Abstract
Background
Most patients requiring an extended right hepatectomy (ERH) have an inadequate standardized future liver remnant (sFLR) and need preoperative portal vein embolization (PVE). However, the clinical and oncologic impact of PVE in such patients remains unclear.
Methods
All consecutive patients from MD Anderson Cancer Center with colorectal liver metastases (CLM) requiring ERH at presentation from 1995 through 2012. The surgical and oncologic outcomes were compared between patients with adequate and inadequate sFLRs at presentation.
Results
Of the 265 patients requiring ERH, 126 (47.5%) had an adequate sFLR at presentation, and 123 of them underwent curative resection. Of the 139 patients (52.5%) who had an inadequate sFLR and underwent PVE, 87 (62.6% PVE) underwent curative resection. Thus, PVE increased the curative resection rate from 123/265 (46.4%) at baseline to 210/265 (79.2%). Among patients who underwent ERH, rates of major complications and 90-day mortality were similar in the non-PVE and PVE groups (22.0% and 4.1% vs. 31% and 7%, respectively); overall survival (OS) and disease-free survival (DFS) were also similar in these 2 groups. Among patients with an inadequate sFLR at presentation, patients who underwent ERH had significantly better median OS (50.2 months) than patients who underwent noncurative surgery (21.3 months) or did not undergo surgery (24.7 months) (p=0.002).
Conclusions
PVE enables curative resection in two-thirds of patients with CLM who have an inadequate sFLR to tolerate ERH at presentation. Patients who undergo curative resection after PVE have OS and DFS equivalent to that of patients who never needed PVE.
INTRODUCTION
For patients with colorectal liver metastases (CLM), complete surgical resection with perioperative chemotherapy has contributed to improved survival over the decades.1 Theoretically, the more hepatic parenchyma is removed, the higher the chance of cure because of clearance of latent tumor cells present in the liver. However, small future liver remnant (FLR) volume is strongly associated with increased postoperative morbidity and mortality rates in patients undergoing major hepatectomy.2–6 Therefore, it is important to leave a sufficient volume of hepatic parenchyma, tailored to the functional reserve of the underlying liver, to secure the safety of hepatic resection.
For many patients with CLM, complete surgical resection of the metastases requires extended right hepatectomy (ERH). ERH is a challenging procedure involving removal of 5 segments of the liver and is associated with reported mortality rates of 6% to 8% even in high-volume hepatobiliary centers.7–10 With routine measurement of FLR volume prior to major hepatectomy, it has been clarified that for ERH to be performed safely, 20% or greater FLR volume compared to the standard liver volume (standardized FLR volume [sFLR volume]) is required for patients with normal livers4 and 30% or greater sFLR volume is required for patients who underwent preoperative chemotherapy lasting more than 3 months.6 However, a previous anatomic study revealed that the left lateral bisegments account for only 16% of the total liver volume.11 Thus, an inadequate sFLR volume often precludes surgery in patients requiring ERH.
Portal vein embolization (PVE) is an effective strategy for producing hypertrophy of the FLR and improving the safety of extended hepatectomy.4, 12–15 This technique has allowed many patients whose tumors were initially unresectable because of small FLR volume to benefit from curative resection. However, several authors have reported that PVE might be associated with interval tumor progression and a worse long-term survival rate after curative resection of CLM.16–19 Thus, the prognostic impact of PVE for patients undergoing curative resection of extensive CLM remains controversial. The objective of this study was to clarify the prognostic impact of PVE by comparing long-term surgical outcomes in patients requiring ERH.
PATIENTS AND METHODS
Patients
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study protocol. From a prospective hepatobiliary database maintained by the Department of Surgical Oncology, 265 patients were identified as having resectable or potentially resectable CLM requiring ERH (resection of partial or whole part of segment IV adding to right hemihepatectomy with or without caudate resection) at presentation from January 1995 to November 2012. The patients were divided into 2 cohorts—one with an adequate sFLR for ERH at presentation according to our volume criteria and one with an inadequate sFLR (disease deemed unresectable). Surgical and oncologic outcomes were compared between the 2 cohorts.
Preoperative management of potential surgical candidates
At the MD Anderson Cancer Center, patients with advanced CLM initially unsuitable for resection are seen by surgeon before initiation of chemotherapy to determine whether they may be potential candidates for surgery. Every potential candidate for hepatectomy underwent 2–3 months of chemotherapy first, and response to chemotherapy and technical resectability were assessed. Surgical indication was determined by 1) chemosensitivity of the tumor (at least stable disease in size-based response criteria20, 21 should be achieved after chemotherapy), 2) absence of extrahepatic metastases, and 3) technical feasibility of curative (R0) resection. In patients indicated for surgery, chemotherapy-free interval was taken for at least 4 weeks prior to surgery to reduce the risk of postoperative complication.
Pre-PVE liver volumetry, calculation of FLR volume, and PVE
All patients with potentially resectable CLM underwent preoperative liver volumetry based on computed tomography (CT), and sFLR volume was estimated according to the previously reported method.22 Enhanced CT scans were performed with a multidetector row CT scanner, with 4, 16, or 64 slices (Light-Speed; GE Healthcare, Piscataway, NJ), using a triphasic liver protocol or single-phase technique at 2.5- to 5-mm-thick slices. Liver volumes was determined by loading the CT images onto an Advantage Workstation 4.1 (GE Medical Systems, Milwaukee, WI). Standardized liver volume was calculated using the following formula: SLV = −794.41 + 1267.28 × body surface area (m2).23 PVE was considered when sFLR was less than 20% in patients with normal liver, or less than 30% in patients with evidence of fibrosis or severe liver injury including sinusoidal obstructive syndrome or steatohepatitis.2, 24, 25 All embolizations were performed by the ipsilateral percutaneous transhepatic approach using tris-acryl microspheres ranging in size from 100 to 700 microns and coils. The right PVE was expanded to segment IV branches because this practice was expected to result in better regeneration of the FLR (i.e., left lateral segment) for ERH.26–29 Post-PVE liver volume assessments were performed with 3-dimensional CT volumetry 2 to 8 weeks after PVE, and resectability was reassessed at that time on the basis of degree of regeneration in nonembolized liver and progression of tumor. Interval chemotherapy was not routinely used to avoid the risk associated with extended chemotherapy.30, 31 If hypertrophy at the first post-PVE volume assessment was insufficient serial radiographic volumetric assessments were performed and systemic therapy was administered if applicable until the sFLR volume was sufficient to permit resection.
Definitions of outcomes
Postoperative complications were classified using standard criteria, and major complications were defined as grade III or higher complications.32 Postoperative hepatic insufficiency was defined as a peak total bilirubin level (normal range, <2 mg/dL) greater than 7mg/dL.33 The mortality rate was assessed at 90 days after surgery.
All patients who underwent surgical resection were followed up every 3–6 months with CT scan and serum carcinoembryonic antigen (CEA) level postoperatively. When an equivocal finding was pointed out on CT scan image with increased serum CEA level, MRI or PET scan was added to confirm recurrence. Overall survival (OS) was calculated from the date of surgery to death or last follow-up date for the non-PVE group and from the date of PVE to death or last follow-up date for the PVE group. Disease-free survival (DFS) was calculated from the date of surgery to first recurrence detected on CT scan or last follow-up date.
Statistical analysis
Statistical analysis was performed using IBM SPSS software (ver19.0. SPSS Inc., IL, USA). Continuous variables were compared using the Mann-Whitney U test. Categorical variables were compared using chi-squared test or Fisher’s exact test as appropriate. Survival curves were generated using the Kaplan-Meier method and compared by the log-rank test. All the tests were two-tailed and statistical significance was defined as p<0.05.
RESULTS
Patient characteristics
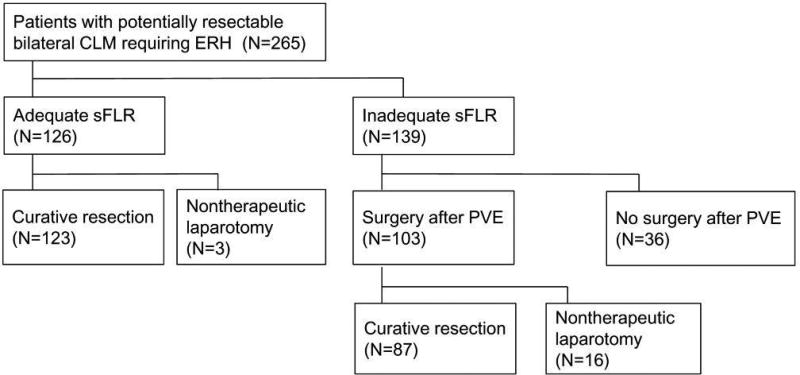
Of the 265 patients requiring ERH at presentation, only 126 (47.5%) had an adequate sFLR to undergo surgery up front; the remaining 139 patients (52.5%) required PVE (Figure 1). Among the 126 patients with an adequate sFLR, 3 patients were unable to undergo resection because of interval disease progression (n=1) or grossly abnormal liver tissue associated with sinusoidal injury (n=1) or steatohepatitis (n=1). Among the 139 patients with an inadequate sFLR at presentation, right PVE plus segment 4 embolization was successfully accomplished at the first session in 134 patients (96.4%), and a median volume increase of 52.4% (range, −60.6%–381%) was achieved at a median time from PVE of 27 days (range, 15–259 days). Among the 139 patients with an inadequate sFLR at presentation, 36 never made it to the operating room because of disease progression (23, 16.5%) including new lesions in FLR (n=13), rapid progression of tumor extending to FLR (n=2), and extrahepatic metastases (n=8), inadequate sFLR growth (n=6, 4.3%), or medical comorbidities (n=7, 5.0%). Another 16 patients (11.5%) underwent nontherapeutic laparotomy due to progression of disease (n=14) or grossly abnormal liver tissue (n=2). Eighty-seven patients (62.6%) eventually underwent curative ERH. As a result, the curative resection rate increased from 123/265 (46.4%) at baseline to 210/265 (79.2%) after application of PVE. PVE did not adversely affect the subsequent treatment outcome except for one patient (0.7%) who presented inadequate regeneration of FLR due to portal thrombosis developed after PVE.
Figure 1.
Clinical outcomes of 265 patients with potentially resectable colorectal liver metastases at presentation.
Abbreviations. sFLR, Standardized future liver remnant; PVE, portal vein embolization.
Surgical risk (American Society of Anesthesiologists score) was higher and number of tumors was significantly greater in the PVE group. Operation time was significantly longer in the PVE group. However, the rates of major complications, postoperative hepatic insufficiency, and 90-day mortality were similar in the non-PVE and PVE groups (Table 1).
Table 1.
Clinical parameters for patients who underwent curative resection with extended right hepatectomy (ERH) without portal vein embolization (PVE) or after PVE.
| Parameter | ERH without PVE (n=123) |
ERH after PVE (n=87) |
P |
|---|---|---|---|
| Age, median (range), y | 56 (25–85) | 53 (33–77) | 0.13 |
| Gender, male, no. (%) | 65 (52.8) | 60 (69) | 0.02 |
| ASA score ≥3, no. (%) | 78 (63.4) | 71 (82) | 0.004 |
| sFLR at presentation, median (IQR), % | - | 18.1 (14.1–22.7) | - |
| sFLR before surgery, median (IQR), % | 27.5 (22.8–33.3) | 27.1 (21.5–34.2) | 0.94 |
| Preoperative chemotherapy, no. (%) | 91 (74.0) | 76 (87) | 0.02 |
| Number of tumors, median (range) | 3 (1–21) | 5 (1–21) | 0.0002 |
| Maximum tumor size, median (range), mm | 30 (10–260) | 33 (5–190) | 0.82 |
| Operation time, median (range), min | 170 (81–551) | 305 (85–745) | <0.0001 |
| Blood loss, median (range), mL | 400 (40–2900) | 500 (50–3500) | 0.37 |
| Transfusion, no. (%) | 30 (24.4) | 15 (17) | 0.34 |
| Major complication, no. (%) | 27 (22.0) | 27 (31) | 0.14 |
| Postoperative hepatic insufficiency, no. (%) | 18 (14.6) | 9 (10) | 0.48 |
| 90–day mortality, no. (%) | 5 (4.1) | 6 (7) | 0.37 |
ASA, American Society of Anesthesiologists; IQR, interquartile range; sFLR, standardized future liver remnant.
Survival outcomes
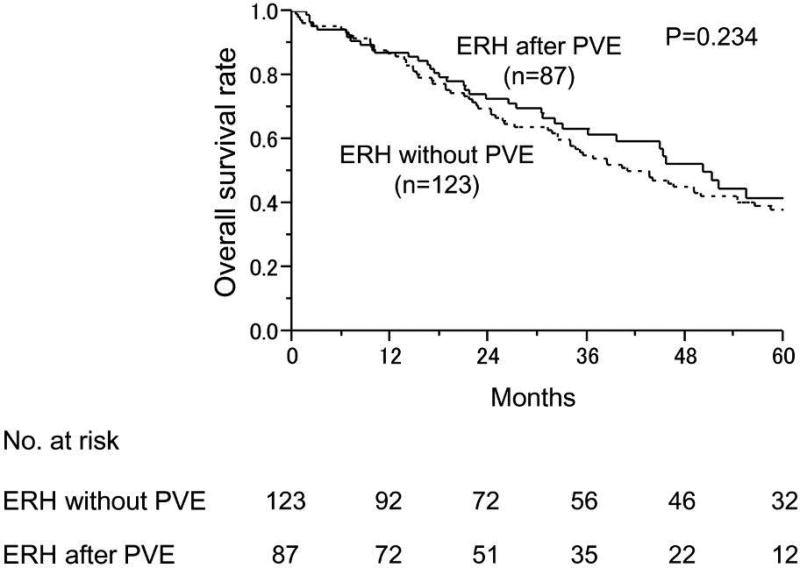
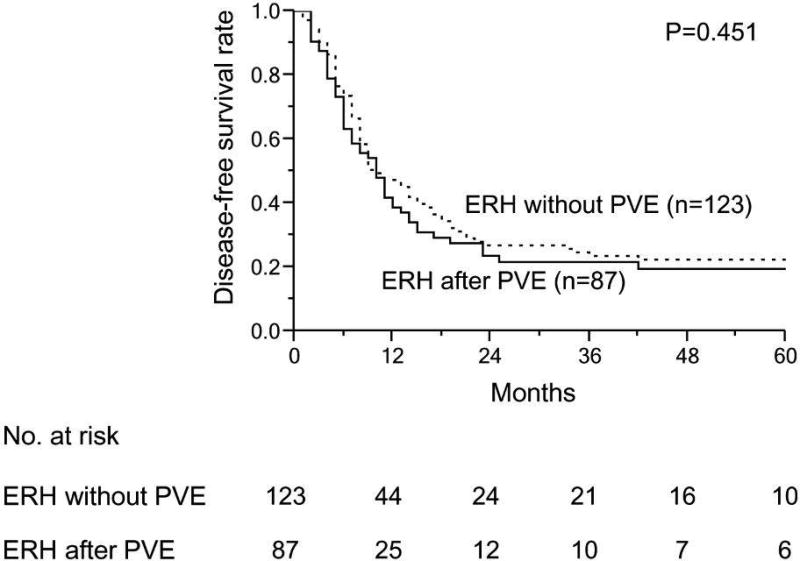
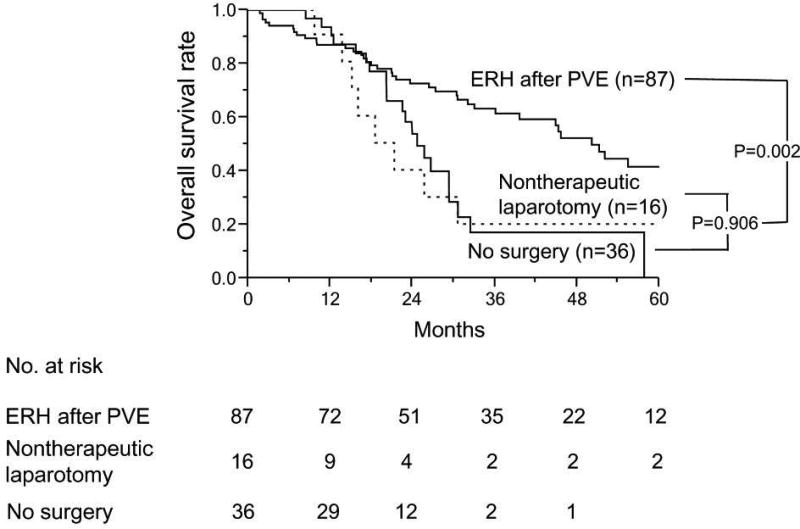
With similar follow-up duration (median 44.1 month for non-PVE group and 50.2 months for PVE group, p=0.234), the 3-year and 5-year survival rates were 54.9% and 38.0%, respectively for the non-PVE group, and 61.4% and 41.6%, respectively for the PVE group (Figure 2A). The DFS duration also was similar between these 2 groups (P=0.451) (Figure 2B). Among patients with an inadequate sFLR at presentation, patients who underwent ERH had significantly better median OS (44.1 months) than patients who underwent noncurative surgery (21.3 months) and those who did not undergo surgery (24.7 months) (P=0.002) (Figure 2C).
Figure 2.
Long-term survival outcomes in patients with colorectal liver metastases. A and B. Overall (A) and disease-free survival rates (B) in patients treated with extended right hepatectomy (ERH) without portal vein embolization (PVE) and after PVE. (C) Overall survival rates in patients who underwent PVE according to surgical treatment.
DISCUSSION
This study confirms that more than 60% of patients who are diagnosed with unresectable CLM because of a small FLR are able to undergo ERH after PVE. The patients who underwent ERH after PVE had operative morbidity and mortality rates similar to those of patients who underwent ERH without PVE, and these 2 groups of patients also had similar OS and DFS.
The efficacy of PVE with regard to increasing the proportion of patients who are candidates for surgery and improving the safety of major hepatectomy has been actively discussed elsewhere.4, 12–15 In the era of effective chemotherapy and multidisciplinary approaches, PVE has been increasingly performed because PVE is expected to increase the resectability of extensive tumors in patients with very small FLR volumes.34 However, although the feasibility and safety of PVE have already been established,13, 34, 35 little evidence is available regarding the prognostic impact of PVE.36–40
Wicherts et al. analyzed 364 patients who underwent major hepatectomy and reported that PVE increased resectability and that patients who were able to undergo resection after PVE had a significantly higher survival rate than patients whose disease remained unresectable after PVE.19 T In contrast to our findings, these authors also found that the survival rate of patients who underwent resection after PVE was inferior to that of patients who did not require PVE. However, in the Wicherts et al. study, the distributions of tumors and extent of surgery were significantly different between these 2 patient groups: the patients who underwent resection after PVE had more advanced disease and a higher incidence of bilobar tumors, and as a result, the proportion of patients who underwent ERH was significantly higher in this group. Therefore, this study left open the question of whether there is a survival difference between patients who undergo curative resection without PVE and those who undergo curative resection after PVE.
The most important result of the current study is that the aggressive surgical approach of PVE followed by ERH did not result in worse short-term or long-term outcomes than up-front ERH. In the current oncologically homogeneous population of patients with CLM requiring resection of the same extent (i.e., ERH), use of PVE clearly increased the number of patients eligible for ERH, and ultimately, two-thirds of patients with an inadequate sFLR to permit ERH at presentation were able to undergo curative resection after PVE. As a result, 79.2% of the 265 patients who required ERH at presentation eventually achieved curative resection with application of PVE. In contrast, survival after nontherapeutic laparotomy was as poor as that in patients who could not proceed to surgery (Figure 2). Thus, PVE allows some patients to have prolonged survival by making possible ERH rather than nonsurgical therapy.
Another noteworthy outcome is that DFS did not differ between patients with PVE followed by ERH and those with up-front ERH. Several authors have reported that PVE can accelerate tumor growth and increase the risk of progression of disease during the waiting time for surgery.17, 18, 41 Indeed, 23 (16.5%) of the 139 patients who underwent PVE in the current study could not proceed to surgery because of interim tumor progression during the waiting time after PVE. However, 87 (62.6%) of the 139 patients who underwent PVE eventually had curative resection without any evidence of disease progression, and the DFS of these patients was equivalent to that of patients who had up-front ERH without PVE (Figure 2). Therefore, with PVE, patients with initially unresectable tumors have a chance to live as long as patients whose disease was resectable at presentation when adequately selected based on the degree of regeneration of FLR and oncologic activity of tumor (i.e., no significant progression) after PVE.
Limitations of the current study include its retrospective nature and a change in the technique of PVE and definition of sFLR early in the study period. However, the study was based on clinical results recorded in a prospective database of patients with CLM, and all consecutive patients who required ERH at consultation were analyzed. Also, long-term oncologic outcome is independent of sFLR or surgical complications. To our knowledge, this is the first study to compare survival outcomes in a homogeneous population requiring resection of similar extent (i.e., ERH) and treated with a uniform multidisciplinary approach with preoperative chemotherapy and PVE. These results warrant a prospective study to validate the true prognostic impact of PVE.
In conclusion, among patients with CLM with an inadequate sFLR to tolerate ERH at presentation, two-thirds of patients are able to undergo ERH after PVE. Patients who undergo ERH after PVE may have OS and DFS equivalent to that of patients who never needed PVE under a constant policy of a multidisciplinary approach for CLM.
Acknowledgments
Source of Funding: The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672
References
- 1.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias D, Ouellet JF, De Baere T, Lasser P, Roche A. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294–299. doi: 10.1067/msy.2002.120234. [DOI] [PubMed] [Google Scholar]
- 4.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 6.Shindoh J, Tzeng CD, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Optimal Future Liver Remnant in Patients Treated with Extensive Preoperative Chemotherapy for Colorectal Liver Metastases. Ann Surg Oncol. 2013 doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halazun KJ, Al-Mukhtar A, Aldouri A, Malik HZ, Attia MS, Prasad KR, et al. Right hepatic trisectionectomy for hepatobiliary diseases: results and an appraisal of its current role. Ann Surg. 2007;246:1065–1074. doi: 10.1097/SLA.0b013e3181492795. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melendez J, Ferri E, Zwillman M, Fischer M, DeMatteo R, Leung D, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. doi: 10.1016/s1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Baer HU, Guastella T, Blumgart LH. Comparison of outcome between extended and nonextended liver resections for neoplasms. Surgery. 1993;114:968–975. [PubMed] [Google Scholar]
- 11.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 13.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 14.Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg. 2012;29:23–29. doi: 10.1159/000335718. [DOI] [PubMed] [Google Scholar]
- 15.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–727. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812–817. doi: 10.1097/SLA.0b013e3182733f09. discussion 817-818. [DOI] [PubMed] [Google Scholar]
- 18.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 19.Wicherts DA, de Haas RJ, Andreani P, Sotirov D, Salloum C, Castaing D, et al. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240–250. doi: 10.1002/bjs.6756. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 23.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 24.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. discussion 680-671. [DOI] [PubMed] [Google Scholar]
- 25.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730. doi: 10.1097/01.sla.0000124385.83887.d5. discussion 730-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 27.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness--study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 28.Truty MJ, Vauthey JN. Uses and limitations of portal vein embolization for improving perioperative outcomes in hepatocellular carcinoma. Semin Oncol. 2010;37:102–109. doi: 10.1053/j.seminoncol.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishi Y, Madoff DC, Abdalla EK, Palavecino M, Ribero D, Chun YS, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–751. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 31.Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493–2500. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862-854. [DOI] [PubMed] [Google Scholar]
- 34.Shindoh J, Tzeng CD, Aloia TA, Curley SA, Huang SY, Mahvash A, et al. Safety and Efficacy of Portal Vein Embolization Before Planned Major or Extended Hepatectomy: An Institutional Experience of 358 Patients. Journal of Gastrointestinal Surgery. 2013 doi: 10.1007/s11605-013-2369-0. in press. [DOI] [PubMed] [Google Scholar]
- 35.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindner P, Cahlin C, Friman S, Hafstrom L, Klingenstierna H, Lonn L, et al. Extended right-sided liver resection for colorectal liver metastases--impact of percutaneous portal venous embolisation. Eur J Surg Oncol. 2006;32:292–296. doi: 10.1016/j.ejso.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Mueller L, Hillert C, Moller L, Krupski-Berdien G, Rogiers X, Broering DC. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15:1908–1917. doi: 10.1245/s10434-008-9925-y. [DOI] [PubMed] [Google Scholar]
- 39.Oussoultzoglou E, Bachellier P, Rosso E, Scurtu R, Lucescu I, Greget M, et al. Right portal vein embolization before right hepatectomy for unilobar colorectal liver metastases reduces the intrahepatic recurrence rate. Ann Surg. 2006;244:71–79. doi: 10.1097/01.sla.0000217609.26178.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Long-term survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16:1202–1207. doi: 10.1245/s10434-008-0269-4. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–727. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]