Abstract
Invasive apocrine carcinomas (IACs), as defined by morphological features, correspond to 0.3–4% of all invasive ductal carcinomas (IDC), and despite the fact that they are histologically distinct from other breast lesions there are currently no standard molecular criteria available for their diagnosis and no unequivocal information as to their prognosis. In an effort to address these concerns we have been using protein expression profiling technologies in combination with mass spectrometry and immunohistochemistry (IHC) to discover specific biomarkers that could allow us to molecularly characterize these lesions as well as to dissect some of the steps in the processes underlying breast apocrine metaplasia and development of precancerous apocrine lesions. Establishing these apocrine‐specific markers as best practice for the routine pathology evaluation of breast cancer, however, will require their validation in large cohorts of patients. Towards this goal we have composed a panel of antibodies against components of an apocrine protein signature that includes probes against the apocrine‐specific markers 15‐prostaglandin dehydrogenase (15‐PGDH), and acyl‐CoA synthetase medium‐chain family member 1 (ACSM1), in addition to a set of categorizing markers that are consistently expressed (AR, CD24) or not expressed (ERα, PgR, Bcl‐2, and GATA‐3) by apocrine metaplasia in benign breast lesions and apocrine sweat glands. This panel was used to analyze a well‐defined cohort consisting of 14 apocrine ductal carcinoma in situ (ADCIS), and 33 IACs diagnosed at the Cancer Institute Hospital, Tokyo between 1997 and 2001. Samples were originally classified on the basis of cellular morphology with all cases having more than 90% of the tumour cells exhibiting cytological features typical of apocrine cells. Using the expression of 15‐PGDH and/or ACSM1 as the main criterion, but taking into account the expression of other markers, we were able to identify unambiguously 13 out of 14 ADCIS (92.9%) and 20 out of 33 (60.6%) IAC samples, respectively, as being of apocrine origin. Our results demonstrate that IACs correspond to a distinct, even if heterogeneous, molecular subgroup of breast carcinomas that can be readily identified in an unbiased way using a combination of markers that recapitulate the phenotype of apocrine sweat glands (15‐PGDH+, ACSM1+, AR+, CD24+, ERα−, PgR−, Bcl‐2−, and GATA‐3−). These results pave the way for addressing issues such as prognosis of IACs, patient stratification for targeted therapeutics, as well as research strategies for identifying novel therapeutic targets for developing new cancer therapies.
Keywords: Breast cancer subtypes, Apocrine carcinoma, Protein signature, 15-PGDH, ACSM1, Patient stratification
Abbreviations
- IHC
Immunohistochemistry
- 2-D PAGE
Two-dimensional polyacrylamide gel electrophoresis
1. Introduction
Breast cancer is not a single disease, but a heterogeneous group of diseases that comprises a wide range of histopathological types. Conventional histopathological criteria classify invasive breast cancer into defined groups: two major subtypes, ductal of no specific type (NST) and lobular accounting for around 75% and 15% of all cases, respectively, in addition to rare special types e.g. tubular, cribriform, mucinous, medullary, metaplastic, apocrine breast carcinomas as well as others (Tavassoli and Devilee, 2003; Page, 2003). Lately, this classification has been refined by molecular characterization of histological special types using immunohistochemistry (IHC) (Weigelt et al., 2008). Transcriptome profiling technologies, on the other hand, have subdivided breast cancer tumours into five clinically relevant clusters with different prognostic characteristics. These include two estrogen receptor alpha (ERα) positive groups (luminal A and B) and three ERα negative groups that include basal‐like, normal breast tissue‐like and ERBB2/HER2‐neu positive lesions (Perou et al., 2000; Sorlie et al., 2001). Patients with an ERα positive phenotype have a good prognosis as compared with basal‐like breast carcinomas (BLBCs), which have a much shorter overall and disease‐free survival period (Sorlie et al., 2003; Chin et al., 2006). BLBCs account for 15–25% of all breast‐cancer cases, and show a high frequency of p53 mutations and genetic abnormalities (Sorlie et al., 2003; Foulkes et al., 2004; Rakha et al., 2006; Langerød et al., 2007 and references therein). Even though there is no consensus concerning the phenotype of these lesions, all available information indicates that these tumours are ERα and PgR negative, and many, but not all, express basal cytokeratins (CK's 5/6, and/or 14 and/or 17), sometimes together with vimentin, and exhibit increased expression of epidermal growth factor receptor (EGFR) (Nielsen et al., 2004; Carey et al., 2006; Rodríguez‐Pinilla et al., 2007; Livasy et al., 2007; Moinfar, 2008; Fadare and Tavassoli, 2008). BLBCs have been shown to be heterogeneous in terms of response to treatment and include ERα‐/PgR‐/Her‐2neu‐ carcinomas, often termed “triple negatives” (Cleator et al., 2007 and references therein). The latter have a poor prognosis and are increased in women with BRCA1 mutations as well as among sporadic breast cancer (Irvin and Carey, 2008; Reis‐Filho and Tutt, 2008; Fadare and Tavassoli, 2008).
Invasive apocrine carcinomas (IACs), as defined by morphological characteristics, are rare corresponding to about 0.3–4% of all invasive ductal carcinomas (IDC) (Azzopardi, 1979; Frable and Kay, 1968; Mossler et al., 1980; Eusebi et al., 1986; O'Malley and Bane, 2008). Despite the fact that these tumours are histologically distinct from other breast lesions there are currently no standard molecular criteria at hand for their diagnosis and no definite information as to their prognosis (O'Malley and Bane, 2008; Japaze et al., 2005; Page, 2005; Wells and El‐Ayat, 2007; Tanaka et al., 2008). In fact, molecular classification studies of breast carcinomas are often inconsistent in their categorization of IACs. In the original groundbreaking gene profiling study by Perou and colleagues, IACs clustered within the basal subtype of breast carcinomas (Perou et al., 2000). More recently, Farmer et al. (2005) identified a discrete subset of breast tumours, characterized by increased androgen signaling and a distinctive expression profile, that they termed “molecular apocrine” as these lesions do not exhibit all the histopathological features of classical apocrine carcinomas. Molecular apocrine carcinomas encompass tumours that share some common expression characteristics with the ERBB2 class (ERα‐/PgR‐/ERBB2+) in the Stanford classification as well as with some lesions that exhibit morphological features of the triple negative group (high grade lesions; ERα‐/PgR‐/ERBB2‐). However, Weigelt et al. (2008) based on the analysis of 6 apocrine carcinomas concluded that these tumours are unlikely to constitute a distinct entity. The lesions, which expressed high protein levels of AR and GCDFP‐15, were shown to exhibit heterogeneous gene expression profiles, to distribute to several molecular subtypes, and to have a higher risk of recurrence. This inconsistency in the classification of IACs as either constituting a distinct entity, with specific clinical outcomes, or not, is likely to arise from the lack of established molecular criteria by which one can unambiguously identify these lesions.
IACs are generally accepted to have a distinct hormonal profile being ERα, and PgR negative, but androgen receptor (AR) positive (Tavassoli et al., 1996; Gatalica, 1997; Selim and Wells, 1999; Sapp et al., 2003). IACs are also negative for the proto‐oncogene Bcl‐2 (Tavassoli et al., 1996) and GATA‐3 (Celis et al., 2008), a transcription factor that maintains luminal epithelial differentiation (Kouros‐Mehr et al., 2006; Asselin‐Labat et al., 2007). Moreover, most apocrine carcinomas are positive for the gross cystic disease fluid protein‐15 (GCDFP‐15) (Mazoujian et al., 1983; Eusebi et al., 1986; Miller et al., 1988; Honma et al., 2005), but this marker has been shown not to be specific for these lesions (Miller et al., 1988; Honma et al., 2005; Celis et al., 2006a; Moriya et al., 2009).
Given the need to develop molecular criteria to reproducibly categorize IACs, and the lack of information as to which is the most appropriate treatment for patients bearing these lesions, we have embarked in a systematic proteomic undertaking aimed at identifying biomarkers that may characterize and subtype these lesions to a greater detail, and to search for targets that may lead to the development of novel targeted therapies and chemoprevention strategies. By comparing the gel‐based protein expression profiles of “blue dome” apocrine cysts, which are commonly present in fibrocystic changes, and normal breast epithelial tissue obtained from the same patient, followed by protein identification using mass spectrometry, antibody preparation, and immunohistochemistry (IHC) for verification of results we have identified several biomarkers that are expressed by benign and non‐obligatory precancerous lesions as well as by some IACs (Celis et al., 2006, 2006, 2007, 2007, 2008).
Recently, we presented evidence that IACs correspond to a distinct molecular subtype of breast carcinomas as determined by the expression of biomarkers such as 15‐prostaglandin dehydrogenase (15‐PGDH), an enzyme involved in prostaglandin synthesis, and a novel form of acyl‐CoA synthetase medium‐chain family member 1 (ACSM1), proteins that are not expressed, at least within the limits of detection of gel‐based proteomics and IHC, in other breast cancers subtypes (Celis et al., 2008). Establishing these apocrine‐specific markers as best practice for the routine pathology evaluation of breast cancer, will require their validation in large cohorts of patients. Towards this goal we have put together a panel of antibodies against components of an apocrine protein signature that includes probes against 15‐PGDH and ACSM1 in addition to a set of categorizing markers that are consistently expressed (AR, CD24) or not expressed (ERα, PgR, bcl‐2, and GATA‐3) by apocrine metaplastic lesions in benign breast lesions (Wells and El‐Ayat, 2007) and apocrine sweat glands. This panel was used to analyze a sample set consisting of 14 apocrine ductal carcinoma in situ (ADCIS), and 33 IACs diagnosed at the Cancer Institute Hospital, Tokyo between 1997 and 2001. In this unique cohort more than 90% of the tumour cells exhibited cytological features typical of apocrine cells (Frable and Kay, 1968; Azzopardi, 1979; Tavassoli and Norris, 1994; Tavassoli and Devilee, 2003; Honma et al., 2007). Besides demonstrating that IACs correspond to a heterogeneous, yet distinct molecular subgroup of breast carcinomas that can be readily identified in an unbiased way using a combination of markers that recapitulate the phenotype of apocrine sweat glands, our results pave the way for addressing issues such as prognosis of IACs, patient stratification for targeted therapeutics, as well as research strategies for identifying novel therapeutic targets for developing new cancer therapies.
2. Results
2.1. Panel of biomarkers/antibodies used to characterize breast apocrine carcinomas by means of IHC
By comparing the gel‐based protein expression profiles of “blue dome” apocrine cysts with normal breast epithelial tissue obtained from the same patient (Celis et al., 2006, 2006, 2007, 2007, 2008), we have identified a number of apocrine protein biomarkers and two of these, 15‐PGDH (Figure 1A) and ACSM1 (Figure 1B), were proven to be specific for benign apocrine lesions as determined by IHC and 2D gel‐based proteomics (Celis et al., 2006, 2006, 2007, 2007, 2008). IHC analysis of well‐defined sets of breast tumour subtypes without apocrine differentiation, has failed to show any reactivity with the 15‐PGDH and ACSM1 antibodies at the dilutions used in this study (Celis et al., 2008). Moreover, we have been unable to detect 15‐PGDH in the more than 200 non‐apocrine tumours analyzed to date in our laboratory using 2D gel‐based proteomics in combination with silver staining (Celis et al., 2008; unpublished observations; see also Figure 9).
Figure 1.
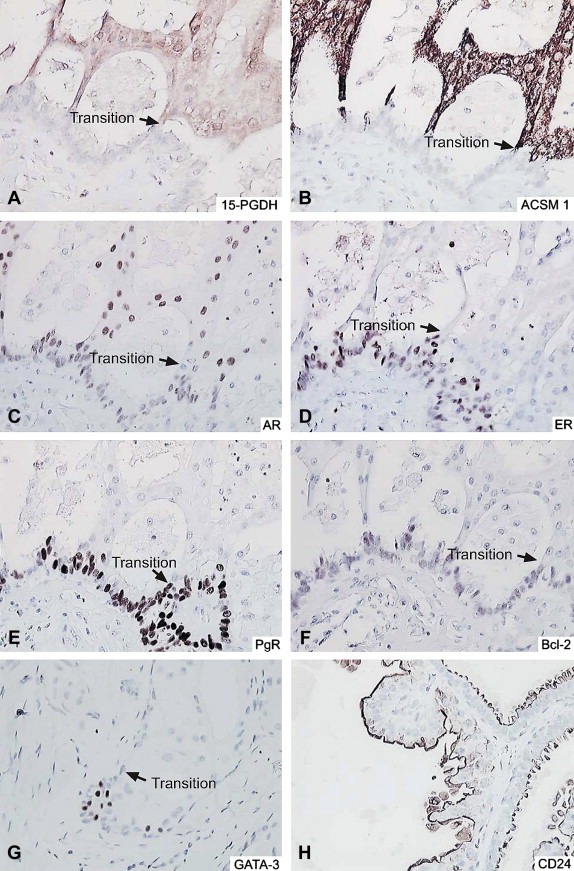
Breast benign lesions immunostained with the battery of antibodies. (A–G) Apocrine change within sclerosing adenosis immunostained with antibodies against (A) 15‐PGDH, (B) ACMS1, (C) AR, (D) ERα, (E) PgR, (F) Bcl‐2 and (G) GATA‐3 respectively. (H) Apocrine cysts immunostained with the CD24 antibody. Arrows indicate the transition to an apocrine phenotype.
Figure 9.
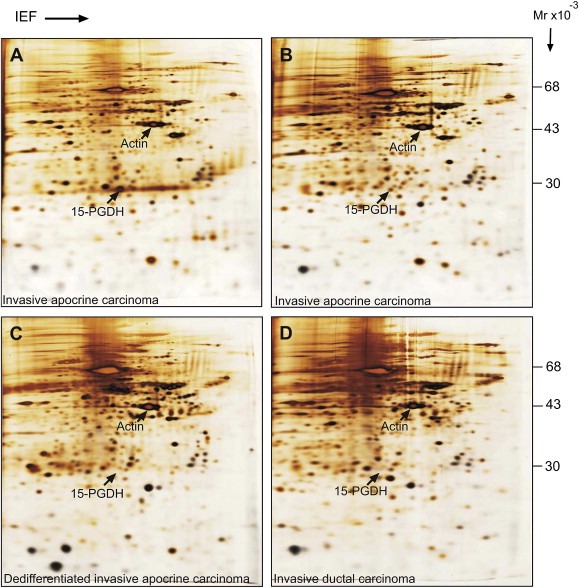
IEF 2D gel analysis of apocrine and non‐apocrine tumours from the DCTB collection. (A and B) Silver stained 2D gels of total protein extracts from 15‐PGDH+, ACSM1+, IACs. (C) Molecularly dedifferentiated. (D) Invasive ductal carcinoma. The position of 15‐PGDH and beta‐actin are indicated for reference. The procedures for 2D gel electrophoresis have been described in detail elsewhere (Celis et al., 2005).
We composed an immunohistochemical panel consisting of a set of antibodies against protein components of an apocrine signature that includes probes against 15‐PGDH and ACSM1 in addition to a set of categorizing markers that have been shown to be consistently expressed (AR, Figure 1C), or not expressed (ERα, Figure 1D; PgR, Figure 1E; Bcl‐2, Figure 1F; GATA‐3, Figure 1G) by benign apocrine lesions (Tavassoli et al., 1996; Gatalica, 1997; Selim and Wells, 1999; Sapp et al., 2003; Celis et al., 2008) to analyze a well‐defined cohort of ADCIS and IACs diagnosed at the Cancer Institute Hospital, Tokyo between 1997 and 2001. We also included in this panel an antibody against CD24, a small cell surface protein that is attached to the cell membrane by a glycosylphosphatidylinositol anchor (Kristiansen et al., 2003; Lim and Oh, 2005) that is only expressed by some breast cancer tumour subtypes (Surowiak et al., 2006; Honeth et al., 2008) and that we found to be expressed by benign apocrine cells (Figure 1H; unpublished observations; this study). IHC analysis of commercially available breast tissue microarrays (BRC1502 and BRC1503; Pantomics, Inc, USA) showed that approximately 30% of breast tumours in these arrays express CD24, a number comparable to previous reports in the literature (Honeth et al., 2008). Representative sections, most of them tandem cut, of a sample with apocrine change within sclerosing adenosis (Figure 1A–G) and an apocrine cyst (Figure 1H) stained with the apocrine immunohistochemical panel are shown for illustration in Figure 1, panels A through H. The transition point between non‐apocrine cell precursors and the apocrine cells is indicated with an arrow for reference.
The following apocrine cellular phenotype can thus be derived from these data: 15‐PGDH+, ACSM1+, AR+, CD24+, ERα−, PgR−, Bcl‐2−, and GATA‐3−. As shown in Figure 2, panels A through H, this apocrine phenotype is recapitulated in apocrine sweat glands indicating that this particular combination of markers is sufficient to define cellular “apocrinicity” indicating that it may represent a potential gold standard for characterizing the breast apocrine phenotype.
Figure 2.
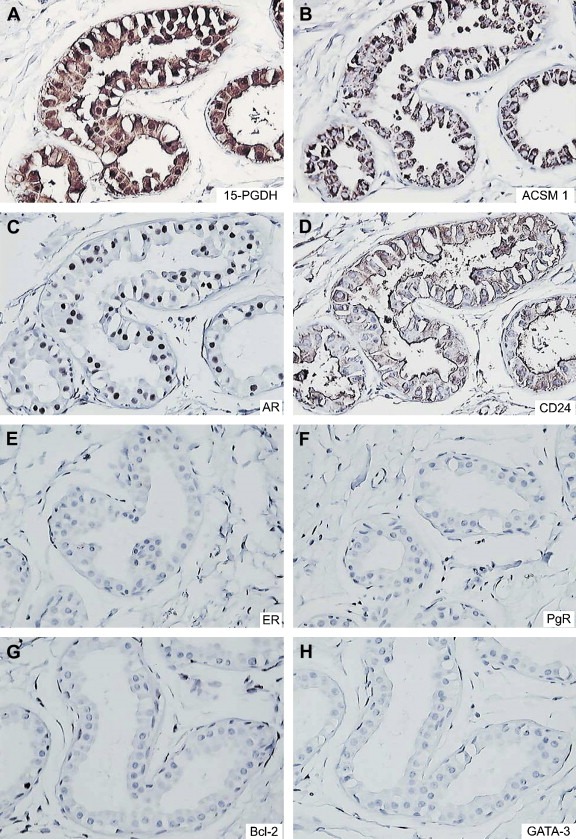
Axillary apocrine sweat glands immunostained with the battery of antibodies. (A) 15‐PGDH, (B) ACSM1, (C) AR, (D) CD24, (E) ERα, (F) PgR, (G) Bcl‐2, and (H) GATA‐3.
2.2. Expression of the components of the apocrine signature by a well‐defined cohort of apocrine carcinomas diagnosed at the Cancer Institute Hospital, Tokyo
To validate the apocrine‐specific panel we used a collection of apocrine samples consisting of 14 ADCIS and 33 IACs diagnosed at the Cancer Institute Hospital, Tokyo between 1997 and 2001 (Honma et al., 2005, 2007). More than 90% of the tumour cells in all samples within this cohort exhibited cytological features typical of apocrine cells (Frable and Kay, 1968; Azzopardi, 1979; Tavassoli and Norris, 1994; Tavassoli and Devilee, 2003; Honma et al., 2007) and as a result, this unique set of samples represents an exceptional resource ideal for validation purposes.
2.2.1. ADCIS
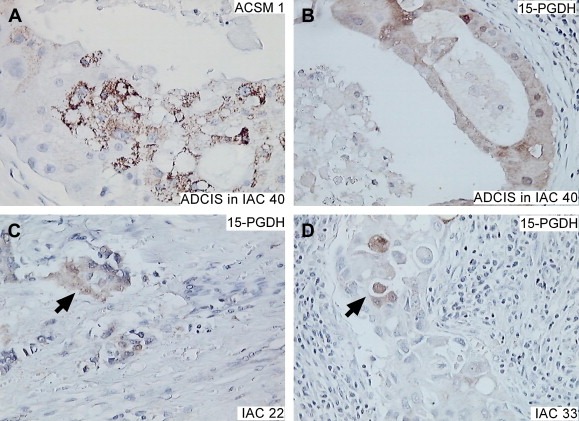
The immuno‐expression of the 8 biomarkers composing the apocrine IHC panel in the 14 ADCIS samples is given in Table 2. Eleven out of the 14 ADCIS (78.7 %) stained positively for both 15‐PGDH (Figure 3A) and ACSM1 (+/+ lesions; Figure 3B). Of the three remaining lesions, two were positive for either 15‐PGDH or ACSM1 with the phenotypes +/− (Figure 3C and D), and −/+ (Figure 3E and F). One single lesion (ADCIS 12) was negative for both markers (−/−; result not shown), but exhibited a few cells that stained weakly with the 15‐PGDH antibody indicating that in this particular case the expression of 15‐PGDH may have been lost during the early stages of tumour development. As expected, all samples expressing one or both of the apocrine‐specific markers were AR+, CD24+ (membranous), ERα−, PgR−, Bcl‐2− and GATA‐3− (Table 2).
Table 3.
Expression of 15‐PGDH, ACSM1 and other markers by IACs.
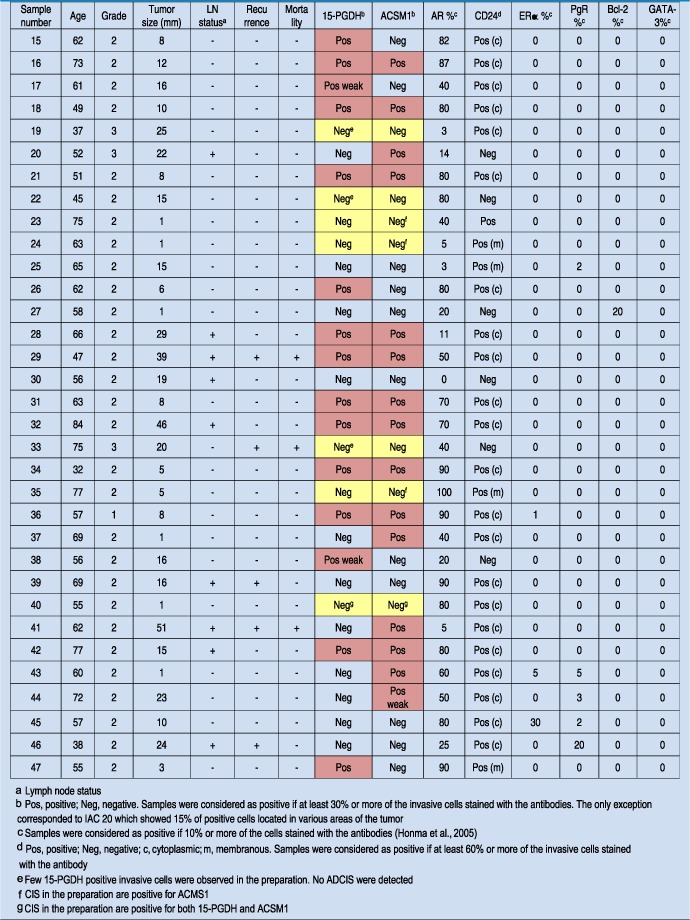
Figure 3.
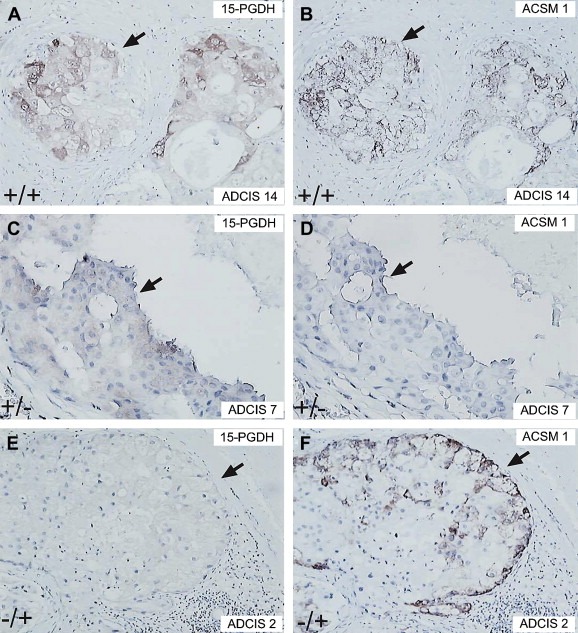
ADCIS immunoreacted with antibodies against 15‐PGDH and ACSM1. (A, C, and D) ADCIS 14, 7 and 2 immunostained with antibodies against 15‐PGDH. (B, D, and F) ADCIS 14, 7 and 2 immunostained with antibodies against ACSM1.
Table 2.
Expression of 15‐PGDH, ACSM1 and other markers by ADCIS.
In one case, that of ADCIS 8 (+/+ phenotype), the sample was originally classified as PgR+ since it contained approximately 30% of cells that stained, albeit very weakly, for PgR (Table 2). Double immunofluorescence staining of this sample with ACSM1 and PgR antibodies showed that the in situ cells that were positive for ACSM1 (Figure 4, Panel 1A) were PgR negative (Figure 4, Panel 1B), whereas the in situ cells that were negative for ACSM1 (Figure 4, Panel 2A) were positive for PgR (Figure 4, Panel 2B). The latter, emphasizes the need to assert the nature of the cells expressing a given antigen as percentages alone may not give a true picture of the situation.
Figure 4.
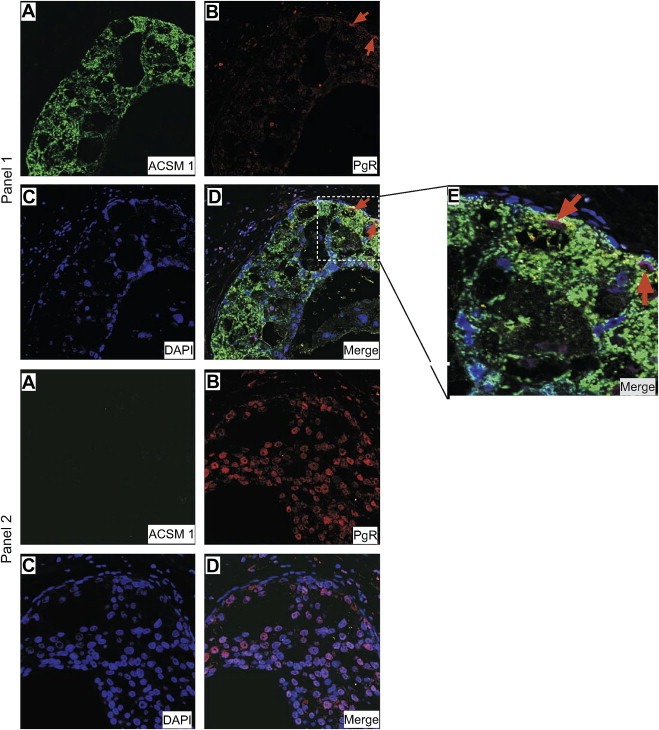
Indirect double‐label immunofluorescence analysis of ADCIS 8 tissue sections reacted with ACMS1 (subpanels A) and PgR (subpanels B) antibodies showed that CIS that stained with ACMS1 (Alexa Fluor® 488; green channel) were PgR (Alexa Fluor® 594; red channel) negative (panel 1), while those negative for ACSM1 were positive for PgR (panel 2). Sections were counterstained with the nuclear stain DAPI (blue channel). An area of the ACSM1+ CIS showing a few PgR positive cells present at the transition point were metaplasia takes place is shown in higher magnification for illustration purposes (subpanel E).
2.2.2. IACs
Ten out of the 33 IACs analyzed (30.3%) were 15‐PGDH+, ACSM1+ (Figure 5A and B), 5 (15.1%) were 15‐PGDH+, ACSM1−, 5 (15.1%) were 15‐PGDH−, ACSM1+, and 13 (39.4 %) were 15‐PGDH−ACSM1− (3, 4).
Figure 5.
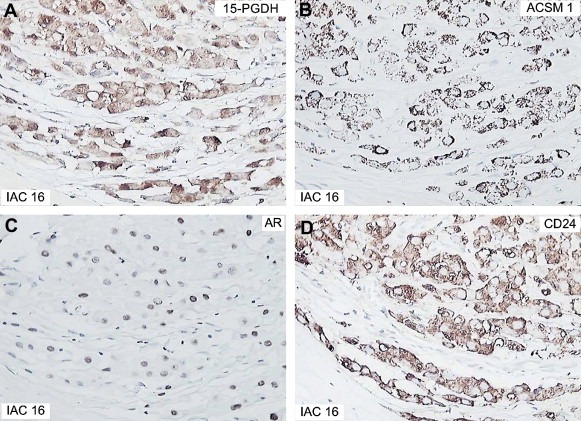
Immunostaining of IAC 16 with various antibodies. (A) 15‐PGDH, (B) ACSM1, (C) AR, and (D) CD24.
Table 4.
Distribution of 15‐PGDH/ACSM1 phenotypes among ADCIS and IACs.
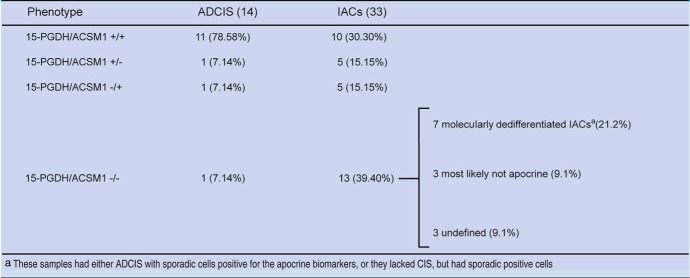
With the exception of IAC 41, which was negative for AR, IACs expressing one or both of the apocrine markers (+/+, +/− and −/+; marked in red in Table 3) were all AR positive (Figure 5C) 18 were CD24 positive (cytoplasmic staining; Figure 5D), and as expected they were all negative for ERα, PgR, Bcl‐2, and GATA‐3 (Table 3).
Careful examination of the tissue sections of the 13 IACs exhibiting the 15‐PGDH−ACSM1− phenotype suggested that this group was in fact heterogeneous being comprised of at least two types of lesions (i) molecularly dedifferentiated IACs that have lost the apocrine markers as a result of progression, but have maintained their apocrine appearance, and or (ii) lesions that morphologically resemble apocrine cells, but that in fact are not of apocrine nature.
-
(i)
Molecularly dedifferentiated IACs: Seven out of the 13 15‐PGDH−, ACSM1−, IACs were identified as putative molecularly dedifferentiated lesions and are marked in yellow in Table 3. Four of these presented with CIS that were either positive for ACSM1 alone (samples 23, 24 (AR negative), and 35, Table 3), or for both 15‐PGDH and ACSM1 (sample 40, Figure 6A and B), indicating that their expression was lost during progression from CIS to invasive disease. In the three other cases, the lesions did not present with CIS but exhibited a few 15‐PGDH positive invasive cells spread throughout the preparations (IACs 19; 22, Figure 6C; and 33, Figure 6D). As shown in Table 3, one of these lesions (IAC 19) was AR negative.
-
(ii)
Lesions that morphologically resemble apocrine cells, but may not correspond to IACs. 15‐PGDH−, ACSM1−, IACs 27, 45 and 46 were most likely not of apocrine origin as these lesions exhibited an unusually high percentage of cells staining with either the Bcl‐2 (IAC 27, a few cells are also CD24‐), the ERα (IAC 45), or the PgR (IAC 46) antibodies (Table 3). All three lesions were AR positive (Table 3), but we have observed that many of the non‐apocrine tumours are also positive for this receptor.
Figure 6.
Immunostaining of 15‐PGDH−, ACSM1−, IACS with the ACSM1 and 15‐PGDH antibodies. (A and B) ADCIS in IAC 8 showing CIS immunostaining with the ACSM1 and 15‐PGDH antibodies. (C and D) Invasive cells in IACs 22 and 33 staining with the 15‐PGDH antibody.
Finally, the three remaining 15‐PGDH−, ACSM1−, IACs (samples 25, 30 and 39) were considered as “undefined” as we found no evidence for the presence of 15‐PGDH or ACSM1 positive cells. Two of these lesions were AR negative (IACS 25 and 30; Table 3).
A summary of the frequencies of the ADCIS and IACs expressing the different phenotypes is presented in Table 4. As a whole we were able to identify with certainty 92.9% of the morphologically diagnosed ADCIS and 60.6% of the IACs as being of apocrine origin. The marked differences in percentages most likely reflect a combination of loss of the apocrine markers by the invasive cells as well as problems associated with morphological identification of the invasive lesions.
3. Discussion
Understanding the mechanisms underlying tumour progression is crucial if one wants to carry out research on early detection and prevention strategies. The approach we have used to characterize apocrine lesions is based on the assumption that IACs evolve from epithelial cells in terminal duct lobular units (TDLU's) in a stepwise manner, going through different stages that may involve metaplasia, hyperplasia, atypia and carcinoma in situ (CIS). Accordingly, we derived specific protein biomarkers for benign apocrine metaplasia by comparing the protein expression profiles of non‐malignant breast epithelium with apocrine macrocyst cells. We then used specific antibody probes against these markers to identify and characterize precancerous and malignant lesions (Celis et al., 2006, 2006, 2007, 2007, 2008).
Here we report on the use of a panel of antibodies against components of an apocrine protein signature that includes probes against 15‐PGDH and ACSM1 (Celis et al., 2006, 2006, 2007, 2007, 2008) in addition to a set of categorizing markers that are consistently expressed (AR, CD24) or not expressed (ERα, PgR, bcl‐2, and GATA‐3) (Tavassoli et al., 1996; Gatalica, 1997; Selim and Wells, 1999; Sapp et al., 2003) by apocrine metaplastic lesions in benign breast lesions (Wells and El‐Ayat, 2007) and apocrine sweat glands, to analyze a well‐defined cohort of ADCIS and IACs in which more than 90% of the tumour cells exhibited cytological features typical of apocrine cells (Azzopardi, 1979; Tavassoli and Norris, 1994; Tavassoli and Devilee, 2003; Honma et al., 2007). Using expression of 15‐PGDH and ACSM1 alone or in combination with each other as the only classification criterion, we were able to identify 92.9% of the morphologically diagnosed ADCIS and 60.6% of the IACs as being of apocrine origin. With one exception, all the lesions were AR+, CD24+, ERα−, PgR−, Bcl‐2−, and GATA‐3−. In the case of the IACs, we could further identify a set of seven putative molecularly dedifferentiated lesions (21.2%) that most likely lost the apocrine markers during progression. Taken together, our results demonstrate that IACs correspond to a heterogeneous, yet distinct molecular subgroup of breast carcinomas that can be readily identified in an unbiased way using a combination of markers that recapitulate the phenotype of apocrine sweat glands.
A recent report by Weigelt et al. (2008) concluded, based on the analysis of 6 apocrine carcinomas that IACs may not correspond to a distinct entity as they have heterogeneous gene expression profiles that relate to several molecular subtypes. The apocrine IACs in Weigelt study had a high‐risk of recurrence and most had a poor 70‐gene prognosis signature (van't Veer et al., 2002; van de Vijver et al., 2002; Paik et al., 2004). In the case of our cohort, five of the IACs harboring patients experienced recurrences. One had a 15‐PGDH+, ACSM1+ phenotype (IAC 29), 1 was −/+ (IAC 41), 1 corresponded to an undifferentiated lesion (IAC 33), and the other 2 (IACs 39, and 46) were most likely not of apocrine origin. With the exception of IAC 33, the 4 other patients with recurrences presented also with nodal metastasis and 3 died from the disease (patients 29, +/+; 33, undifferentiated and 41; −/+). In the case of the ADCIS, no recurrences have been observed, although two patients died due to causes unrelated to the disease (patients 1 and 3). The malignant potential of the ADCIS, however, is underlined by the fact that we detected ADCIS in several of the IACs.
It is quite likely that the apparent dissimilarities between our results and those reported by Weigelt and colleagues may reflect in part differences in the cytological parameters used to diagnose the apocrine carcinomas (Rosen, 2001; Wells and El‐Ayat, 2007) as well as the fact that sub‐grouping based on genome‐wide mRNA expression profiling may not be directly comparable with sub‐typing based on a limited, even if specific, number of protein markers. Parameters such as architectural patterns and nuclear features are difficult to evaluate and as a consequence there is an intrinsic variation in their application from pathologist to pathologist, a reality that further emphasizes the need to derive objective parameters to define these lesions. In addition, the fact that two lesions are morphologically similar does not necessary mean that they are molecularly equivalent. This is well illustrated by our studies on ADCIS 8, a sample that was composed of 30% of CIS lesions positive for 15‐PDGH and ACMS1 (+/+), and 70% negative for these markers (−/−) (Figure 4). About 30% of the cells in the −/− CIS expressed very low levels of PgR, and careful analysis of the +/+ CIS revealed very few PgR positive cells present at the transition point where metaplasia takes place (Figure 4, see also Figure 1E). Previously, we have presented evidence indicating that the precursor cells giving rise to apocrine metaplasia have the phenotype 15‐PGDH−, ACMS1−, ER+, PgR+, and CK15+ (Figure 7A, compare with Figure 1A–D; Celis et al., 2007, 2008). Since CK15 positive cells are easier to identify than weakly staining, sparse PgR positive cells, we stained sections of ADCIS 8 with ACMS1 and CK15 antibodies to determine if CK15 positive cells were present, and if so, were they present in the +/+ and/or −/− CIS. As shown in Figure 7, the +/+ CIS contained CK15 positive cells (Figure 7B) contiguous to apocrine cells (Figure 7C); −/− CIS, on the other hand, also contained CK15 positive precursor cells (Figure 7D), but the flat cells contiguous to them were ACMS1 negative (Figure 7E). All CIS, however, contained cells that were CD24+ (Figure 7F), suggesting that the CK15+ precursors may give rise to apocrine as well as non‐apocrine lesions depending on the order of events leading to loss of the ER and PgR markers (Celis et al., 2006, 2008) and the effect of the local microenvironment (Figure 8). At present we cannot eliminate the possibility that these precursors correspond to two different types of cells and further studies will be needed to answer this question. As a whole, we believe that these results are quite important as they may explain in part the variability in the incidence of apocrine carcinomas (0.3–4%; Azzopardi, 1979; Frable and Kay, 1968; Mossler et al., 1980; Eusebi et al., 1986; O'Malley and Bane, 2008) and may stimulate studies intended to correlate apocrine‐like morphologies with well‐defined sets of molecular markers parameters.
Figure 7.
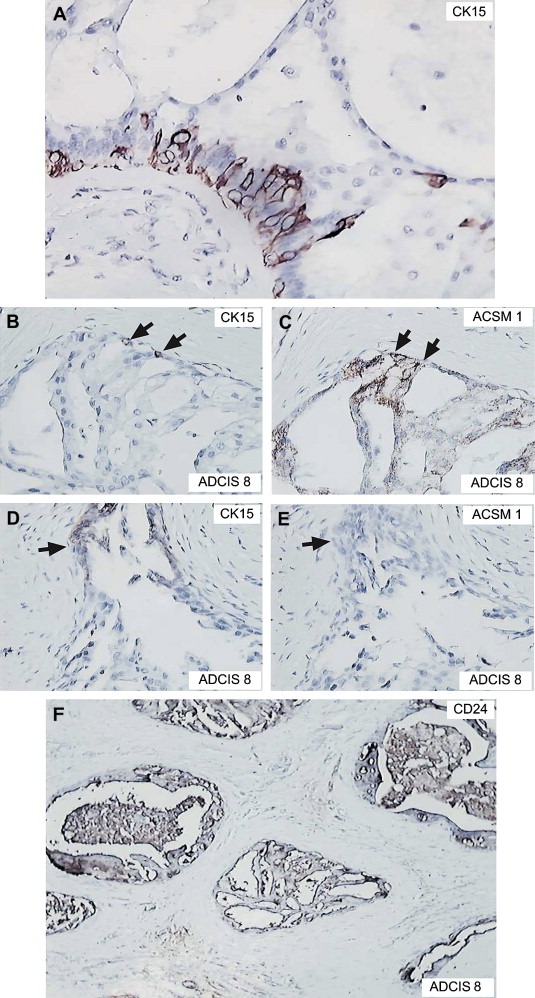
Immunostaining of apocrine lesions with various antibodies. (A) Apocrine change within sclerosing adenosis immunostained with an antibody against CK15 (AVIVA, dilution 1: 15,000). (B and C) CIS in ADCIS 8 stained with the CK15 and ACSM1 antibodies, respectively. (C and D) another CIS in ADCIS 8 stained with the CK15 and ACSM1 antibodies, respectively. (E) ADCIS 8 stained with the CD24 antibody.
Figure 8.
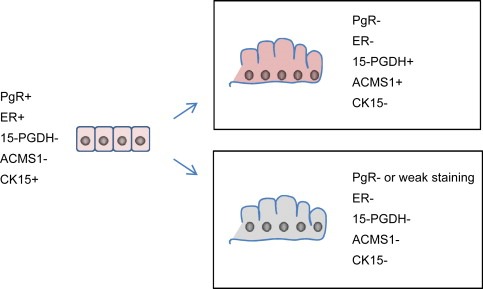
Phenotype of putative cell precursor giving rise to apocrine (15‐PGDH+, ACSM1+) and non‐apocrine cells (15‐PGDH−, ACSM1−) in ADCIS 8. At present we cannot eliminate the possibility that the precursors correspond to two different types of cells. It has not been possible to determine the CD24 status of the precursor cells.
Another source of heterogeneity that can be surmised from our observations is that some ADCIS and IACs may lose some of the markers as well as critical morphological features as result of progression making it difficult to distinguish them from other tumour types (Honma et al., 2005; Celis et al., 2008). Here, we were able to putatively identify seven 15‐PGDH/ACSM1 −/− IACs as these lesions exhibited an apocrine morphology as well as sporadic staining of invasive cells for 15‐PDGH in various areas of the preparations, or CIS that were positive for ACMS1 alone, or both for 15‐PGDH and ACMS1. So far, 2D gel‐based analysis of IACS available at the Danish Centre for Translational Breast Cancer Research (DCTB) frozen tissue collection have shown that presumed molecularly dedifferentiated IACs express undetectable levels of 15‐PGDH (Figure 9A; compare with Figure 9B and C, 15‐PGDH+, ACSM1+, IACs) and that their expression patterns very much resemble those of invasive ductal carcinomas (Figure 9D). Thus, the identification of protein biomarkers that characterize molecularly dedifferentiated IACs is not expected to be an easy task as these lesions are supposed to be rare and their systematic proteomic analysis (Celis and Gromov, 1999, 2007, 2008) may require access to large biobanks with frozen tissue samples (Ericsson et al., 2006; Riegman et al., 2008). Clearly, at present we have no direct evidence supporting the contention that the 15‐PGDH/ACSM1 −/− IACs we have identified are in fact dedifferentiated and further studies will be necessary to shed some light as to their origin.
3.1. Conclusions and future perspectives
We have established an immunohistochemical panel that defines the apocrine phenotype and that can be used to identify apocrine carcinomas and to stratify patients in an objective manner. Given the rarity of these lesions, validation of this panel for use in routine pathology will necessarily have to be done in a multi‐centre, international setup. Once these objective criteria for classification of IACs are validated it will be possible to address questions concerning clinical outcome for these lesions as well as strategies for the identification of potential molecular targets for drug development (Aggarwal et al., 2007; Ben‐Kasus et al., 2007; Marrer and Dieterle, 2008; Hodgson et al., 2009) as today's targeted therapies are beset with problems associated with safety, efficacy and resistance.
A prerequisite for successful tailored therapies is the development of molecular criteria for patient stratification so that each and every patient will receive the most adequate treatment based on therapies that spare healthy cells. Our studies have so far succeeded in partially characterizing the phenotype of early non‐obligatory precancerous lesions, ADCIS, and IACs (Celis et al., 2006, 2006, 2007, 2007, 2008) and as a result, we are in a unique position to start unraveling the state of signaling pathways and protein networks in these lesions. Identification of a critical node or interactions within the network is a potential starting point for developing drugs that inhibit the molecular target(s) that is/are vital to the continued existence of the lesion (Levitzki, 1997; Aggarwal et al., 2007; Chautard et al., in press; Winkler, 2008). Currently, we are profiling signaling pathways using phosphopeptide specific antibodies that are commercially available and we plan to determine the levels of cellular effectors and signaling molecules using tumour tissue extracts from the DCTB IAC frozen tumour collection in combination with the PanoramaTM Ab Microarray‐Cell Signaling array (Gromov et al., 2008).
Considering that the apocrine lesions are CD24+ it seems feasible to employ cell‐sorting strategies to isolate this particular cell type using as a source of sample material both benign apocrine cysts and fresh IACs collected prospectively. Moreover, it may be possible to grow these cells in three dimensional cultures (Lee et al., 2007; Kenny et al., 2007; Krause et al., 2008), a fact that will provide us with relevant cellular models, that may recapitulate the in vivo situation, to perform pathway perturbation studies in a holistic, system biology type of approach, to search for novel targets for therapeutic intervention (Ideker et al., 2001; Fishman and Porter, 2005; Schiess et al., 2009).
It should be added that some of the biomarkers we identified so far as being expressed by apocrine cells such as 15‐PGDH, HMG‐CoA reductase, and COX‐2 (Celis et al., 2006a), are well known therapeutic targets (Cho and Tai, 2002; Fujimura et al., 2007; Murtola et al., 2008; Agrawal and Fentiman, 2008; Moreno et al., 2005; Hull, 2005; Demierre et al., 2005) with pharmacological agents already available e.g. pravastatin, lovastatin, Ph CL 28A, nafazatrom, celecoxib, or rofecoxib. At least in the case of COX‐2, one has reason to suspect a causal relationship (Subbaramaiah et al., 2002) to the development of breast fibrocystic changes, implying that COX‐2 inhibitors are potential chemoprevention agents for apocrine breast cyst formation and development.
4. Experimental procedures
4.1. Samples
14 ADCIS and 33 IACs diagnosed at the Cancer Institute Hospital, Tokyo between 1997 and 2001 were analyzed (Honma et al., 2005, 2007). The age range was 32–84 with a mean average of 59 years. Hematoxylin/eosin stained slides were reviewed by two pathologists (F. A. and N. H). Apocrine carcinoma was defined as a carcinoma in which more than 90% of the tumour cells exhibited cytological features typical of apocrine cells.
4.2. Antibodies
The sources of the antibodies used in this study as well as the dilutions utilized in IHC are given in Table 1. The specificity of the antibodies against the apocrine‐specific markers was determined in every case by 2‐D PAGE immunoblotting (Celis et al., 2008).
Table 1.
Source of the Antibodies and Dilution used for IHC.
| Target protein | Antibody | Manufacturer/Provider | Dilution |
|---|---|---|---|
| ACSM1a | Rabbit polyclonal EP 071751 | Eurogentec (Liege, Belgium) | Dil 1:400 |
| 15‐PGDHa | Rabbit polyclonal A‐1294 | J.E.Celis | Dil 1:1000 |
| ERa | Mouse monoclonal Clone 1D5 | DakoCytomation (Glostrup, Denmark) | Prediluted |
| PgR | Mouse monoclonal Clone 1A6 | DakoCytomation (Glostrup, Denmark) | Prediluted |
| PgR | Mouse mononoclonal Clone PgR 636 | DakoCytomation (Glostrup, Denmark) | Dil 1:200 |
| AR | Mouse monoclonal AR27 | NovoCastra Laboratories Ltd, New Castle,UK | Dil 1:100 |
| AR | Mouse monoclonal Clone AR441 | DakoCytomation (Glostrup, Denmark) | Dil 1:100 |
| Bcl‐2 | Mouse monoclonal Clone 124 | DakoCytomation (Glostrup, Denmark) | Dil 1:20 |
| GATA‐3 | Mouse monoclonal Clone sc‐268 | Santa Cruz Biotechnology Inc | Dil 1:500 |
| CD24 | Mouse monoclonal Ab‐2 Clone SN3b | LAB VISION NeoMarkers | Dil 1:200 |
The specificity of the antibodies against the apocrine‐specific markers was determined by 2‐D PAGE immunoblotting (Celis et al., 2008).
4.3. Immunohistochemistry (IHC)
The procedures for IHC have been described in detail in previous publications (Honma et al., 2008, 2005, 2007). IHC for AR, ERα, PgR, and Bcl‐2 was performed in Tokyo, while immunostaining for 15‐PGDH, ACSM1, CD24, and GATA‐3 was performed in Copenhagen. In a few cases confirmatory stainings for AR, PgR and ERα were performed in Copenhagen. In most cases the data is expressed as % of positive cells. Samples were considered as positive if 10% or more of the cells stained with the antibodies (Honma et al., 2005). For 15‐PGDH and ACSM1 we used the following cut‐off values: ADCIS: positive if 60% or more of the CIS contained at least 30% of the cells reacting with the antibodies. IACs: positive if 30% or more of the invasive cells reacted with the antibodies.
4.4. Immunofluorescence on paraffin sections
Five‐μm sections cut from paraffin blocks of breast tissue samples were mounted on Super Frost Plus slides (Menzel‐Gläser, Braunschweig, Germany), baked at 60°C for 60min, deparaffinized, and rehydrated through graded alcohol rinses. Heat induced antigen retrieval was performed by immersing the slides in Tris/EDTA pH 9.0 buffer (10mM Tris, 1mM EDTA) and microwaving in a 750W microwave oven for 8min. Following antigen retrieval sections were treated with Image‐iT FX™ signal enhancer (Molecular Probes, USA) to block non‐specific staining and subsequently incubated with the relevant primary antibodies at appropriate dilutions. Detection of immune complexes was done with species specific secondary antibodies conjugated to Alexa Fluor® 488 and Alexa Fluor® 594 (Molecular Probes, OR, USA). Nuclear material was counterstained with TO‐PRO‐3. The sections were washed three times with cold phosphate‐buffered saline (PBS) between incubations. Normal rabbit or mouse serum instead of primary antibody was used as a negative control. Sections were imaged using a confocal laser scanning microscope (Zeiss LSM510Meta) (Moreira et al., 2005).
Acknowledgements
We would like to thank Dorte Holm, Lene Jørgensen, Kitt Christensen, Genkichi Iwakoshi, Tanya Moore, Hanne Nors, Sofia Svensson, and Signe Trentemøller for expert technical assistance. This work was supported by the Danish Cancer Society through the budget of the Institute of Cancer Biology, and by grants from the Danish Medical Research Council, Novo Nordisk, the John and Birthe Meyer Foundation, the Kai Lange and Gundhild Kai Lange Fond, the Saint Albans Church, the Lisa and Gudmund Jørgensens Fond and the “Race against Breast Cancer” foundation. The ear‐marked support of the Marketing Department at the Danish Cancer Society through their fundraising activities on behalf of DCTB is greatly appreciated.
Celis Julio E., Cabezón Teresa, Moreira José M.A., Gromov Pavel, Gromova Irina, Timmermans-Wielenga Vera, Iwase Takuji, Akiyama Futoshi, Honma Naoko, Rank Fritz, (2009), Molecular characterization of apocrine carcinoma of the breast: Validation of an apocrine protein signature in a well‐defined cohort, Molecular Oncology, 3, doi: 10.1016/j.molonc.2009.01.005.
References
- Aebersold, R. , Mann, M. , 2003. Mass spectrometry-based proteomics. Nature. 422, 198–207. [DOI] [PubMed] [Google Scholar]
- Aggarwal, B.B. , Sethi, G. , Baladandayuthapani, V. , Krishnan, S. , Shishodia, S. , 2007. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J. Cell. Biochem.. 102, 580–592. [DOI] [PubMed] [Google Scholar]
- Agrawal, A. , Fentiman, I.S. , 2008. NSAIDs and breast cancer: a possible prevention and treatment strategy. Int. J. Clin. Pract.. 62, 444–449. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat, M.L. , Sutherland, K.D. , Barker, H. , Thomas, R. , Shackleton, M. , Forrest, N.C. , Hartley, L. , Robb, L. , Grosveld, F.G. , van der Wees, J. , Lindeman, G.J. , Visvader, J.E. , 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol.. 9, 201–209. [DOI] [PubMed] [Google Scholar]
- Azzopardi, J. , 1979. Problems in Breast Pathology WB Saunders; Philadelphia, PA: pp. 23–87 [Google Scholar]
- Ben-Kasus, T. , Schechter, B. , Sela, M. , Yarden, Y. , 2007. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol. Oncol.. 1, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, L.A. , Perou, C.M. , Livasy, C.A. , Dressler, L.G. , Cowan, D. , Conway, K. , Karaca, G. , Troester, M.A. , Tse, C.K. , Edmiston, S. , Deming, S.L. , Geradts, J. , Cheang, M.C. , Nielsen, T.O. , Moorman, P.G. , Earp, H.S. , Millikan, R.C. , 2006. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 295, 2492–2502. [DOI] [PubMed] [Google Scholar]
- Celis, J.E. , Gromov, P. , 1999. 2D protein electrophoresis: can it be perfected?. Curr. Opin. Biotechnol. 10, 16–21. [DOI] [PubMed] [Google Scholar]
- Celis, J.E. , Trentemølle, S. , Gromov, P. , 2005. Gel-Based proteomics: high-resolution two-dimensional gel electrophoresis of proteins. Isoelectric Focusing (IEF) and Nonequilibrium pH Gradient Electrophoresis (NEPHGE) In Celis J.E., Carter N., Hunter T., Simons K., Small J.V., Shotton D.(Eds.), third ed. A Cell Biology. Laboratory Handbook. vol. 4, Elsevier; AP San Diego: [Google Scholar]
- Celis, J.E. , Gromov, P. , Moreira, J.M. , Cabezón, T. , Friis, E. , Vejborg, I.M. , Proess, G. , Rank, F. , Gromova, I. , 2006. Apocrine cysts of the breast: biomarkers, origin, enlargement, and relation with cancer phenotype. Mol. Cell. Proteomics. 5, 462–483. [DOI] [PubMed] [Google Scholar]
- Celis, J.E. , Gromova, I. , Gromov, P. , Moreira, J.M. , Cabezon, T. , Friis, E. , Rank, F. , 2006. Molecular pathology of breast apocrine carcinomas: a protein expression signature specific for benign apocrine metaplasia. FEBS Lett.. 580, 2935–2944. [DOI] [PubMed] [Google Scholar]
- Celis, J.E. , Moreira, J.M. , Gromova, I. , Cabezon, T. , Gromov, P. , Shen, T. , Timmermans-Wielenga, V. , Rank, F. , 2007. Characterization of breast precancerous lesions and myoepithelial hyperplasia in sclerosing adenosis with apocrine metaplasia. Mol. Oncol.. 1, 97–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis, J.E. , Gromova, I. , Cabezon, T. , Gromov, P. , Shen, T. , Timmermans-Wielenga, V. , Rank, F. , Moreira, J.M.A. , 2007. Identification of a subset of breast carcinomas characterized by expression of cytokeratin15: relationship between CK15+ progenitor/amplified cells and pre-malignant lesions and invasive disease. Mol. Oncol.. 1, 321–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis, J.E. , Gromov, P. , Cabezón, T. , Moreira, J.M. , Friis, E. , Jirström, K. , Llombart-Bosch, A. , Timmermans-Wielenga, V. , Rank, F. , Gromova, I. , 2008. 15-prostaglandin dehydrogenase expression alone or in combination with ACSM1 defines a subgroup of the apocrine molecular subtype of breast carcinoma. Mol. Cell. Proteomics. 7, 1795–1809. [DOI] [PubMed] [Google Scholar]
- Chautard, E., Thierry-Mieg, N., Ricard-Blum, S. Interaction networks: from protein functions to drug discovery. Pathol. Biol., in press. [DOI] [PubMed]
- Chen, E.I. , Yates, J.R. , 2007. Cancer proteomics by quantitative shotgun proteomics. Mol. Oncol.. 1, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, K. , DeVries, S. , Fridlyand, J. , Spellman, P.T. , Roydasgupta, R. , Kuo, W.L. , Lapuk, A. , Neve, R.M. , Qian, Z. , Ryder, T. , Chen, F. , Feiler, H. , Tokuyasu, T. , Kingsley, C. , Dairkee, S. , Meng, Z. , Chew, K. , Pinkel, D. , Jain, A. , Ljung, B.M. , Esserman, L. , Albertson, D.G. , Waldman, F.M. , Gray, J.W. , 2006. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell.. 10, 529–541. [DOI] [PubMed] [Google Scholar]
- Cho, H. , Tai, H.H. , 2002. Inhibition of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by cyclooxygenase inhibitors and chemopreventive agents. Prostaglandins Leukot. Essent. Fatty Acids. 67, 461–465. [DOI] [PubMed] [Google Scholar]
- Cleator, S. , Heller, W. , Coombes, R.C. , 2007. Triple-negative breast cancer: therapeutic options. Lancet Oncol.. 8, 235–244. [DOI] [PubMed] [Google Scholar]
- Demierre, M.F. , Higgins, P.D. , Gruber, S.B. , Hawk, E. , Lippman, S.M. , 2005. Statins and cancer prevention. Nat. Rev. Cancer. 5, 930–942. [DOI] [PubMed] [Google Scholar]
- Ericsson, C. , Franzén, B. , Nistér, M. , 2006. Frozen tissue biobanks. Tissue handling, cryopreservation, extraction, and use for proteomic analysis. Acta Oncol.. 45, 643–661. [DOI] [PubMed] [Google Scholar]
- Eusebi, V. , Millis, R.R. , Cattani, M.G. , Bussolati, G. , Azzopardi, J.G. , 1986. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am. J. Pathol.. 123, 532–541. [PMC free article] [PubMed] [Google Scholar]
- Fadare, O. , Tavassoli, F.A. , 2008. Clinical and pathologic aspects of basal-like breast cancers. Nat. Clin. Pract. Oncol.. 5, 149–159. [DOI] [PubMed] [Google Scholar]
- Farmer, P. , Bonnefoi, H. , Becette, V. , Tubiana-Hulin, M. , Fumoleau, P. , Larsimont, D. , Macgrogan, G. , Bergh, J. , Cameron, D. , Goldstein, D. , Duss, S. , Nicoulaz, A.L. , Brisken, C. , Fiche, M. , Delorenzi, M. , Iggo, R. , 2005. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 24, 4660–4671. [DOI] [PubMed] [Google Scholar]
- Fishman, M.C. , Porter, J.A. , 2005. Pharmaceuticals: a new grammar for drug discovery. Nature. 437, 491–493. [DOI] [PubMed] [Google Scholar]
- Foulkes, W.D. , Brunet, J.S. , Stefansson, I.M. , Straume, O. , Chappuis, P.O. , Bégin, L.R. , Hamel, N. , Goffin, J.R. , Wong, N. , Trudel, M, , Kapusta, L. , Porter, P. , Akslen, L.A. , 2004. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res.. 64, 830–835. [DOI] [PubMed] [Google Scholar]
- Frable, W.J. , Kay, S. , 1968. Carcinoma of the breast. Histologic and clinical features of apocrine tumors. Cancer. 21, 756–763. [DOI] [PubMed] [Google Scholar]
- Fujimura, T. , Ohta, T. , Oyama, K. , Miyashita, T. , Miwa, K. , 2007. Cyclooxygenase-2 (COX-2) in carcinogenesis and selective COX-2 inhibitors for chemoprevention in gastrointestinal cancers. J. Gastrointest. Cancer. 38, 78–82. [DOI] [PubMed] [Google Scholar]
- Gatalica, Z. , 1997. Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol. Res. Pract.. 193, 753–758. [DOI] [PubMed] [Google Scholar]
- Gromov, P. , Celis, J.E. , Gromova, I. , Rank, F. , Timmermans-Vilenga, V. , Moreira, J.M.A. , 2008. A single lysis solution for the analysis of tissue samples by different proteomic technologies. Mol. Oncol.. 2, 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson, D.R. , Whittaker, R.D. , Herath, A. , Amakye, D. , Clack, G. , 2009. Biomarkers in oncology drug development. Mol. Oncol.. 3, (1) 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeth, G. , Bendahl, P.O. , Ringnér, M. , Saal, L.H. , Gruvberger-Saal, S.K. , Lövgren, K. , Grabau, D. , Fernö, M. , Borg, A. , Hegardt, C. , 2008. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res.. 10, R53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma, N. , Takubo, K. , Akiyama, F. , Sawabe, M. , Arai, T. , Younes, M. , Kasumi, F. , Sakamoto, G. , 2005. Expression of GCDFP-15 and AR decreases in larger or node-positive apocrine carcinomas of the breast. Histopathology. 47, 195–201. [DOI] [PubMed] [Google Scholar]
- Honma, N. , Takubo, K. , Akiyama, F. , Kasumi, F. , Sawabe, M. , Arai, T. , Hosoi, T. , Yoshimura, N. , Harada, N. , Younes, M. , Sakamoto, G. , 2007. Expression of oestrogen receptor-beta in apocrine carcinomas of the breast. Histopathology. 50, 425–433. [DOI] [PubMed] [Google Scholar]
- Hull, M.A. , 2005. Cyclooxygenase-2: how good is it as a target for cancer chemoprevention?. Eur. J. Cancer. 41, 1854–1863. [DOI] [PubMed] [Google Scholar]
- Ideker, T. , Galitski, T. , Hood, L. , 2001. A new approach to decoding life: systems biology. Annu. Rev. Genomics Hum. Genet.. 2, 343–372. [DOI] [PubMed] [Google Scholar]
- Irvin, W.J. , Carey, L.A. , 2008. What is triple-negative breast cancer?. Eur. J. Cancer. 44, 2799–2805. [DOI] [PubMed] [Google Scholar]
- Japaze, H. , Emina, J. , Diaz, C. , Schwam, R.J. , Gercovich, N. , Demonty, G. , Morgenfeld, E. , Rivarola, E. , Gil Deza, E. , Gercovich, F.G. , 2005. ‘Pure’ invasive apocrine carcinoma of the breast: a new clinicopathological entity?. Breast. 14, 3–10. [DOI] [PubMed] [Google Scholar]
- Kenny, P.A. , Lee, G.Y. , Myers, C.A. , Neve, R.M. , Semeiks, J.R. , Spellman, P.T. , Lorenz, K. , Lee, E.H. , Barcellos-Hoff, M.H. , Petersen, O.W. , Gray, J.W. , Bissell, M.J. , 2007. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol.. 1, 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr, H. , Slorach, E.M. , Sternlicht, M.D. , Werb, Z. , 2006. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 127, 1041–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen, G. , Schlüns, K. , Yongwei, Y. , Denkert, C. , Dietel, M. , Petersen, I. , 2003. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Br. J. Cancer. 88, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, S. , Maffini, M.V. , Soto, A.M. , Sonnenschein, C. , 2008. A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng. Part C Methods. 14, 261–271. [DOI] [PubMed] [Google Scholar]
- Langerød, A. , Zhao, H. , Borgan, Ø. , Nesland, J.M. , Bukholm, I.R. , Ikdahl, T. , Kåresen, R. , Børresen-Dale, A.L. , Jeffrey, S.S. , 2007. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res.. 9, R30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G.Y. , Kenny, P.A. , Lee, E.H. , Bissell, M.J. , 2007. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods. 4, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki, A. , 1997. Targeting signal transduction for disease therapy. Med. Oncol. 14, 83–89. [DOI] [PubMed] [Google Scholar]
- Lim, S.C. , Oh, S.H. , 2005. The role of CD24 in various human epithelial neoplasias. Pathol. Res. Pract.. 201, 479–486. [DOI] [PubMed] [Google Scholar]
- Livasy, C.A. , Perou, C.M. , Karaca, G. , Cowan, D.W. , Maia, D. , Jackson, S. , Tse, C.K. , Nyante, S. , Millikan, R.C. , 2007. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum. Pathol.. 38, 197–204. [DOI] [PubMed] [Google Scholar]
- Marrer, E. , Dieterle, F. , 2008. Biomarkers in oncology drug development: rescuers or troublemakers?. Expert Opin. Drug Metab. Toxicol.. 4, 1391–1402. [DOI] [PubMed] [Google Scholar]
- Mazoujian, G. , Pinkus, G.S. , Davis, S. , Haagensen, D.E. , 1983. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am. J. Pathol.. 110, 105–112. [PMC free article] [PubMed] [Google Scholar]
- Miller, W.R. , Shivas, A.A. , Franchimont, P. , Haagensen, D.E. , 1988. Breast gross cystic disease protein 15 in human breast cancer in culture. Eur. J. Cancer Clin. Oncol.. 24, 223–228. [DOI] [PubMed] [Google Scholar]
- Moinfar, F. , 2008. Is ‘basal-like’ carcinoma of the breast a distinct clinicopathological entity? A critical review with cautionary notes. Pathobiology. 75, 119–131. [DOI] [PubMed] [Google Scholar]
- Moreno, J. , Krishnan, A.V. , Swami, S. , Nonn, L. , Peehl, D.M. , Feldman, D. , 2005. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res.. 65, 7917–7925. [DOI] [PubMed] [Google Scholar]
- Moreira, J.M. , Ohlsson, G. , Rank, F.E. , Celis, J.E. , 2005. Down-regulation of the tumor suppressor protein 14-3-3sigma is a sporadic event in cancer of the breast. Mol. Cell. Proteomics. 4, 555–569. [DOI] [PubMed] [Google Scholar]
- Moriya, T. , Kozuka, Y. , Kanomata, N. , Tse, G.M. , Tan, P.H. , 2009. The role of immunohistochemistry in the differential diagnosis of breast lesions. Pathology. 41, 68–76. [DOI] [PubMed] [Google Scholar]
- Mossler, J.A. , Barton, T.K. , Brinkhous, A.D. , McCarty, K.S. , Moylan, J.A. , McCarty, K.S. , 1980. Apocrine differentiation in human mammary carcinoma. Cancer. 46, 2463–2471. [DOI] [PubMed] [Google Scholar]
- Murtola, T.J. , Visakorpi, T. , Lahtela, J. , Syvälä, H. , Tammela, T.Lj. , 2008. Statins and prostate cancer prevention: where we are now, and future directions. Nat. Clin. Pract. Urol.. 5, 376–387. [DOI] [PubMed] [Google Scholar]
- Nielsen, T.O. , Hsu, F.D. , Jensen, K. , Cheang, M. , Karaca, G. , Hu, Z. , Hernandez-Boussard, T. , Livasy, C. , Cowan, D. , Dressler, L. , Akslen, L.A. , Ragaz, J. , Gown, A.M. , Gilks, C.B. , van de Rijn, M. , Perou, C.M. , 2004. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res.. 10, 5367–5374. [DOI] [PubMed] [Google Scholar]
- O'Malley, F.P. , Bane, A. , 2008. An update on apocrine lesions of the breast. Histopathology. 52, 3–10. [DOI] [PubMed] [Google Scholar]
- Page, D.L. , 2003. Special types of invasive breast cancer, with clinical implications. Am. J. Surg. Pathol.. 27, 832–835. [DOI] [PubMed] [Google Scholar]
- Page, D.L. , 2005. Apocrine carcinomas of the breast. Breast. 14, 1–2. [DOI] [PubMed] [Google Scholar]
- Paik, S. , Shak, S. , Tang, G. , Kim, C. , Baker, J. , Cronin, M. , Baehner, F.L. , Walker, M.G. , Watson, D. , Park, T. , Hiller, W. , Fisher, E.R. , Wickerham, D.L. , Bryant, J. , Wolmark, N. , 2004. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med.. 351, 2817–2826. [DOI] [PubMed] [Google Scholar]
- Perou, C.M. , Sørlie, T. , Eisen, M.B. , van de Rijn, M. , Jeffrey, S.S. , Rees, C.A. , Pollack, J.R. , Ross, D.T. , Johnsen, H. , Akslen, L.A. , Fluge, O. , Pergamenschikov, A. , Williams, C. , Zhu, S.X. , Lønning, P.E. , Børresen-Dale, A.L. , Brown, P.O. , Botstein, D. , 2000. Molecular portraits of human breast tumours. Nature. 406, 747–752. [DOI] [PubMed] [Google Scholar]
- Rakha, E.A. , Putti, T.C. , Abd El-Rehim, D.M. , Paish, C. , Green, A.R. , Powe, D.G. , Lee, A.H. , Robertson, J.F. , Ellis, I.O. , 2006. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J. Pathol.. 208, 495–506. [DOI] [PubMed] [Google Scholar]
- Reis-Filho, J.S. , Tutt, A.N. , 2008. Triple negative tumours: a critical review. Histopathology. 52, 108–118. [DOI] [PubMed] [Google Scholar]
- Riegman, P.H.J. , Morente, M.M. , Betsou, F. , de Blasio, P. , Geary, P. , 2008. Biobanking for better healthcare. Mol. Oncol.. 2, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Pinilla, S.M. , Sarrió, D. , Honrado, E. , Moreno-Bueno, G. , Hardisson, D. , Calero, F. , Benítez, J. , Palacios, J. , 2007. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J. Clin. Pathol.. 60, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, P. , 2001. Invasive duct carcinoma. Assessment of prognosis, morphologic prognostic markers, and tumor growth rate. In Rosen P., Rosen's Breast Pathology. second ed. Lippincott Williams & Wilkins; Philadelphia: [Google Scholar]
- Sapp, M. , Malik, A. , Hanna, W. , 2003. Hormone receptor profile of apocrine lesions of the breast. Breast J.. 9, 335–356. [DOI] [PubMed] [Google Scholar]
- Selim, A.G. , Wells, C.A. , 1999. Immunohistochemical localisation of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to oestrogen and progesterone receptors. J. Clin. Pathol.. 52, 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiess, R. , Wollscheid, B. , Aebersold, R. , 2009. Targeted proteomic strategy for clinical biomarker discovery. Mol. Oncol.. 3, (1) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie, T. , Perou, C.M. , Tibshirani, R. , Aas, T. , Geisler, S. , Johnsen, H. , Hastie, T. , Eisen, M.B. , van de Rijn, M. , Jeffrey, S.S. , Thorsen, T. , Quist, H. , Matese, J.C. , Brown, P.O. , Botstein, D. , Eystein Lønning, P. , Børresen-Dale, A.L. , 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U.S.A.. 98, 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie, T. , Tibshirani, R. , Parker, J. , Hastie, T. , Marron, J.S. , Nobel, A. , Deng, S. , Johnsen, H. , Pesich, R. , Geisler, S. , Demeter, J. , Perou, C.M. , Lonning, P.E. , Brown, P.O. , Borresen-Dale, A.L. , Botstein, D. , 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. U.S.A.. 100, 8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah, K. , Norton, L. , Gerald, W. , Dannenberg, A.J. , 2002. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J. Biol. Chem.. 277, 18649–18657. [DOI] [PubMed] [Google Scholar]
- Surowiak, P. , Materna, V. , Györffy, B. , Matkowski, R. , Wojnar, A. , Maciejczyk, A. , Paluchowski, P. , Dziegiel, P. , Pudełko, M. , Kornafel, J. , Dietel, M. , Kristiansen, G. , Zabel, M. , Lage, H. , 2006. Multivariate analysis of oestrogen receptor alpha, pS2, metallothionein and CD24 expression in invasive breast cancers. Br. J. Cancer. 95, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K. , Imoto, S. , Wada, N. , Sakemura, N. , Hasebe, K. , 2008. Invasive apocrine carcinoma of the breast: clinicopathologic features of 57 patients. Breast J.. 14, 164–168. [DOI] [PubMed] [Google Scholar]
- Tavassoli, F.A. , Norris, H.J. , 1994. Intraductal apocrine carcinoma: a clinicopathologic study of 37 cases. Mod. Pathol.. 7, 813–818. [PubMed] [Google Scholar]
- Tavassoli, F.A. , Purcell, C.A. , Bratthauer, G.L. , Man, Y. , 1996. Androgen receptor expression along with loss of Bcl-2, ER, and PR expression in benign and malignant apocrine lesions of the breast: implications of therapy. Breast J.. 2, 261–269. [Google Scholar]
- In Tavassoli F.A., Devilee P.(Eds.), World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; Lyon: [Google Scholar]
- van de Vijver, M.J. , He, Y.D. , van't Veer, L.J. , Dai, H. , Hart, A.A. , Voskuil, D.W. , Schreiber, G.J. , Peterse, J.L. , Roberts, C. , Marton, M.J. , Parrish, M. , Atsma, D. , Witteveen, A. , Glas, A. , Delahaye, L. , van der Velde, T. , Bartelink, H. , Rodenhuis, S. , Rutgers, E.T. , Friend, S.H. , Bernards, R. , 2002. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med.. 347, 1999–2009. [DOI] [PubMed] [Google Scholar]
- van't Veer, L.J. , Dai, H. , van de Vijver, M.J. , He, Y.D. , Hart, A.A. , Mao, M. , Peterse, H.L. , van der Kooy, K. , Marton, M.J. , Witteveen, A.T. , Schreiber, G.J. , Kerkhoven, R.M. , Roberts, C. , Linsley, P.S. , Bernards, R. , Friend, S.H. , 2002. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 415, 530–536. [DOI] [PubMed] [Google Scholar]
- Wells, C.A. , El-Ayat, G.A. , 2007. Non-operative breast pathology: apocrine lesions. J. Clin. Pathol.. 60, 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt, B. , Horlings, H.M. , Kreike, B. , Hayes, M.M. , Hauptmann, M. , Wessels, L.F. , de Jong, D. , Van de Vijver, M.J. , Van't Veer, L.J. , Peterse, J.L. , 2008. Refinement of breast cancer classification by molecular characterization of histological special types. J. Pathol.. 216, 141–150. [DOI] [PubMed] [Google Scholar]
- Winkler, D.A. , 2008. Network models in drug discovery and regenerative medicine. Biotechnol. Annu. Rev.. 14, 143–170. [DOI] [PubMed] [Google Scholar]


