations in colorectal cancer. Clin Chim Acta. 2013;425:1–2. doi: 10.1016/j.cca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Thierry AR, Mouliere F, El Messaoudi S, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumo DNA. Nat Med. 2014;20:430–5. doi: 10.1038/nm.3511. [DOI] [PubMed] [Google Scholar]
- 44.Angelsen JH, Horn A, Eide GE, Viste A. Surgery for colorectal liver metastases: the impact of resection margins on recurrence and overall survival. World J Surg Oncol. 2014;12:127. doi: 10.1186/1477-7819-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]