Abstract
In human breast cancer, overexpression of the protooncogene MET is strongly associated with poor prognosis and high risk of metastasis. It stands out as a reliable prognostic indicator of survival and defines a set of tumors exclusive of those that express HER2 or hormone receptors. Studies have shown that overexpression of mutant forms of MET cause cancer in mice. However, MET mutations have not been found in human breast cancer, and the consequences of overexpression of normal MET are unknown. To investigate the role of MET and other putative oncogenes in breast cancer, we developed an experimental system that involves retroviral delivery of genes into primary mammary epithelial cells, followed by transplantation of the transduced cells into mammary fat pads. Using this approach, we found that overexpression of wild-type MET leads to the development of nonprogressive neoplasms. The lesions progressed to mammary adenocarcinoma when a second protooncogene, MYC, was overexpressed, indicating that MET and MYC cooperate in mammary tumorigenesis. Both the nonprogressive neoplasms and adenocarcinomas display characteristics consistent with transformation and expansion of mammary progenitor cells. The approach described here should provide a useful model with which to efficiently test effects of various genes on tumor development in the breast.
Keywords: breast cancer, mouse models, retrovirus
The MET protooncogene encodes a transmembrane receptor tyrosine kinase, Met, that has been widely implicated in tumorigenesis. Mutations that result in constitutive activation of Met are common in hereditary papillary renal carcinoma and have also been reported in hepatocellular carcinoma, gastric cancer, ovarian cancer, and squamous cell carcinoma. In addition, the wild-type MET gene is amplified or overexpressed in many other types of human cancer, including breast cancer (1). Several studies have found that high levels of Met are associated with poor prognosis and high risk of metastasis in breast cancer (2–4). Overexpression of Met occurs in a group of tumors distinct from those that express estrogen and progesterone receptors and those that overexpress HER2 (5), suggesting that inhibition of Met may provide an opportunity to treat tumors for which no targeted therapy is available.
Little is known about the tumorigenic function of MET in vivo. Overexpression of constitutively activated MET mutants under the metallothionein-1 promoter led to the development of metastatic mammary tumors in transgenic mice (6, 7), and replacement of the wild-type MET allele with various MET mutants resulted in the development of several types of tumors (8). Although these results provide insight into the tumorigenic capabilities of mutant Met proteins, our goal was to generate a mouse model in which the effects of wild-type MET overexpression could be studied, because MET is not mutated in human breast cancer.
Existing mouse models of breast cancer exhibit significant differences in pathology and metastasis from the human disease (9). This may be partly attributed to different breast anatomy and physiology between the two species. It is also possible that the majority of genetically engineered mouse models, which target expression of oncogenes to the mammary gland under promoters that are preferentially expressed in differentiated cells, do not recapitulate spontaneous transformation of various cell types in the human breast. Thus, mouse models in which different cell types can be targeted for transformation might prove useful. A recent report described that overexpression of certain oncogenes, but not others, results in expansion of the mouse mammary progenitor cell population, and this correlates with heterogenous tumor phenotypes (10). This phenomenon supports a current model for human breast cancer in which stem or progenitor cells become transformed, resulting in cellular heterogeneity within tumors as well as phenotypic diversity between tumors (11, 12). Effects of oncogene activation in mammary progenitor cells are difficult to examine, because mammary-specific promoters are highly responsive to steroid hormones (13, 14) and are highly expressed in more differentiated steroid receptor-positive cells. Specific targeting of transgene expression in mammary progenitor cells has not yet been described.
In the developing mammary gland, stem and progenitor cells are present within terminal end buds and along mature ducts and give rise to both myoepithelial and luminal epithelial lineages (15–17). Molecular characterization of mammary progenitor cells has revealed that they are contained within a population of cells that express Sca-1 (15). However, ≈20% of mammary epithelial cells (MEC) are Sca-1-positive, indicating that not all Sca-1-positive cells are progenitor cells. A second more restricted marker of these progenitor cells is cytokeratin 6 (CK6). CK6-positive cells are contained within the Sca-1-positive population (10). CK6 is normally expressed in the mammary gland during embryonic stages and within terminal end buds during ductal morphogenesis, but CK6-positive cells are rare in mature differentiated glands (18).
To better understand the contribution of genes such as MET to breast cancer without restricting expression to differentiated cells, we optimized a retroviral system to introduce genes of interest into the mammary gland with a vector that was likely to express MET in stem and/or progenitor cells. Using this system, we found that overexpression of MET resulted in the development of multiple microscopic foci of neoplasia that failed to progress to full-blown malignancies. Although overexpression of MET alone did not result in the development of tumors, we found that an additional genetic stimulus, overexpression of MYC, cooperated with MET in mammary tumorigenesis. Overexpression of MYC alone resulted in epithelial hyperplasia as previously reported in studies of transgenic mice (19–21). The nonprogressive neoplasia (NPN) and tumors resulting from MET and MET/MYC overexpression, respectively, displayed cellular markers characteristic of mammary progenitor cells, suggesting that MET may play an important role in transformation and expansion of these cells.
Materials and Methods
Transgenic Mice. Mouse mammary tumor virus (MMTV)-rtTA transgenic mice were obtained from Lewis Chodosh (University of Pennsylvania School of Medicine, Philadelphia) (22). TRE-MET transgenic mice were described previously (23). MMTV-rtTA/TRE-MET mice were maintained on chow containing 200 mg/kg doxycycline to induce transgene expression.
Preparation of Ecotropic Retrovirus. BOSC-23 cells were cultured in DMEM with glutamine and 10% FBS. To prepare virus, the cells were cotransfected with pCL-Eco plasmid (24) and the pMIG retroviral vector, which was obtained from Yosef Refaeli (National Jewish Medical and Research Center, Denver) (25). One day after transfection, the medium was changed, and collection of virus-containing medium began on day 2. The medium was filtered through a 0.45-μm filter. Collection of virus from the same cells continued for 1 more day.
Preparation of Primary Mouse MEC. For preparation of primary mouse MEC, 10- to 12-wk-old donor mice were killed, and only the nos. 3, 4, and 5 gland pairs were harvested to minimize contamination with muscle and other tissues. Inguinal lymph nodes were removed before isolation of the no. 4 glands. MEC cultures were prepared as described (26), except that glands were minced with razor blades for 5 min and then digested in 5 ml of collagenase buffer (RPMI with 10 mM Hepes/2.5% FBS/100 μg/ml penicillin/streptomycin) per gram of tissue in the presence of collagenase (1 mg/ml, Sigma) for 1 h at 37°C while shaking at 200 rpm. The cells, still aggregated as mammary organoid structures, were washed five times with collagenase buffer, including two rapid-pulse centrifugation steps to remove single cells such as fibroblasts and enrich for large mammary organoids, as described (27). Organoids were pooled and plated over an area of 45–90 cm2 per gram of tissue (weighed before collagenase treatment) in six-well tissue culture plates (Corning). The organoids were plated in MEC medium [50% DMEM with glutamine/50% F-12 with glutamine/10 mM Hepes/10% FBS/5 μg/ml insulin/1 μg/ml hydrocortisone (Sigma)/10 ng/ml recombinant epidermal growth factor (Roche Applied Science)/50 μg/ml gentamicin/100 μg/ml penicillin/streptomycin] and allowed to attach overnight.
Optimization of Retroviral Infections. Analysis of factors that contribute to successful infection of primary MEC was carried out by using GFP as a marker of infected cells and systematically altering the following parameters: the method used to isolate MEC, cell density during infection, the presence of various growth factors in the medium, retroviral packaging cell lines, polybrene supplementation, length and speed of centrifugation during infection, and the number of successive infections. Factors that proved most important for successful infection of primary MEC are the following: (i) glands should not be overly minced before treatment with collagenase; (ii) to produce the highest viral titer, BOSC-23 cells should be transfected with additional plasmid encoding viral packaging proteins (vs. BOSC-23 cells alone or 293T cells transfected with the packaging proteins); and (iii) polybrene concentration should not exceed 1 μg/ml (data not shown). Using these conditions, we obtained 25–65% of cells expressing GFP after two successive infections, measured by FACS analysis (Fig. 6B, which is published as supporting information on the PNAS web site). We found no significant improvement in infection efficiency when a third spin infection was carried out (data not shown). Cells were infected on 2 consecutive days by replacing the MEC medium with BOSC-23 medium containing packaged retrovirus and supplemented with polybrene (1 μg/ml, Sigma). The plates were centrifuged at room temperature for 1 h at 600 × g. The conditioned medium was then discarded, and fresh MEC medium was added to the cells.
Transplantation of Infected MEC into Cleared Mammary Fat Pads. On the fourth day in culture, MEC were trypsinized to a single cell suspension, washed with PBS, and counted. The cells were resuspended at a concentration of 108 cells per ml in PBS and transferred to ice. Three-week-old recipient mice were anesthetized with avertin (125–400 mg/kg), and 106 MEC were injected in a volume of 10 μl into the cleared inguinal fat pads, as described (28).
Whole Mounts and Tissue Processing. Glands were dissected from mice and flattened between two glass slides for visualization of GFP expression on a fluorescent dissecting microscope. The glands were then fixed in 4% paraformaldehyde on ice for 4–16 h. Carmine alum staining of whole mounts and processing of tissue for histology was performed by using standard methods.
Immunohistochemistry. Tissue sections were stained by using ABC Elite and MOM immunohistochemistry reagents and avidin/biotin blocking reagents (Vector Laboratories). Antibodies used were anti-cytokeratin 18 (1:30; Progen, Heidelberg), anti-cytokeratin 14 (1:10,000; Covance, Princeton), anti-CK6 (1:100; Covance), antiphospho-tyrosine-Met (1:25; Cell Signaling Technology, Beverly, MA), and anti-human Met (1:500; Zymed).
Results
Overexpression of MET via Retroviral Transduction in Primary MEC Resulted in Focal NPN. To determine whether overexpression of wild-type MET contributes to the development of breast cancer, we took two approaches. First, we obtained transgenic mice that express the tetracycline-responsive transactivator protein under the MMTV promoter (MMTV-rtTA) and crossed them to transgenic mice that express MET under the tetracycline response element (TRE-MET). Despite extensive analysis, we could not discern any consistent adverse effect of MET overexpression in MMTV-rtTA/TRE-MET animals, even though MET was appropriately overexpressed, and the protein was activated by phosphorylation (Fig. 7, which is published as supporting information on the PNAS web site).
As a second approach to overexpress MET, we devised a system in which we hoped to target gene expression to multiple cell types in the mammary gland, including mammary progenitor cells. We infected primary MEC with pMIG, a retroviral vector derived from mouse stem cell virus, which was originally developed for its ability to be expressed in embryonic stem cells (29). We optimized retroviral infection of MEC, as described in Materials and Methods. Using an ecotropic envelope, we were able to consistently achieve infection of 25–65% of primary MEC, as determined by FACS analysis using GFP as a marker of infected cells (Fig. 6B). After infection, the MEC were transplanted into cleared inguinal fat pads of 21-day-old syngenic mice. Reconstituted glands harvested 8–10 wk after transplantation showed normal outgrowth of GFP+ cells and, as expected, all fat pads were filled with ductal structures arising solely from the transplanted cells; control fat pads that were cleared of endogenous epithelium but did not receive injections of cells remained empty (data not shown). Because the transplanted MEC gave rise to GFP+ outgrowths, a portion of the infected cells were indeed mammary progenitor cells; only progenitor cells are capable of giving rise to a mammary outgrowth after transplantation (15, 30).
To conditionally overexpress wild-type human MET in the mouse mammary gland, we infected primary MEC from mature virgin TRE-MET female mice with a retrovirus expressing a cassette consisting of the tetracycline-repressible transactivator protein (tTA) followed by an internal ribosome entry site and GFP (pMIG-tTA; diagrammed in Fig. 6A). As controls, TRE-MET transgenic MEC were infected with empty pMIG vector, which expresses GFP alone (25). Animals were treated with doxycycline to repress expression of MET or were left untreated to allow for MET overexpression.
Ten weeks after transplantation, mammary gland outgrowths that overexpressed MET displayed abnormal lobule-like structures (Fig. 1 B, D, and F). Glands arising from MEC expressing GFP alone had no abnormalities (see Figs. 1 A, C, and E and 2 A, C, and E). Hematoxylin/eosin staining of sections from mammary gland outgrowths revealed that the “lobules” in MET-expressing glands were actually disorganized clusters of epithelial cells (Fig. 2B). Immunohistochemical staining showed that the cytokeratin 18-positive luminal epithelial cells were abnormally clustered together, and often there was little or no discernable lumen (Fig. 2D). Likewise, the cytokeratin 14-positive myoepithelial cells were either scattered about or organized around filled islands of cells (Fig. 2F). Histological analysis confirmed that the epithelial cells were atypical with large nuclei, condensed chromatin, large nucleoli, and little cytoplasm (data not shown). TRE-MET/pMIG-tTA mammary outgrowths from mice that were treated with doxycycline to repress expression of MET were not distinguishable from those expressing GFP alone (Fig. 2G and data not shown).
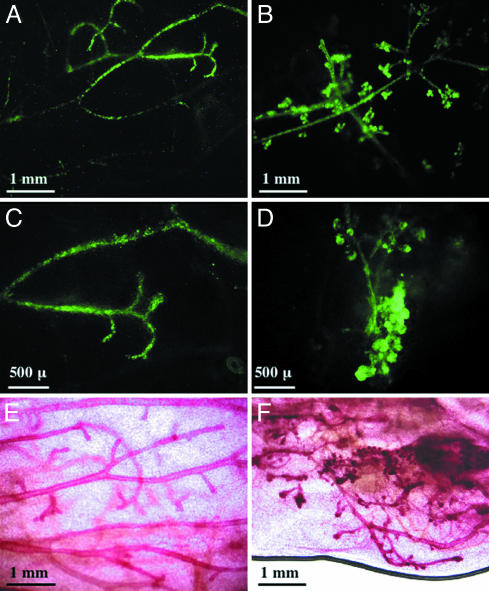
Fig. 1.
Overexpression of wild-type MET resulted in abnormal mammary gland development. Mammary gland outgrowths expressing either GFP alone (A, C, and E) or MET and GFP (B, D, and F) were evaluated by whole-mount microscopy 10 wk after transplantation. Infected cells were visualized by GFP fluorescence (A–D), and the entire gland was visualized by carmine alum staining (E and F).
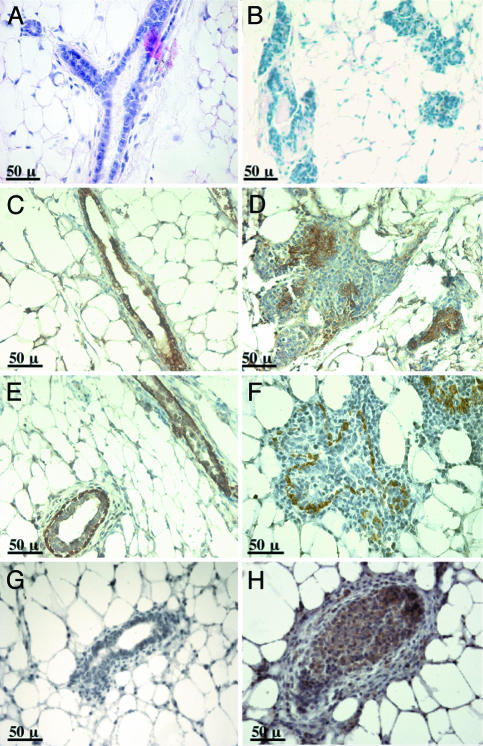
Fig. 2.
Overexpression of MET resulted in ductal filling and loss of organization. Glands expressing GFP (A, C, and E) or MET and GFP (B, D, and F) were sectioned and stained with hematoxylin/eosin (A and B). Alternatively, sections were stained with antibodies specific for cytokeratin 18 to visualize luminal epithelial cells (C and D) or with antibodies against cytokeratin 14 to detect myoepithelial cells (E and F). Sections from mice that received MEC in which MET expression was repressed by treatment with doxycycline (G) or from mice that received MEC overexpressing MET (H) were stained with antibodies specific for tyrosine-phosphorylated Met.
Based on accepted nomenclature according to the Annapolis guidelines (www.nih.gov/Annapolis-guidelines) for mouse mammary pathology, the phenotype induced by overexpression of MET most closely resembles mammary intraepithelial neoplasia, a spectrum of intraluminal epithelial proliferations with cytologic atypia that includes in situ carcinomas (31). However, many of the lesions resulting from overexpression of MET do not have an intact myoepithelial layer, indicating the neoplasias are not strictly intraluminal. Because the lesions do not progress to tumors (see below), we refer to the MET-induced lesions as NPN. A total of 26 glands expressing MET were analyzed in five independent experiments. Multiple microscopic neoplastic foci were observed in ≈77% (20/26) of the glands after successful transplantation. No green cells were detected in 6/26 of the glands despite successful mammary reconstitution. An additional nine glands were analyzed from mice maintained on doxycycline to repress MET expression. All nine glands appeared normal (as shown in Fig. 2G).
Immunohistochemical staining with antibodies specific for Met (not shown) and tyrosine-phosphorylated Met (Fig. 2H) verified that Met protein was present and actively signaling in epithelial cells that filled the ducts but not in the surrounding stroma. There was evidence for an inflammatory response, manifested by the presence of increased numbers of CD45+ leukocytes compared to the control outgrowths (data not shown); however, these cells represented only a small portion of the stroma in MET-expressing outgrowths. Our data show that overexpression of wild-type MET in the mouse mammary gland leads to NPN with loss of ductal organization. The phenotype was observed by 8 wk after transplantation but did not progress; no tumors or noticeable worsening of the neoplastic lesions was detected over a period of 8 months.
To rule out the possibility that the observations made with overexpression of MET via retroviral transduction/transplantation were an artifact of the procedure, we tested whether MEC expressing MET under control of the MMTV promoter (which has no effect in the intact mammary gland; Fig. 7) would develop NPN upon infection and transplantation. For these experiments, we isolated MEC from 10- to 12-wk-old MMTV-rtTA/TRE-MET transgenic mice, infected them with pMIG, and transplanted them into cleared fat pads of 21-day-old mice that were treated with doxycycline throughout the experiment to induce MET expression. Ten weeks after transplantation, the resulting glands did not show any abnormalities by fluorescent whole-mount analysis (data not shown). These results show that the retroviral infection/transplantation technique does not promote abnormal ductal outgrowth.
MET and MYC Cooperate in Mammary Gland Tumorigenesis. Because mammary outgrowths expressing MET via retroviral transduction do not progress to tumors over the course of several months, we speculated that the transplants might represent an ideal model in which to directly test cooperation of MET with other oncogenes in tumor progression. It has been demonstrated that multiparous transgenic mice expressing the ligand for Met, hepatocyte growth factor/scatter factor develop mammary tumors with an average latency of 8 months, and among other alterations, the tumors were shown to contain excessive amounts of activated Myc protein (32). MYC has been implicated in many types of human cancer, and it is overexpressed in a subset of breast cancers (33). Although MET and MYC loci are coamplified in some gastric cancers (34, 35), it is not known whether MET and MYC are cooverexpressed or whether these genes cooperate in the genesis of breast cancer.
To test whether overexpression of MYC would cause the MET-induced NPN to advance to tumors, we infected MEC from TRE-MET transgenic mice with pMIG-tTA alone or pMIG-tTA and pMIG-MYC. Alternatively, we infected MEC from nontransgenic mice with pMIG-MYC alone. Eight weeks later, we examined the mammary outgrowths. Although MET expression alone induced NPN (Fig. 3 A–C), expression of MYC alone caused increased branching and hyperplastic lateral budding (Fig. 3 D–F), as previously described in transgenic mouse models (19, 21). Coexpression of MET and MYC resulted in high-grade neoplasia in which ductal organization was no longer recognizable by 8 wk after transplantation (Fig. 3 G–I). Glands expressing both MET and MYC displayed a highly disorganized clustering of cells, demonstrated by staining for cytokeratin 14 (Fig. 3 C, F, and I). Whereas myoepithelial cells could still be detected surrounding ductal structures in outgrowths overexpressing MYC alone (Fig. 3F), outgrowths expressing either MET alone (Fig. 3C) or MET and MYC (Fig. 3I) were no longer organized and often did not contain an intact myoepithelial cell layer.
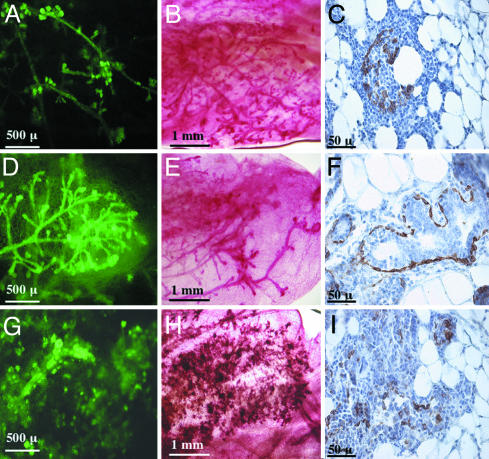
Fig. 3.
MET cooperates with MYC to promote progression of neoplasia in the mammary gland. Whole mounts of mammary gland outgrowths harvested 8 wk after transplantation were viewed by fluorescent microscopy (A, D, and G) and by carmine alum staining and light microscopy (B, E, and H). Sections from mammary glands isolated at this stage were also stained with antibodies against the myoepithelial marker cytokeratin 14 (C, F, ad I). Glands that expressed MET alone (A–C), MYC alone (D–F), or MET and MYC (G–I) are shown.
Although no tumors were observed in glands expressing either MET or MYC alone, mammary glands coexpressing MET and MYC progressed to focal palpable tumors by 10 wk. Histological analysis of tumors at 4 months revealed mammary adenocarcinoma (Fig. 4A), with features typical of highly proliferating lesions including prominent nucleoli and high mitotic indices (Fig. 4B). A total of 25 glands in two independent experiments were analyzed for cooperation between MET and MYC. Tumor penetrance was 100% after successful transplantation. An additional 17 glands were analyzed from mice that received doxycycline to repress MET expression (MYC was expressed alone). All of these glands developed various degrees of hyperplasia, as shown in Fig. 3 D–F.
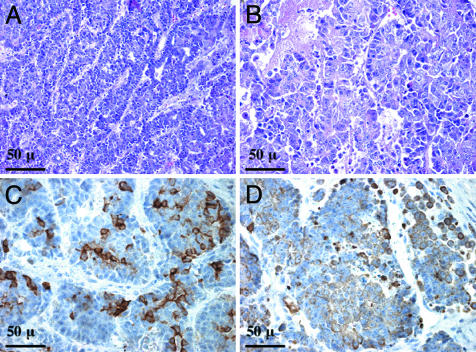
Fig. 4.
MET and MYC cooperate in mammary tumorigenesis. Sections of tumors resulting from expression of both MET and MYC were stained with hematoxylin/eosin (A and B). The tumors were isolated 4 months posttransplantation. Sections were also stained with antibodies against cytokeratin 14 (C) and cytokeratin 18 (D).
Overexpression of MET in Neoplasms Correlates with an Abundance of the Early Developmental Marker CK6. Tumors resulting from overexpression of MET and MYC were remarkable in that they contained cells from both myoepithelial and luminal epithelial cell lineages, as illustrated by staining for cytokeratins 14 and 18, respectively (Fig. 4 C and D). A recent report revealed that mammary hyperplasias and tumors that are composed of heterogenous cell types also express early progenitor cell markers such as Sca-1 and CK6 (10). To determine whether tumors arising from MET/MYC overexpression might be a result of progenitor cell expansion, we examined the expression of CK6 by immunohistochemistry. Fig. 5A shows that MET/MYC-induced tumors contained numerous CK6-positive cells and thus fit into a class of tumors in which heterogeneity of cell types correlates with aberrant expression of the early developmental marker CK6.
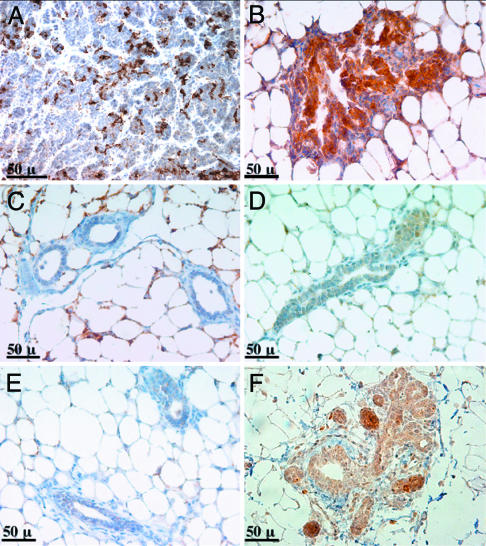
Fig. 5.
Neoplastic lesions induced by MET express the mammary progenitor cell marker CK6. Immunohistochemistry with antibodies specific for CK6 was carried out on sections from the following samples: adenocarcinoma harvested 4 months after coexpression of MET and MYC (A), MET-induced neoplasia harvested 10 wk after transplantation (B), 10-wk mammary outgrowths that expressed GFP alone (C), and mammary glands from 10-wk-old virgin wild-type (D) or MMTV-rtTA/TRE-MET (E) mice. A mammary gland from a 1-year-old MMTV-rtTA/TRE-MET mouse containing a rare focal hyperplasia is shown in F.
Because MET/MYC tumors display characteristics consistent with expansion of a progenitor cell population, we investigated whether NPN might be the result of MET overexpression in progenitor cells. We found that lesions resulting from overexpression of MET contained large numbers of CK6-positive cells (Fig. 5B), whereas mammary outgrowths expressing only GFP and mammary glands from unmanipulated mice contained few or no CK6-positive cells (Fig. 5 C and D). Mammary glands from most MMTV-rtTA/TRE-MET transgenic mice also had virtually no CK6-positive cells and were not distinguishable from wild-type controls (Fig. 5E). Rare hyperplasias observed in MMTV-rtTA/TRE-MET mice >1 year of age were CK6-positive (Fig. 5F). Because MET is overexpressed and activated in glands from all MMTV-rtTA/TRE-MET mice (Fig. 7), we conclude that expression of CK6 is not merely a result of MET activity.
Discussion
Development of Breast Cancer Models Using Ecotropic Retroviral Infection and Transplantation of Primary MEC. We report the optimization of a system to efficiently overexpress genes of interest in the mouse mammary gland. This method has allowed us to generate mouse models of breast cancer that are different from the commonly used transgenic models. Overexpression of wild-type MET using our system resulted in multiple microscopic foci of neoplasia that did not progress to malignancy in the time frame of our experiments (>8 months) unless MYC was also overepxressed. This model should be useful to study progression of cancer and for the identification of additional oncogenic insults that can cooperate with MET in tumorigenesis.
In contrast to the results obtained by overexpression of wild-type MET into MEC via retroviral transduction and transplantation, overexpression of wild-type MET under the MMTV promoter did not result in abnormal mammary glands. It is formally possible that the MMTV promoter does not elicit MET expression at the exact developmental time or at levels above a required threshold to achieve a neoplastic phenotype. Because MET overexpression and activation are readily detectable at similar time points in both systems by parallel immunohistochemistry, however, it is more likely that the difference in outcomes lies in the cell types in which the promoters are expressed. Overexpression of MET by the MMTV promoter may occur mainly in differentiated, hormone-responsive cells, and that may not result in cell transformation. In contrast, the retroviral vector used in the present work expresses in a variety of cell types (data not shown), including progenitor cells that give rise to mammary gland outgrowth (as in Fig. 1). Furthermore, repopulation of the mammary gland after transplantation of MEC requires progenitor cell expansion (15, 30), so mature outgrowths that overexpress MET presumably arose from progenitor cells overexpressing MET.
MET-Induced Neoplastic Lesions Display Characteristics of Mammary Progenitor Cells. Luminal epithelial cells and myoepithelial cells within the mammary gland are derived from a common progenitor (36, 37). Most mouse mammary tumors display a loss of cytokeratin 14-positive myoepithelial cells as the luminal epithelium expands. However, we found that tumors resulting from overexpression of MET and MYC in our system contained both luminal and myoepithelial cells, suggesting that the tumors might arise from an expanded population of bipotential mammary progenitor cells.
Expansion of mammary progenitor cells in this model is further supported by our finding that NPN initiated by overexpression of MET alone consisted of CK6-positive cells. An abundance of CK6-positive cells is thought to reflect expansion of a progenitor cell population (10). It has previously been found that expression of MET increases 5-fold during differentiation of human breast progenitor cells in vitro (37). Although MET expression may be regulated during differentiation of breast epithelium, the outcome of aberrant expression of MET in progenitor cells has not directly been tested.
Similar to our results, hyperplasias and tumors induced by Wnt-1, β-catenin, or Myc are heterogenous in nature and express both CK6 and Sca-1. On the other hand, hyperplasias and tumors driven by Neu, H-Ras, or the polyomavirus middle T antigen do not express CK6 or Sca-1 and are much more homogenous in nature (10). Because of the presence of early developmental markers and the coexistence of multiple epithelial cell lineages, the former group of tumors is thought to arise from expansion of a transformed progenitor cell population, whereas the latter group might arise from transformation of a more differentiated cell type. Thus far, tumors suspected to arise from a putative progenitor cell population all express oncogenes that lead to activation of components of the Wnt signaling pathway (10). Our data remain consistent with this: β-catenin was found in the cytoplasm and nuclei of cells within MET-induced NPN, indicating that it is active (Fig. 8, which is published as supporting information on the PNAS web site). Tumors arising in WAP-HGF transgenic animals also displayed activation of β-catenin (32), and MET has been implicated in β-catenin activation in other cell types as well (38, 39).
It is important to note that not all hyperplasias or tumors arising from the retroviral transduction/transplantation system express CK6. For example, we have found that tumors arising from expression of the Polyomavirus middle T antigen were CK6-negative whether the oncogene was expressed by retroviral transduction/transplantation (Fig. 9, which is published as supporting information on the PNAS web site) or from the MMTV promoter (10). In contrast, hyperplasias resulting from overexpression of MYC were CK6-positive in both MMTV-MYC transgenic mice (10) and in outgrowths resulting from retroviral transduction of MYC (Fig. 9). Based on these results, it is likely that expression of an oncogene in progenitor cells (as with the retroviral transduction/transplant system) is not sufficient for generation of tumors that consist of an expanded progenitor cell population. Instead, transformation and expansion of progenitor cells may also require the function of particular oncogenes.
There is a clear association between CK6 expression and the Sca-1+ mammary progenitor cell population: CK6+ cells are present in the Sca-1+ cell population and absent in the Sca-1– cell population (10). CK6 expression might be a better marker of mammary progenitor cells, because it is more restrictive than Sca-1 expression (10). Still, it should be noted that CK6+ cells have not yet been proven to be bona fide progenitor cells that are capable of giving rise to multiple cell types in the mammary gland. This will require either identification of new progenitor cell markers in the mammary gland, or a method to sort viable cells based on expression of intracellular markers such as CK6 without permeating the cell membrane.
MET and Metastasis. Met activity has been implicated in cell migration and invasion, and there is a strong clinical association between MET overexpression and poor prognosis due to metastasis. We did not detect metastasis from MET/MYC-induced mouse mammary tumors (unpublished data), although our experimental system is capable of giving rise to metastatic tumors initiated by other protooncogenes (unpublished data). Our data suggest that, at least in mice, MET overexpression is not sufficient for tumor metastasis. We have not ruled out that MET plays a role in this process, perhaps in cooperation with other factors.
Supplementary Material
Acknowledgments
We thank Luda Urisman for excellent technical assistance, as well as Robert Cardiff and Kirk Jones for consultation with histology. We also thank Rong Wang (University of California, San Francisco) and Lewis Chodosh for providing transgenic mice and Yosef Refaeli for providing the pMIG vector. We are grateful to Joan Brugge, Mikala Egeblad, Thea Tlsty, Zena Werb, and Max Wicha for critical reading of the manuscript. This work was supported by the G. W. Hooper Research Foundation and National Institutes of Health Grant CA44338 (to J.M.B.). A.L.W. is supported by Susan G. Komen Breast Cancer Foundation Grant PDF0201190. B.E.W. is supported by the Department of Defense Breast Cancer Program (Grant 17-03-1-0498) and the National Cancer Institute (Grant U01 CA84343).
Author contributions: A.L.W. designed research; A.L.W., S.K., and B.E.W. performed research; A.L.W. and S.K. contributed new reagents/analytic tools; A.L.W., S.K., and J.M.B. analyzed data; and A.L.W. and J.M.B. wrote the paper.
Abbreviations: CK6, cytokeratin 6; MEC, mammary epithelial cells; MMTV, mouse mammary tumor virus; NPN, nonprogressive neoplasia.
References
- 1.Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 915–925. [DOI] [PubMed] [Google Scholar]
- 2.Beviglia, L., Matsumoto, K., Lin, C. S., Ziober, B. L. & Kramer, R. H. (1997) Int. J. Cancer 74, 301–309. [DOI] [PubMed] [Google Scholar]
- 3.Camp, R. L., Rimm, E. B. & Rimm, D. L. (1999) Cancer 86, 2259–2265. [DOI] [PubMed] [Google Scholar]
- 4.Ghoussoub, R. A., Dillon, D. A., D'Aquila, T., Rimm, E. B., Fearon, E. R. & Rimm, D. L. (1998) Cancer 82, 1513–1520. [DOI] [PubMed] [Google Scholar]
- 5.Tolgay Ocal, I., Dolled-Filhart, M., D'Aquila, T. G., Camp, R. L. & Rimm, D. L. (2003) Cancer 97, 1841–1848. [DOI] [PubMed] [Google Scholar]
- 6.Liang, T. J., Reid, A. E., Xavier, R., Cardiff, R. D. & Wang, T. C. (1996) J. Clin. Invest. 97, 2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffers, M., Fiscella, M., Webb, C. P., Anver, M., Koochekpour, S. & Vande Woude, G. F. (1998) Proc. Natl. Acad. Sci. USA 95, 14417–14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graveel, C., Su, Y., Koeman, J., Wang, L. M., Tessarollo, L., Fiscella, M., Birchmeier, C., Swiatek, P., Bronson, R. & Vande Woude, G. (2004) Proc. Natl. Acad. Sci. USA 101, 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiff, R. D. (2001) Microsc. Res. Tech. 52, 224–230. [DOI] [PubMed] [Google Scholar]
- 10.Li, Y., Welm, B., Podsypanina, K., Huang, S., Chamorro, M., Zhang, X., Rowlands, T., Egeblad, M., Cowin, P., Werb, Z., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 15853–15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dontu, G., Al-Hajj, M., Abdallah, W. M., Clarke, M. F. & Wicha, M. S. (2003) Cell Prolif. 36 Suppl. 1, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dontu, G., El-Ashry, D. & Wicha, M. S. (2004) Trends Endocrinol. Metab. 15, 193–197. [DOI] [PubMed] [Google Scholar]
- 13.Bayna, E. M. & Rosen, J. M. (1990) Nucleic Acids Res. 18, 2977–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer, T. K., Fryer, C. J., Lee, H. L., Zaniewski, E., Liang, T. & Mymryk, J. S. (1995) J. Steroid Biochem. Mol. Biol. 53, 421–429. [DOI] [PubMed] [Google Scholar]
- 15.Welm, B. E., Tepera, S. B., Venezia, T., Graubert, T. A., Rosen, J. M. & Goodell, M. A. (2002) Dev. Biol. 245, 42–56. [DOI] [PubMed] [Google Scholar]
- 16.Daniel, C. W., DeOme, K. B., Young, J. T., Blair, P. B., and Faulkin, L. J., Jr. (1968) Proc. Natl. Acad. Sci. USA 61, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenney, N. J., Smith, G. H., Lawrence, E., Barrett, J. C. & Salomon, D. S. (2001) J. Biomed. Biotechnol. 1, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, G. H., Mehrel, T. & Roop, D. R. (1990) Cell Growth Differ. 1, 161–170. [PubMed] [Google Scholar]
- 19.Stewart, T. A., Pattengale, P. K. & Leder, P. (1984) Cell 38, 627–637. [DOI] [PubMed] [Google Scholar]
- 20.Rose-Hellekant, T. A. & Sandgren, E. P. (2000) Oncogene 19, 1092–1096. [DOI] [PubMed] [Google Scholar]
- 21.D'Cruz, C. M., Gunther, E. J., Boxer, R. B., Hartman, J. L., Sintasath, L., Moody, S. E., Cox, J. D., Ha, S. I., Belka, G. K., Golant, A., et al. (2001) Nat. Med. 7, 235–239. [DOI] [PubMed] [Google Scholar]
- 22.Gunther, E. J., Belka, G. K., Wertheim, G. B., Wang, J., Hartman, J. L., Boxer, R. B. & Chodosh, L. A. (2002) FASEB J. 16, 283–292. [DOI] [PubMed] [Google Scholar]
- 23.Wang, R., Ferrell, L. D., Faouzi, S., Maher, J. J. & Bishop, J. M. (2001) J. Cell Biol. 153, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. (1996) J. Virol. 70, 5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Parijs, L., Refaeli, Y., Lord, J. D., Nelson, B. H., Abbas, A. K. & Baltimore, D. (1999) Immunity 11, 281–288. [DOI] [PubMed] [Google Scholar]
- 26.Pullan, S. E., Streuli, C.H. (1996) in Epithelial Cell Culture, ed. Harris, A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 97–121.
- 27.Simian, M., Hirai, Y., Navre, M., Werb, Z., Lochter, A. & Bissell, M. J. (2001) Development (Cambridge, U.K.) 128, 3117–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rijnkels, M. & Rosen, J. M. (2001) J. Cell Sci. 114, 3147–3153. [DOI] [PubMed] [Google Scholar]
- 29.Grez, M., Akgun, E., Hilberg, F. & Ostertag, W. (1990) Proc. Natl. Acad. Sci. USA 87, 9202–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kordon, E. C. & Smith, G. H. (1998) Development (Cambridge, U.K.) 125, 1921–1930. [DOI] [PubMed] [Google Scholar]
- 31.Cardiff, R. D., Anver, M. R., Gusterson, B. A., Hennighausen, L., Jensen, R. A., Merino, M. J., Rehm, S., Russo, J., Tavassoli, F. A., Wakefield, L. M., et al. (2000) Oncogene 19, 968–988. [DOI] [PubMed] [Google Scholar]
- 32.Gallego, M. I., Bierie, B. & Hennighausen, L. (2003) Oncogene 22, 8498–8508. [DOI] [PubMed] [Google Scholar]
- 33.Liao, D. J. & Dickson, R. B. (2000) Endocr. Relat. Cancer 7, 143–164. [DOI] [PubMed] [Google Scholar]
- 34.Hara, T., Ooi, A., Kobayashi, M., Mai, M., Yanagihara, K. & Nakanishi, I. (1998) Lab. Invest. 78, 1143–1153. [PubMed] [Google Scholar]
- 35.Nessling, M., Solinas-Toldo, S., Wilgenbus, K. K., Borchard, F. & Lichter, P. (1998) Genes Chromosomes Cancer 23, 307–316. [DOI] [PubMed] [Google Scholar]
- 36.Williams, J. M. & Daniel, C. W. (1983) Dev. Biol. 97, 274–290. [DOI] [PubMed] [Google Scholar]
- 37.Dontu, G., Abdallah, W. M., Foley, J. M., Jackson, K. W., Clarke, M. F., Kawamura, M. J. & Wicha, M. S. (2003) Genes Dev. 17, 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danilkovitch-Miagkova, A., Miagkov, A., Skeel, A., Nakaigawa, N., Zbar, B. & Leonard, E. J. (2001) Mol. Cell. Biol. 21, 5857–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monga, S. P., Mars, W. M., Pediaditakis, P., Bell, A., Mule, K., Bowen, W. C., Wang, X., Zarnegar, R. & Michalopoulos, G. K. (2002) Cancer Res. 62, 2064–2071. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.