Abstract
Objective
To assess whether providing ventilation during delayed cord clamping (V-DCC) increases placental transfusion compared with delayed cord clamping alone (DCC only).
Study design
Inborn premature infants (230-316 week gestational age) were randomized to receive at least 60 seconds of delayed cord clamping with ventilation (V-DCC: initial continuous positive airway pressure [CPAP]) with addition of positive pressure ventilation if needed) or without assisted ventilation (DCC only). For the DCC only group, infants were dried and stimulated by gently rubbing the back if apneic. The primary outcome was the peak hematocrit in the first 24 hours of life. Delivery room outcomes were analyzed from video recordings and a data acquisition system. Hemodynamic measurements were performed using functional echocardiography (fECHO), near infrared spectroscopy (NIRS), and electrical cardiometry (EC).
Results
There was no difference in the primary outcome of peak hematocrit in the first 24 hours of life. The onset of breathing was similar between both groups (25±20 and 27±28 seconds, p=0.627). However, infants receiving DCC received a greater duration of stimulation than VDCC (41±19 and 20±21 seconds p=0.002). There were no differences in delivery room interventions, early hemodynamics (cerebral oxygenation by NIRS, cardiac output and stroke volume by EC, or superior vena cava flow by fECHO) or neonatal outcomes.
Conclusions
Ventilation during delayed cord clamping was feasible but did not lead to any measurable clinical improvements immediately after delivery or reduce subsequent neonatal morbidity. Caretakers should consider providing adequate stimulation prior to cord clamping.
Trial registration
Keywords: delayed cord clamping, resuscitation, cesarean section, intraventricular hemorrhage
Recently the International Liaison Committee on Resuscitation (ILCOR) recommended delayed cord clamping (DCC) for longer than 30 seconds in both term and premature infants who do not require resuscitation.(1) DCC in preterm infants has been associated with a reduction in intraventricular hemorrhage (IVH) and less need for blood transfusions. A recent Cochrane meta-analysis of 10 randomized controlled trials of DCC compared with immediate cord clamping reported a lower incidence of IVH in those receiving DCC [35J260 (13%)] vs controls [56/279 (20%)], or a 35% relative decrease.(2) This evidence and the ILCOR statement should have changed the standard of care for all preterm deliveries by eliminating immediate cord clamping. Interestingly, in the largest published meta-analysis, DCC reduced overall IVH without a significant reduction in severe (grade 3 or 4) IVH.(2) One possibility is that the volume of placental transfusion may be diminished during cesarean delivery, which is the mode of delivery for approximately 70% of premature infants.(3) Trials comparing DCC to immediate cord clamping found no difference in hematocrit in infants delivered by cesarean. (4-6) The ACOG statement acknowledges that there are limited data to determine whether DCC performed during cesarean delivery can improve placental transfusion.(7) We have previously shown that a placental transfusion with DCC is inferior to umbilical cord milking at cesarean but not during vaginal delivery. (8)
An important consideration is whether resuscitation interventions would be beneficial during a delayed placental transfusion, perhaps by increasing the accepting reservoir for placental blood through decreasing pulmonary vascular resistance. We hypothesized that the provision of early–CPAP during DCC, with addition of positive pressure ventilation (PPV) if the infant was apneic, would increase placental transfusion at cesarean delivery as determined by an increased hematocrit. In addition, we hypothesized that such an approach would improve circulatory hemodynamics, such as cerebral oxygenation and \systemic blood flow measured by echocardiography. Unlike previous trials of DCC that excluded infants that needed resuscitation, we encouraged inclusion of these infants to ensure the most compromised infants received DCC. We also separately stratified and randomized infants after vaginal delivery as the effects of ventilation in this subgroup are unknown.
Methods
This was a randomized blinded controlled trial conducted at Sharp Mary Birch Hospital for Women & Newborns (SMBHWN) and approved by the institutional review board (ClinicalTrials.gov: NCT02231411). Pregnant women who were dated by their earliest ultrasound at <32 weeks gestation were approached for consent. Antenatal consent was not always practical because it would potentially exclude the sickest newborns born to women unable to provide consent before emergent preterm delivery. DCC and providing CPAP or PPV were both separate but standard treatments in the first minute of life and were not felt to add risk because they are routinely provided after delivery. The World Health Organization (WHO) has recommended if there is experience in providing ventilation without cutting the umbilical cord, ventilation can be initiated before cord cutting. (9) Our obstetricians were already routinely providing DCC and were aware that they could override the protocol and perform early clamping if necessary. Therefore, we requested that the IRB grant a deferred waiver of informed consent based on the inability to conduct the trial without a waiver, and minimal risk of either intervention. Parents were approached for antenatal consent if there was adequate time and opportunity (ie, not in active labor) for consent prior to delivery. If antenatal consent was not possible, parents were notified of the intervention by the obstetrician or research team at delivery and were approached immediately after birth to provide written consent to enroll their newborn, and for continued data collection and study related procedures (eg, echocardiograms, additional monitoring). If a parent did not want to enroll their child in the study, we removed the subject from our study. Exclusion criteria included monochorionic multiples, placenta previa, concern for abruption, Rh sensitization, hydrops, and congenital anomalies. If at the time of delivery, an abruption occurred, the cord was immediately clamped and cut and the subject was removed from the trial.
Infants were randomized by opaque, sealed envelopes immediately before delivery. A computer-generated randomization table was used. Subjects were stratified by gestational age and mode of delivery (23-27 6/7 or 28-31 6/7 weeks) to ensure that an equal number of infants born at <28 weeks gestation were in each arm. The research team opened the randomization cards when notified of a subject's impending birth, reviewed the protocol with the obstetricians and recorded the time from delivery until the clamping and cutting of the umbilical cord in both groups. Research staff recorded the type of ventilation (CPAP and/or PPV) and the time elapsed from when the infant was delivered until the time the umbilical cord was clamped. The time to first breath (chest movement) and/or cry was recorded.
When feasible, video recordings were obtained by fixed cameras (GoPro, Cardiff, CA) affixed to the LifeStart Trolley (Inditherm, United Kingdom). The camera was positioned as another method to determine whether the baby was breathing. DCC was performed by the obstetric team by having the delivering obstetrician place the infant on the LifeStart trolley (with a chemical heat mattress placed on top) covered in a sterile drape. The bed was lowered to at least the level of the uterine incision at cesarean delivery or below the mother's introitus at vaginal delivery. A neonatal provider (typically a neonatal nurse practitioner or member of the research team), was draped in sterile gown and handed a facemask connected to a T-piece after the infant was born to avoid contamination of the field. If randomized to V-DCC, the infant was briefly dried and if apneic (no initial gasping or crying efforts) was briefly stimulated by rubbing the infant's back with warm dry towels. The provider then placed the CPAP mask on the infant and delivered a CPAP of 5 cm H20 using a NeoPuff Infant Resuscitator (Fisher and Paykel, New Zealand) at an FiO2 of 0.21. The mask and T-piece were connected to a colorimetric carbon dioxide (CO2) detector (NeoStatCO2, Mercury Medical). The time that the CO2 detector changed color was called out by the provider and recorded by the research staff. If the infant was still apneic PPV (peak inspiratory pressure set at 20 cm H2O) was provided. Lack of color change during PPV demonstrated to the provider that the airway was potentially obstructed, the pressure was insufficient to expand the lungs, that there was excessive air leak, or there was no or inadequate pulmonary blood flow. If there was no color change, the provider either repositioned and adjusted the mask and airway and could deliver prolonged inflations (up to 5 seconds) and repeat if needed. Once ventilation was established, the infant then remained on CPAP of 5 cm H20 until the cord was clamped.
If the infant was randomized to DCC only, the infant was dried and stimulated (by gently rubbing the back with warm sterile towels) if apneic, until the onset of breathing. The occurrence and duration of stimulation and the onset of breathing were recorded by the research team and confirmed by video recordings when available. Once the cord was clamped and cut the infant was handed through a window in the resuscitation suite where the neonatal team (blinded to the intervention) resuscitated the infant according to our unit's protocol, which was that all infants <32 weeks initially received CPAP (at 5 cm H20) or PPV with PEEP (at starting PIP of 20 cm H2O) given by a T-piece with an initial FiO2 of 0.3.
The near infrared (NIRS, FORESIGHT, Casmed, Branford, CT) and electrocardiometry (EC, Cardiotronic, La Jolla, CA) sensors were placed in the NICU once the infant was considered stable by the medical team (1-3 hours of life). The NIRS sensor was placed on the infant's forehead and recorded cerebral oxygen (StO2). The EC sensors were placed on the infant's chest and recorded stroke volume (SV) and cardiac output (CO). Cerebral StO2, CO, and SV were recorded every two seconds and linked with recordings from the bedside monitor (heart rate, arterial oxygenation, and respiratory rate). These variables were recorded for the first 24 hours. A single functional echocardiogram (fECHO) was also performed in infants enrolled in the study within the first 12 HOL to evaluate measures of systemic blood flow.(10, 11) Following study enrollment, relevant data was collected from the mother and the infants.
Stratification was blocked in groups of 8 to allow for an equal distribution of infants <28 weeks and infants from 28 to <32 weeks gestation and by mode of delivery (cesarean and vaginal delivery). Multiples were included and randomized individually. All aspects of data analysis and statistical investigations were performed by our statisticians. We postulated that at least 60 infants in each group born by cesarean delivery would be needed to show at least a 15% difference in peak hematocrits in the first 24 hours with a two-sided alpha of 0.05 and 80% power in those delivered by cesarean. Because approximately 80% of our preterm infants are born by cesarean delivery, we estimated the sample size to be approximately 150 infants (120 cesarean and 30 vaginal delivery). Data was also collected on vaginal delivery and analyzed separately. A subgroup analysis on the entire cohort was also performed on whether infants breathed spontaneously before the cord was clamped. Student's (unpaired) T-test was used for continuous variables and Chi-squared test for categorical variables. We compared continuous data measures averaged over each minute of life using multi-variate linear regression analysis. We used linear, logistic or generalized linear models to evaluate demographic variables and clinical outcome variables using PASW Statistics 20.0 (Chicago, IL).
Results
150 newborns were enrolled (N=75 in each arm) August 2014-October 2015, 125 by cesarean delivery, (Figure 1; available at www.jpeds.com). All infants were analyzed and data were analyzed by intent to treat. There was no difference in the primary outcome of peak hematocrit in the first 24 hours of life. 95% of infants received at least a 60-second delay in cord clamping and 78% were able to be placed on the LifeStart bed. The reason for early clamping was due to the obstetrician's assessment that the infant was too unstable. There were 80 available video recordings (37 DCC only vs. 43 V-DCC).
Figure 1.
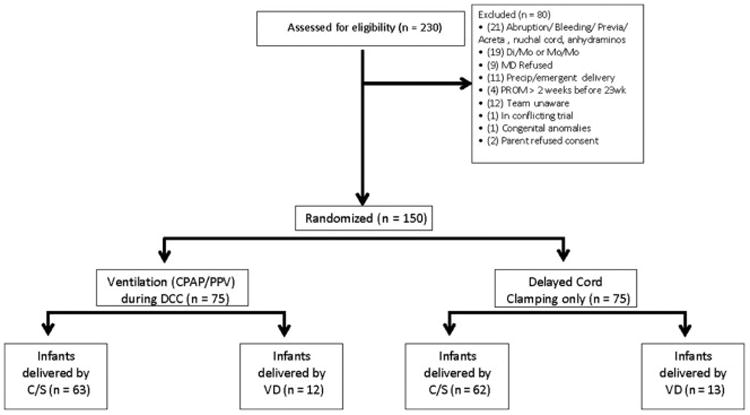
(online) CONSORT Diagram.
Demographics for infants delivery by cesarean are shown in Table I. Neonatal outcomes and interventions performed during DCC in infants delivered by cesarean are shown in Tables II and III. There were more males in the V-DCC group delivered by cesarean (Table II). Although a similar number of infants received stimulation, there was prolonged stimulation for those randomized to the DCC only group (Table III). Eight babies in the DCC only group did not initiate breathing during delayed cord clamping, as assessed by observation and video.
Table 1. Perinatal Outcomes For Infants delivered by cesarean.
| DCC (N=62) | V-DCC (N=63) | p-value | |
|---|---|---|---|
| Gestational Age (Weeks) | 28.47 ± 2.17 | 28.25 ± 2.41 | 0.659 |
| Birthweight (Grams) | 1174.19 ± 407.037 | 1184.94 ± 355.72 | 0.875 |
| Male | 23 (37.09) | 35 (55.55) | 0.049 |
| Antenatal Steroids (≥1 dose) | 60 (97) | 61 (97) | 0.619 |
| Antenatal Magnesium | 58 (93.54) | 55 (87.30) | 0.189 |
| Diabetes (Gestational or Type 1 or 2) | 11 (17.74) | 7 (11.11) | 0.319 |
| Chorioamnionitis | 15 (24.19) | 16 (25.39) | 0.999 |
| Pregnancy-Induced Hypertension | 18 (29.03) | 17 (26.98) | 0.844 |
| Labor or Uterotonics Given Before Delivery | 41 (66.12) | 40 (63.49) | 0.999 |
| General Anesthesia | 2 (3.22) | 0 (0) | 0.244 |
| Lowest Maternal Hb (48 Hours After Deliver), g/dL | 10.58 ± 1.78 | 10.20 ± 1.98 | 0.263 |
p<0.05. Data are presented as mean ± SD; and n (%)
DCC: Delayed Cord Clamping; V-DCC: Ventilation during Delayed Cord Clamping;
Table 2. Neonatal Outcomes for Infants delivered by cesarean.
| DCC Only (N=62) | VDCC (N=63) | p-value | |
|---|---|---|---|
| Median Apgar 1 Minute [IQR] | 7 (5,7) | 7 [4,7] | 0.164 |
| Median Apgar 5 Minute [IQR] | 8 [7,8] | 8 [7,8] | 0.616 |
| Umbilical Cord Gas pH (venous) | 7.30 ± 0.08 | 7.32 ± 0.07 | 0.031 |
| Positive Pressure Ventilation in Delivery Room | 42 (68) | 39 (63) | 0.575 |
| Intubation in Delivery Room | 28 (45) | 24 (38) | 0.471 |
| Surfactant given in NICU (after delivery room) | 25 (40) | 20 (32) | 0.355 |
| Admission Hemoglobin | 16.35 ± 2.17 | 16.57 ± 1.86 | 0.530 |
| Peak Hct % In 1st 24 Hours | 52.1 ± 6.6 | 52.4 ± 6.5 | 0.803 |
| Placenta Weight (Grams) | 225 ± 80 | 218 ± 65 | 0.618 |
| Temperature At 5 Minutes Of Life | 36.27 ± 0.38 | 36.72 ± 0.36 | 0.972 |
| Admission Temperature (C) | 36.84 ± 0.38 | 36.82 ± 0.39 | 0.796 |
| Peak Bilirubin (g/dL) | 7.60 ± 2.05 | 7.68 ± 2.33 | 0.841 |
| Duration of Phototherapy (days) | 3.8 ± 1.5 | 4.1 ± 1.7 | 0.323 |
| Polycythemia (Hct >65) | 1 (2) | 2 (3) | 0.999 |
| Urine output over the first 72 hours (ml/kg/d) | 3.9 ± 0.9 | 3.9 ± 1.3 | 0.994 |
| Median Duration Of Mechanical Ventilation (Days) [IQR] | 1 [0,4] | 1[0,5] | 0.999 |
| Required Blood Transfusion | 26 (42) | 27 (43) | 0.999 |
| Retinopathy Necessitating Surgery | 1 (2) | 3 (5) | 0.619 |
| Necrotizing Enterocolitis | 1 (2) | 1 (2) | 0.999 |
| Intraventricular Hemorrhage | 4 (6) | 9 (14) | 0.241 |
| Severe Intraventricular Hemorrhage (≥Grade 3) | 1 (2) | 2 (3) | 0.999 |
| Periventricular Leukomalacia | 0 | 1 (2) | 0.999 |
| Spontaneous Intestinal Perforation | 2 (3) | 3 (5) | 0.999 |
| Oxygen At 36 Weeks Corrected | 9 (15) | 9 (14) | 0.999 |
| Use of Postnatal Steroids | 8 (13) | 7 (11) | 0.789 |
| Sepsis (Culture Positive) | 4 (6) | 2 (3) | 0.440 |
| Death | 2 (3) | 4 (6) | 0.680 |
p<0.05. Data are presented as mean ± SD; Median, IQR [25th, 75th interquartile range] and n (%) DCC: Delayed Cord Clamping; VDCC: Ventilation during Delayed Cord Clamping;
Table 3. Interventions performed during delayed cord clamping for infants in delivered by cesarean.
| DCC Only (N=62) | VDCC (N=63) | p-value | |
|---|---|---|---|
| Duration of DCC (Seconds) | 62.87 ± 8.77 | 65.93 ± 8.82 | 0.054 |
| Received Stimulation | 31 (50) | 14 (22) | 0.001 |
| Duration Of Stimulation Provided (Seconds) | 40.93 ± 19.32 | 20 ± 20.93 | 0.002 |
| Time To Breathe (Seconds) | 24.984 ± 19.80 | 27.07 ± 27.60 | 0.627 |
| Colorimetric CO2 change | N/A | 57 (90) | N/A |
| Time To CO2 Change (Seconds) | N/A | 34 ± 30 | N/A |
| Required CPAP only | N/A | 26 (41) | N/A |
| Required PPV | N/A | 37 (58) | N/A |
| Duration Of PPV (Seconds) | N/A | 38 ±11 | N/A |
| Breathed Before Cord Clamping | 56 (90) | 58 (92) | 0.763 |
p<0.05. Data are presented as mean ± SD; Median, and n (%). DCC: Delayed Cord Clamping; VDCC: Ventilation during Delayed Cord Clamping; CPAP: Continuous Positive Airway Pressure; PPV: Positive Pressure Ventilation
There were no differences in the primary outcomes of Hct in the first 24 hours of life or placental weights in infants delivered by cesarean. There also were no differences in any neonatal outcomes or hemodynamics by echocardiography (Tables II and IV). There were also no differences in shunt (atrial or ductus arteriosus) direction or peak pulmonary arterial pressure (data not shown). There were no differences in delivery room interventions, oxygen administered, heart rate or saturations in the first 10 minutes of life (Figure 2; available at www.jpeds.com). Other outcomes such as maximum FiO2, maximum peak inspiratory pressure, duration of CPAP or PPV, time to reach heart rate >100 beats per minute, time of resuscitation were also not different (data not shown). There were no differences in blood pressure, heart rate, cerebral oxygenation, arterial oxygenation, cardiac output or stroke volume over the first 24 hours of life (Figure 3; available at www.jpeds.com).
Table 4. Hemodynamic Outcomes for infants Delivered by cesarean.
| DCC Only (N=62) | VDCC (N=63) | p-value | |
|---|---|---|---|
| SVC Flow, ml/kg per min | 86.16 ± 32.15 | 83.19 ± 25.813 | 0.570 |
| RVO, ml/kg per min | 255.77 ± 70.521 | 240.94 ± 69.94 | 0.240 |
| Diameter Of Atrial Shunt, mm | 1.46 ± 0.42 | 1.38 ± 0.54 | 0.378 |
| Diameter Of PDA, mm | 1.40 ± 0.47 | 1.51 ± 0.49 | 0.234 |
| LVO, ml/kg per min | 197.37 ± 65.70 | 184.97 ± 60.02 | 0.273 |
| Need For Pressors (N) | 9 (14.51) | 13 (20.63) | 0.482 |
| PDA Necessitating Treatment (N) | 16 (25.80) | 12(19.04) | 0.398 |
| PDA Ligation (N) | 2 (3.22) | 3 (4.76) | 0.508 |
There were no significant differences between groups. Data are presented as mean ± SD; and N (%). PDA, patent ductus arteriosus.
Figure 2.
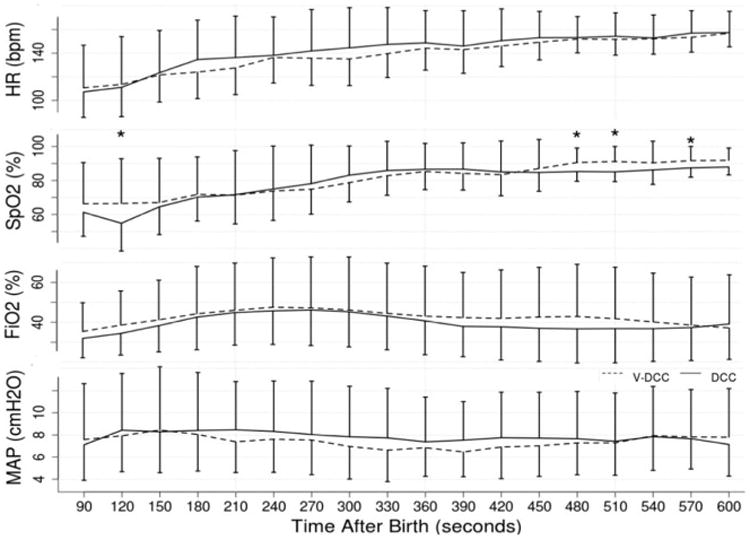
(online) Heart rate (HR), peripheral arterial oxygen saturation (SpO2), administered inspired fractional oxygen (FiO2), Mean Airway pressure (Map) over the first 10 minutes of resuscitation (600 seconds). BPM – beats per minute, VDCC – ventilation during delayed cord clamping, DCC – delayed cord clamping only. *p<0.05 compared to interventional group (VDCC)
Figure 3.
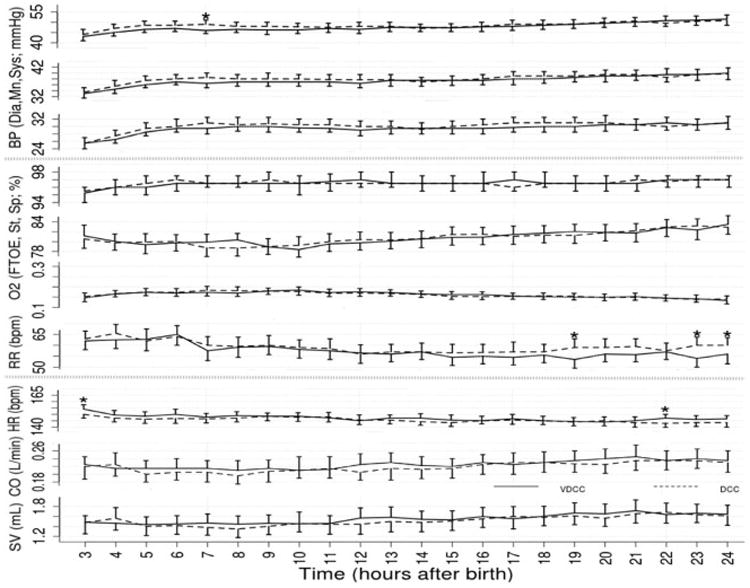
(online) Blood pressure (BP), cerebral oxygenation (StO2), arterial oxygenation (SpO2), fractional oxygen extraction (FTOE), respiratory rate (RR), heart rate (HR), cardiac output (CO) and stroke volume (SV) changes over the first 24 hours of life in neonates receiving DCC or VDCC. Dia- diastolic, Mn- Mean, Sys- Systolic, bpm- beats per minute, VDCC-ventilation during delayed cord clamping, DCC- delayed cord clamping alone. *p<0.05 compared to interventional group (VDCC)
In the V-DCC group there were 75 babies, with 63 were delivered by cesarean. In this group 73 infants had a colorimetric device utilized; of these, 67 had CO2 detected. In addition, 70/75 infants were observed to have breathing movements prior to cord clamping, and 5 babies had no observable breathing movement. At the time of cord clamping, 3 infants who were observed to be breathing had no evidence of colorimetric CO2 change. None of these 3 infants had evidence of severe IVH or death.
Comparison of infants delivered who breathed spontaneously (N=137) or did not breathe before cord clamping (N=13) showed no differences in hematocrit or perinatal or neonatal outcomes in the first 24 hours. The 13 infants who did not breathe before cord clamping (mean time 110 ± 31 seconds) had a higher rate of delivery room PPV (100 vs. 6%, p=0.008) and intubation (81 vs. 38%, p=0.008) compared to the group that breathed (mean time 19 ± 14 seconds), and 2 died (with significant hypotension/acidosis but without severe IVH). Of the 137 infants who breathed before cord clamping, 6 died (5 with severe IVH, 2 with severe hypotension/acidosis, p=0.14, compared to non-breathing infants), and 7 developed severe IVH (p=1.0 compared to non-breathing infants).
There were no differences in any neonatal outcomes in the infants delivered vaginally (N=25) (Tables VI, VII, and VIII; available at www.jpeds.com). The cord pH of DCC only infants was slightly lower (Table VI). Of the 8 infants that did not receive a full 60 seconds of DCC, 1 infant had a grade 4 IVH and was withdrawn from support.
Table 6. (online) Neonatal Outcomes for Infants with Vaginal Delivery.
| DCC Only (N=13) | VDCC (N=12) | p-value | |
|---|---|---|---|
| Median Apgar 1 minute [IQR] | 7 [4,7] | 6 [4,8] | 0.378 |
| Median Apgar 5 minute [IQR] | 8 [6,8] | 8 [7,8] | 0.593 |
| Positive Pressure Ventilation In The Delivery Room | 10 (77) | 5 (42) | 0.111 |
| Intubation In The Delivery Room | 5 (38) | 3 (25) | 0.673 |
| Surfactant given in NICU (after delivery room) | 4 (31) | 4 (33) | 0.999 |
| Admission Hemoglobin | 16.5 ± 1.6 | 16.4 ± 2.1 | 0.892 |
| Peak Hct In 1st 24 Hours | 51.3 ± 5.8 | 51 ± 6.3 | 0.913 |
| Placenta Weight (Grams) | 263 ± 70 | 259 ± 64 | 0.878 |
| Temperature At 5 Minutes Of Life | 37.1 ± 0.5 | 36.9 ± 0.4 | 0.249 |
| Admission Temperature (C) | 37.0 ± 0.6 | 37.0 ± 0.4 | 0.983 |
| Peak Bilirubin (mg/dL) | 8.4 ± 1.7 | 9.1 ± 2.5 | 0.428 |
| Duration of Phototherapy (days) | 4.6 ± 2.2 | 4.3 ± 1.1 | 0.724 |
| Polycythemia (Hct >65) | 0 (0) | 1 (8.3) | 0.480 |
| Urine output over the first 72 hours (ml/kg/d) | 3.5 ± 1.2 | 3.6 ± 0.7 | 0.933 |
| Median Duration Of Mechanical Ventilation (days) [IQR] | 1 [0,8] | 0 [0,5] | 0.999 |
| Required Blood Transfusion | 3 (23) | 3 (25) | 0.999 |
| Retinopathy Necessitating Surgery | 1 (8) | 1 (8) | 0.999 |
| Necrotizing Enterocolitis | 0 (0) | 1 (8) | 0.999 |
| Intraventricular Hemorrhage | 4 (31) | 3 (25) | 0.999 |
| Severe Intraventricular Hemorrhage (≥grade 3) | 2 (15) | 2 (17) | 0.999 |
| Periventricular Leukomalacia | 0 | 0 | 0.999 |
| Spontaneous Intestinal Perforation | 4 (31) | 1 (8) | 0.322 |
| Duration Of DCC (Seconds) | 67.00 ± 16.39 | 65.08 ± 5.33 | 0.703 |
| Received Stimulation | 3 (23) | 2 (16) | 0.999 |
| Duration Of Stimulation Provided (Seconds) | 39.00 ± 20.07 | 19.00 ± 22.62 | 0.373 |
| Time To Breathe (Seconds) | 20.50 ± 15.61 | 53.61 ± 107.53 | 0.302 |
| Colorimetric CO2 Change | N/A | 10 (83) | N/A |
| Time To CO2 Change (Seconds) | N/A | 20 ± 17 | N/A |
| Required CPAP Only | N/A | 7 (58) | N/A |
| Required CPAP/PPV | N/A | 5 (42) | N/A |
| Duration Of PPV (Seconds) | N/A | 61.33 ± 13.50 | N/A |
| Breathed Before Cord Clamping | 11 (84.61) | 12 (100) | 0.480 |
| Oxygen At 36 Weeks Corrected | 1 (8) | 2 (17) | 0.593 |
| Use of postnatal steroids | 1 (8) | 2 (17) | 0.999 |
| Sepsis (Culture Positive) | 1 (8) | 1 (8) | 0.999 |
| Death | 1 (8) | 1 (8) | 0.999 |
p<0.05. Data are presented as mean ± SD; Median, IQR [25th, 75th interquartile range] and n (%) DCC: Delayed Cord Clamping; VDCC: Ventilation during Delayed Cord Clamping; CPAP: Continuous Positive Airway Pressure; PPV: Positive Pressure Ventilation
Table 7. Interventions performed during DO for infants with vaginal delivery.
| DCC only (n = 13) | V-DCC (n = 12) | P value | |
|---|---|---|---|
| Duration of DCC, s | 67.00 ± 16.39 | 65.08 ± 5.33 | .703 |
| Received stimulation | 3 (23) | 2 (16) | .999 |
| Duration of stimulation provided, s | 39.00 ± 20.07 | 19.00 + 22.62 | .373 |
| Time to breathe, s | 20.50 ± 15.61 | 53.61 + 107.53 | .302 |
| Colorimetric CO2 change | N/A | 10 (83) | N/A |
| Time to CO2 change, s | N/A | 20 ± 17 | N/A |
| Required CPAP only | N/A | 7 (58) | N/A |
| Required CPAP/PPV | N/A | 5 (42) | N/A |
| Duration of PPV, s | N/A | 61.33 ± 13.50 | N/A |
| Breathed before cord clamping | 11 (84.61) | 12 (100) | .480 |
N/A, not available.
Data are presented as mean ± SD; median, and n (%).
Table 8. (online) Hemodynamic Outcomes for infants for Infants with Vaginal Delivery.
| DCC Only (N=13) | VDCC (N=12) | p-value | |
|---|---|---|---|
| SVC Flow, ml/kg per min | 90.46 ± 49.15 | 85.17 ± 26.97 | 0.745 |
| RVO, ml/kg per min | 246.08 ± 100.81 | 238.50 ± 67.29 | 0.829 |
| Diameter Of Atrial Shunt, mm | 1.47 ± 0.42 | 1.49 ± 0.69 | 0.914 |
| Diameter Of PDA, mm | 1.39 ± 0.47 | 1.46 ± 0.28 | 0.681 |
| LVO, ml/kg per min | 179.69 ± 54.43 | 192.00 ± 73.51 | 0.637 |
| Need For pressors (N) | 3 (23) | 2 (16.66) | 0.999 |
| PDA Necessitating Treatment (N) | 3 (23) | 2 (16.66) | 0.999 |
| PDA Ligation (N) | 2 (15.38) | 1 (8.33) | 0.999 |
There were no significant differences between groups. Data are presented as mean ± SD; and N (%). PDA, patent ductus arteriosus.
Discussion
Our single center study evaluated the feasibility and efficacy of the use of CPAP and PPV during DCC in premature newborns. Animal studies and one epidemiological study suggest cord clamping should not occur until the newborn is breathing.(12),(13) It has also been suggested that DCC results in inadequate placental transfusion in depressed infants that are not breathing during the delay.(14) Nevill et al compared non-breathing with breathing newborns that received DCC and found that non-breathing infants had a lower 1 minute Apgar score, were more likely to be intubated, and had greater risk of chronic lung disease or severe IVH.(14) We found that the use of CPAP +/- PPV in the first 60 seconds of DCC, in a population of relatively mature preterm infants (mean gestational age 28 weeks) had no effect on placental transfusion, physiological variables in the first 24 hours of life, or neonatal outcomes when compared with stimulation alone.
One unexpected outcome of our finding was the high number of infants that began to establish respirations during DCC. Previous trials suggested that up to 1/3 of infants receiving DCC do not breathe prior to cord clamping, but have several important differences relative to our current study.(14)' (8) First, we included stimulation during DCC as part of the protocol for both groups by a neonatal provider who scrubbed in for the delivery. While both groups had a similar frequency of the initial use of stimulation, the duration of stimulation to establish breathing in the control arm (DCC only) was longer. We acknowledge that the use of a face mask and PPV are potentially equally or more stimulating, and in the V-DCC arm, the provider had to stop stimulation and begin to use CPAP in the intervention arm (V-DCC). Practically speaking a single provider could only perform one intervention at a time so these results were expected. Both interventions likely reduced the number of infants that remained apneic during DCC. Second, the duration of the delay was longer at 60 seconds, providing more time to allow the infant to initiate breathing. Previous studies evaluating breathing used a delay of up to 45 seconds. Third, our use of video recordings of the deliveries and the use of a CO2 detector during ventilation provided objective documentation of respiratory movements and gas exchange improving detection of respiration and appropriate ventilation rather than relying on the provider's assessment of breathing. Our group has had extensive experience with both the use of CO2 detectors during bag mask ventilation(15, 16) and video recordings of neonatal resuscitation.(17) To our knowledge, no study has included these components in a trial of DCC. We were reassured that >90% of infants that established respirations had colorimetric CO2 change before cord clamping was performed.
In the US and other developed countries, ILCOR has recommended that newborns that are apneic or limp at birth (i.e. require resuscitation) receive immediate cord clamping.(1) On the other hand, promoting breathing during DCC has been an integral component of the educational program developed by Helping Babies Breathe to teach neonatal resuscitation techniques to birth attendants in resource-limited areas.(18) The initial algorithm includes emphasis on the “golden minute,” stating that if a newborn is not crying or breathing at birth, drying and stimulating the baby should be performed before the cord is cut.(18)
The rationale for the use of PPV during delayed cord clamping came from animal studies(19),(12) and has been included in the WHO Neonatal Resuscitation guidelines which state that “If there is experience in providing effective PPV without cutting the cord, ventilation can be initiated before cutting the cord. (9)” Bhatt (2013) demonstrated a 50% drop in pulmonary blood flow (PBF) in anesthetized fetal lambs receiving immediate cord clamping, due to the cessation of umbilical venous blood flow (oxygenated blood) from the placenta.(12) Polglase et al demonstrated improved cerebral and systemic oxygenation in preterm lambs if ventilation was provided prior to cord clamping. (19) These data suggest providing lung recruitment with PPV during cord transfusion might improve pulmonary blood flow and prevent sudden changes in cerebral perfusion pressures, which may contribute to the occurrence of severe IVH. However, in this model all the lambs received immediate intubation, general anesthesia that required immediate PPV, and ventilation via an endotracheal tube that bypassed the upper airway. Ventilation in the current trial was by bag and mask which may be inefficient because of upper airway obstruction. The preterm lambs in the Polglase et al study were anesthetized via the placenta by maternal general anesthesia and without any respiratory effort. In such lambs, occlusion of the umbilical cord (without spontaneous respiration) results in “asphyxiation”, bradycardia and reduced cardiac output.
These are important differences when considering extrapolation of such animal research to the human newborn infant. Our trial demonstrates that preterm infants given early stimulation with 60 seconds of DCC are fully capable of spontaneous breathing without any evidence of bradycardia or hypoxemia, and do not require nor benefit from face mask PPV.
An important limitation of our trial is that we did not hypothesize that stimulation during DCC would be equivalent to PPV during DCC. Therefore, our study did not have sufficient power to conclude with certainty that these two interventions are interchangeable. Our trial had extensive monitoring of several other delivery room physiological measurements (heart rate, arterial oxygenation, ventilation), detailed NIRS and cardiac output changes over the first 24 hours of life, and echocardiograms in the first 12 hours of life, none of which suggested physiological benefits of early ventilation. However, we were not able to perform measures of cerebral (NIRS) and systemic blood flow (echocardiograms) in the delivery room. It is possible that other early subtle differences were not detected in a small trial. In addition, our trial had few extremely low birth weight and asphyxiated babies that may have responded differently. While a larger trial could help to clarify the possible clinical benefits, our results suggested minimal physiological improvements with bag and mask CPAP during DCC compared to stimulation alone. In the 8 subjects in the trial that did not receive a full 60 seconds of delay due to early cord clamping, there was no significant increases in any adverse outcomes. Non-breathing infants did not have an increase in death or severe IVH compared to those that did breathe during DCC. These outcomes suggest that performing DCC in infants that do not breathe may not increase the risk of adverse outcomes, but larger trials are needed to confirm this hypothesis.
In conclusion, the provision of gentle tactile stimulation during DCC may hasten the establishment of spontaneous respirations and provide a similar placental transfusion compared to CPAP +/- PPV during DCC. The advantage of this approach is that stimulation can be provided by a single person (often the obstetrician) without the need of respiratory equipment or a neonatal provider. We speculate that spontaneous respirations induced by stimulation prior to cord clamping may have reduced the difference in the primary outcome compared to the CPAP with PPV group.
Table 5. (online) Perinatal Outcomes For Infants with Vaginal Delivery.
| DCC (N=13) | VDCC (N=12) | p-value | |
|---|---|---|---|
| Gestational Age (Weeks) | 28 ± 3 | 29 ± 3 | 0.747 |
| Birthweight (Grams) | 1260 ± 419 | 1435 ± 424 | 0.308 |
| Male | 9 (69) | 10 (83) | 0.645 |
| Antenatal Steroids (≥1 dose) | 13 (13) | 11 (11) | 0.48 |
| Antenatal Magnesium | 12 (90) | 10 (83) | 0.593 |
| Diabetes (Gestational or Type 1 or 2) | 3 (23) | 1 (8) | 0.593 |
| Chorioamnionitis | 7 (54) | 7 (58) | 0.999 |
| Pregnancy-Induced Hypertension | 0 (0) | 0 (0) | N/A |
| Labor or Uterotonics Given Before Delivery | 0 (0) | 0 (0) | N/A |
| General Anesthesia | 0 (0) | 0 (0) | N/A |
| Lowest Maternal Hb (<48 Hours After Delivery), g/dL | 11.70 ± 0.42 | 9.76 ± 2.58 | 0.392 |
p<0.05. Data are presented as mean ± SD; and n (%)*p<0.05. Data are presented as mean ± SD; Median, and n (%). DCC: Delayed Cord Clamping; VDCC: Ventilation during Delayed Cord Clamping
Acknowledgments
We thank the following investigators for <how they contributed to the study>: Paul Wozniak, Maynard Rasmussen, David Kaegi, Nancy Wight, Jack Anderson, Zahra Ghorishi, Graham Bernstein, Allison Graham, Donna Garey, Melissa Brown, Debbie Dennington, Debra Petruzzelli, Sarah Gonzalez, Kasim Hassen, Stephanie Freeman, Danielle Lazarus, and Randy Nopasri.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (<grant number>).
Abbreviations
- IVH
Intraventricular Hemorrhage
- V-DCC
ventilation during delayed cord clamping
- DCC
delayed cord clamping
- ILCOR
international liaison committee on resuscitation
- CPAP
continuous positive airway pressure
- PPV
positive pressure ventilation
- NIRS
near infrared spectroscopy
- EC
electric cardiometry
- fECHO
functional echocardiogram,
- CO2
carbon dioxide
- HBB
helping babies breathe
- WHO
World Health Organization
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 suppl 1):S204–S41. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 2.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. The Cochrane database of systematic reviews. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Bettegowda VR, Dias T, Davidoff MJ, Damus K, Callaghan WM, Petrini JR. The relationship between cesarean delivery and gestational age among US singleton births. Clin Perinatol. 2008;35(2):309–23. v–vi. doi: 10.1016/j.clp.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Strauss RG, Mock DM, Johnson K, Mock NI, Cress G, Knosp L, et al. Circulating RBC volume, measured with biotinylated RBCs, is superior to the Hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion. 2003;43(8):1168–72. doi: 10.1046/j.1537-2995.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 5.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants' blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117(1):93–8. doi: 10.1542/peds.2004-1773. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell M, Henderson-Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. Journal of paediatrics and child health. 1997;33(4):308–10. doi: 10.1111/j.1440-1754.1997.tb01606.x. [DOI] [PubMed] [Google Scholar]
- 7.Raju TN. Committee Opinion No.543: Timing of umbilical cord clamping after birth. Obstetrics and gynecology. 2012;120(6):1522–6. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 8.Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical Cord Milking Versus Delayed Cord Clamping in Preterm Infants. Pediatrics. 2015;136(1):61–9. doi: 10.1542/peds.2015-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Guidelines Approved by the Guidelines Review Committee. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 10.Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. The Journal of pediatrics. 2004;145(5):588–92. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 11.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F188–94. doi: 10.1136/fn.82.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. The Journal of physiology. 2013;591(Pt 8):2113–26. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersdal HL, Linde J, Mduma E, Auestad B, Perlman J. Neonatal outcome following cord clamping after onset of spontaneous respiration. Pediatrics. 2014;134(2):265–72. doi: 10.1542/peds.2014-0467. [DOI] [PubMed] [Google Scholar]
- 14.Nevill E, Meyer MP. Effect of delayed cord clamping (DCC) on breathing and transition at birth in very preterm infants. Early human development. 2015;91(7):407–11. doi: 10.1016/j.earlhumdev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Finer NN, Rich W, Wang C, Leone T. Airway obstruction during mask ventilation of very low birth weight infants during neonatal resuscitation. Pediatrics. 2009;123(3):865–9. doi: 10.1542/peds.2008-0560. [DOI] [PubMed] [Google Scholar]
- 16.Garey DM, Ward R, Rich W, Heldt G, Leone T, Finer NN. Tidal volume threshold for colorimetric carbon dioxide detectors available for use in neonates. Pediatrics. 2008;121(6):e1524–7. doi: 10.1542/peds.2007-2708. [DOI] [PubMed] [Google Scholar]
- 17.Carbine DN, Finer NN, Knodel E, Rich W. Video recording as a means of evaluating neonatal resuscitation performance. Pediatrics. 2000;106(4):654–8. doi: 10.1542/peds.106.4.654. [DOI] [PubMed] [Google Scholar]
- 18.Singhal N, Lockyer J, Fidler H, Keenan W, Little G, Bucher S, et al. Helping Babies Breathe: global neonatal resuscitation program development and formative educational evaluation. Resuscitation. 2012;83(1):90–6. doi: 10.1016/j.resuscitation.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Polglase GR, Dawson JA, Kluckow M, Gill AW, Davis PG, Te Pas AB, et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PloS one. 2015;10(2):e0117504. doi: 10.1371/journal.pone.0117504. [DOI] [PMC free article] [PubMed] [Google Scholar]


