Abstract
Antibodies have become valuable therapeutic agents for targeting of extracellular proteins in various diseases, including cancer, autoimmunity and cardiovascular disorders. For breast cancer, antibodies targeting the human HER2 have been shown to result in cell growth inhibition both in vitro and in patients with breast tumors. There is evidence to suggest that targeting multiple HER2 epitopes may result in increased growth inhibition making it interesting to find antibodies targeting new epitopes. Here, we report on a new scheme to discover antibodies directed to new epitopes using the extracellular domain of the HER2 as a model. Polyclonal antibodies were generated using recombinant protein fragments and affinity purified fractions of the antibodies were functionally characterized and precisely epitope mapped using bacterial surface display. Polyclonal antibodies towards a 127 amino acid recombinant protein fragment spanning between domains II and III of the HER2 were shown to bind to human ductal carcinoma cell line BT474 resulting in growth inhibition. Affinity purification demonstrated that antibodies to two separate regions from the N‐ and C‐terminal end of the fragment exhibited the growth inhibition. Epitope mapping of the C‐terminal antibodies revealed a 25 amino acid region (LPESFDGDPASNTAPLQPEQLQVF) with two distinct epitopes mediating efficient growth inhibition. The results suggest that antibodies directed towards this region of domain III of the HER2, distinct from the well‐known monoclonal antibodies trastuzumab and pertuzumab, bind to the HER2 on living cells and exhibit growth inhibition. The work describes a new strategy to develop antibodies directed to non‐overlapping epitopes and shows a path of pursuit to explore the epitope space of a target protein.
Keywords: Antibody, Growth inhibition, HER2, Epitope mapping
1. Introduction
The human epidermal growth factor receptor 2 (HER2/neu/ErbB2) (Schechter et al., 1985) is a 185kDa transmembrane glycoprotein that is a member of the epidermal growth factor receptor (EGFR) family of tyrosine kinase receptors, also including the EGFR (HER1), HER3 and HER4 receptors (Yarden and Sliwkowski, 2001). HER2 lacks a specific ligand and acts as the preferred partner for ligand‐mediated heterodimerization with the other three EGFR family members leading to structural activation of the intracellular tyrosine kinase domain, thus mediating signal transduction through several pathways (Yarden and Sliwkowski, 2001) resulting in mitogenesis, apotopsis, angiogenesis and cell differentiation (Menard et al., 2000). The extracellular domain of the HER2 with 630 amino acids contains four subdomains I–IV (Figure 1B) and structural studies have revealed that the dimerization loop in domain II is constantly exposed, which aids in explaining why HER2 is the preferred partner for dimerization with ligand‐activated EGFR, HER3 and HER4. In case of overexpression of HER2, as described in about 20–30% of breast cancer patients (Slamon et al., 1987), this conformational feature facilitates the transforming potential of the homodimer, in the absence of any ligand (Yarden and Sliwkowski, 2001).
Figure 1.
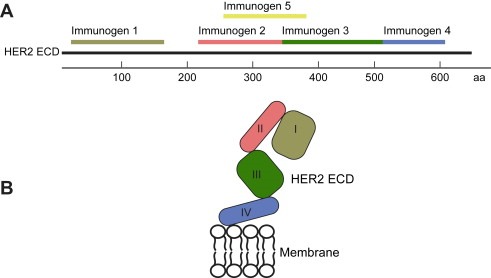
Immunogen location on the extracellular domain of HER2. Five protein fragments covering the extracellular region of the HER2 protein were cloned and expressed as recombinant protein fragments (A). Immunogens 1–4 (residues 42–186, 236–363, 324–530, 531–626) were chosen to resemble the four domains of HER2‐ecd (B), whereas Immunogen 5 (residues 347–492) was chosen on low sequence identity to other human proteins (Lindskog et al., 2005).
Several compounds have been described designed to treat cancer patients with overexpression of the HER2 (Pal and Pegram, 2007). The most known is trastuzumab, also denoted Herceptin (Genentech Inc., San Francisco, CA), which has been approved as first‐line therapy in patients with HER2‐overexpressing metastatic breast cancer. Numerous reports have shown increased disease‐free survival using injections of trastuzumab often in combination with other drugs, such as the taxanes paclitaxel or docetaxel (Popat and Smith, 2008). Trastuzumab binds HER2 on the C‐terminal portion of domain IV at a site that contains the binding pocket for the extended domain II loop of the inactive states of the HER1 and HER3 receptors (Cho et al., 2003). The interaction of the antibody is mediated by three loop regions on the target protein HER2, which are formed by residues 557–561, 570–573 and 593–603. Another monoclonal antibody pertuzumab, also called Omnitarg (Genentech Inc., San Francisco, CA), inhibits the growth of tumors displaying both low and high HER2 receptor levels, in contrast to trastuzumab (Agus et al., 2002), which is only effectively inhibiting highly overexpressing tumors. Pertuzumab binds HER2 near the center of domain II and the binding is predicted to sterically block the region necessary for dimerization with the other HER‐receptors (Franklin et al., 2004). The interaction with the antibody is primary mediated by HER2 residues in region Ser267–Lys333. In particular, the residues His267, Val308, Ser310, Leu317, His318 and Lys333 showed to reduce antibody binding significantly when subjected to alanine mutation studies (Franklin et al., 2004). Hence, the two monoclonal antibodies with proven clinical effects bind to the HER2 receptor in two different regions corresponding to domain II and IV, which indicate the potential to target and effect HER2 at different sites.
The medical need for targeting multiple parts of the receptor has shown to be increasingly important as the clinical problem of trastuzumab resistance is becoming more frequent. As of today, the majority of the initially responding patients with metastatic breast cancer demonstrate disease progression within 1year of treatment with trastuzumab (Nahta et al., 2006). There are several reports suggesting a synergistic treatment such as using several antibodies towards the same target (Pal and Pegram, 2006) and since the immune response consists of antibodies generated towards different parts of the antigen this can be achieved by using polyclonal antibodies. For clinical applications, it is also possible to use a panel of monoclonal antibodies as has been shown for tumor antigens (Sharon et al., 2005). An interesting clinical strategy is thus to generate antibodies with separate and distinct epitopes and then to use these antibodies in combination. For the HER2, there are several reports suggesting that targeting multiple epitopes may result in increased growth inhibition (Nahta et al., 2006; Pal and Pegram, 2007). Spiridon et al. (2002) showed in a human BT474 cell line assay that the IC50 of growth inhibition was 3–200 fold lower for three monoclonal antibodies directed to the HER2 when used in combination with trastuzumab as compared to that of either antibody alone or trastuzumab. Similar results were demonstrated by in vivo experiments using mice xenotransplanted with BT474 cells (Spiridon et al., 2002). There are also data to suggest synergy by the combination of trastuzumab and pertuzumab. Treatment of BT474 cells with a combination of the antibodies resulted in higher growth inhibition and significant decreased levels of phosphor‐serine 473, indicating decreased signaling from the PI3K/Akt pathway, compared to either antibody alone (Nahta et al., 2004). A recent clinical study of trastuzumab insensitive patients showed a potential clinical benefit for a combined treatment using pertuzumab together with trastuzumab (Portera et al., 2008). In a recent phase II clinical trial (Gelmon et al., 2008) nearly one in four patients saw their tumors disappear (complete tumor response, 8%) or shrink (partial tumor response, 16%). In a further 25% of patients with progressing cancer, stabilization was observed for at least 6months. The combination of pertuzumab and trastuzumab was well tolerated in this group of patients and no patients were withdrawn from the trial with treatment‐related adverse events.
A need therefore exist to further explore the epitope space of potential clinical targets in a systematic manner. Here, we describe a strategy to map new epitopes to protein targets, in which affinity purified polyclonal antibodies are used and functionally effective fractions are epitope mapped using a new bacterial display technique. Potential epitopes can subsequently be employed to generate humanized, recombinant human or fully human monoclonal antibodies (Ben‐Kasus et al., 2007) to be used alone or in cocktails (Logtenberg, 2007). We have applied the strategy to explore the possibility to identify functional antibodies directed to new HER2 epitopes. Several effective antibodies towards new epitopes not previously described on the domain III of HER2 were discovered.
2. Results
2.1. Generation of antibodies
Five protein fragments spanning the extracellular (ecto) domain (ecd) of the HER2 receptor were selected as protein epitope signature tags (PrESTs) used as immunogens (Figure 1A). Immunogens 1–4 were chosen to resemble the four domains of HER2‐ecd (Figure 1B), whereas Immunogen 5 was chosen based on low sequence identity with other human proteins using PrEST design software (Lindskog et al., 2005). The five recombinant protein fragments correspond to the ecd protein residues 42–186, 236–363, 364–530, 531–626 and 347–492 respectively (Figure 1A). The first four fragments were designed to encompass complete domains of the extracellular part of the HER2 receptor to facilitate possible correct native fold in the heterologous host. The fifth fragments does not contain complete domains and one might speculate that this fragment does not yield native folded domains with the consequence that antibodies towards this antigen preferentially will be directed towards linear epitopes. In addition, a protein fragment from the intracellular region of the HER2 was designed (Immunogen intra). The corresponding gene fragments were cloned by RT‐PCR from human RNA and sequence‐verified by DNA sequencing (Agaton et al., 2004). Recombinant protein fragments were expressed in Escherichia coli and affinity purified using a His6 affinity tag and the sizes were verified with mass spectrometry. The protein fragments were immunized in separate rabbits and sera were used for affinity purifications using the immunogens as ligands to generate fragment‐specific polyclonal antibodies (Nilsson et al., 2005), abbreviated Ab–D1, Ab–D2, Ab–D3, Ab–D4 and Ab–5 for the five separate immunogens towards the extracellular domain of HER2 and one for the intracellular part (Ab–intra). All sera contained adequate antibody responses for purification and specificity validation to generate antigen purified monospecific antibodies (Nilsson et al., 2005).
2.2. Flow cytometric binding analysis
The five affinity purified polyclonal antibodies Ab–D1, Ab–D2, Ab–D3, Ab–D4 and Ab–5, were subjected to flow cytometric analysis to screen for potential binding to native HER2 protein expressed on the cell surface of human breast tumor cell line BT474 (Figure 2). Cell binding ability was evaluated for all five antibodies, including two positive controls (trastuzumab and pertuzumab) and the negative control (Ab–intra) targeting the inaccessible intracellular part of HER2 receptor. Antibodies Ab–D1, Ab–D2 and Ab–D3 showed no significant cell binding as compared to Ab–intra or naked unlabeled cells. Weak cell binding was shown for Ab–D4 whereas Ab–5 showed a significant binding, although lower than the positive controls. No specific binding to neuroblastoma SH–SY5Y could be detected for either of the polyclonal antibodies when tested using same conditions as for BT474 (data not shown). The results show that the antibody fraction Ab–5 recognizes the native HER2 receptors on live cells.
Figure 2.
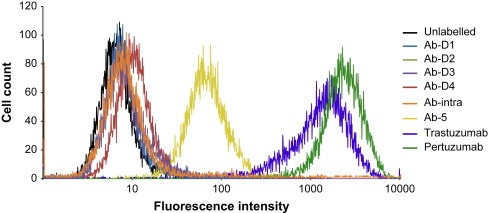
Flow cytometric analysis of antibodies. The five monospecific antibodies Ab–D1, Ab–D2, Ab–D3, Ab–D4 and Ab–5, were subjected to flow cytometric analysis to screen for potential binding to native HER2 on the cell surface of the ductal carcinoma cell line BT474. Cell binding ability was evaluated for all five monospecific antibodies as well as two commercially available monoclonal antibodies (Trastuzumab/Pertuzumab) and one negative control (Ab–intra) targeting an inaccessible intracellular part of HER2.
2.3. Immunohistochemistry of cancer patients
The polyclonal antibody fraction Ab–5 was further analyzed by immunohistochemistry on normal and cancer tissues. As expected for HER2 targeting antibodies, a weak staining was observed in normal breast glandular cells, in contrast to a distinct membranous staining for the breast cancer patients (Figure 3A). A similar staining was observed for a commercial antibody (Dako cytomation A/S, Glostrup, Denmark) recognizing the HER2 receptor (data not shown). The results show that the Ab–5 antibody can recognize the HER2 receptor in a specific manner in formalin‐fixed, paraffin‐imbedded samples from cancer patients.
Figure 3.
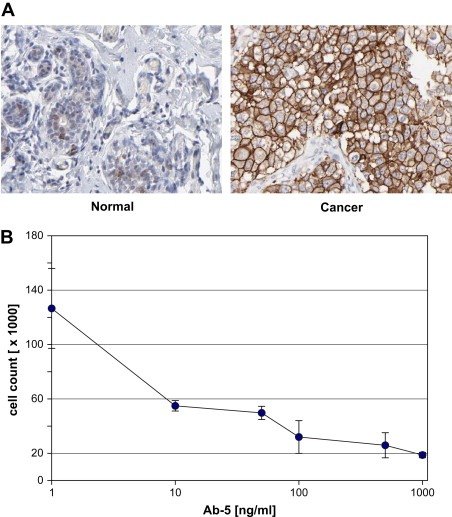
Immunohistochemistry and cell growth inhibition. Ab–5 was analyzed by immunohistochemistry on normal and cancer tissues (Agaton et al., 2004; Nilsson et al., 2005). Weak membranous/cytoplasmic staining can be seen on normal breast glandular cells, in contrast to a clear membranous staining for breast ductal carcinoma over‐expressing HER2 (A). A dose‐dependent effect on cell growth of breast cancer cells treated with increasing concentration of antibody Ab–5 was shown in a 5day titration study using human BT474 cells (B).
2.4. Cell growth inhibition
The antibody Ab–5 was further tested for growth inhibition of BT474 breast tumor cells. A dosage dependent effect on survival of breast cancer cells was shown in a 5day titration study. A clear increased effect was observed for concentrations spanning in the range of 10–100ng/ml (Figure 3B). The results indicate that Ab–5 can inhibit cell growth in a dose‐dependent manner.
2.5. Affinity‐fractionation
The polyclonal fraction Ab–5 was further characterized by affinity purification using different parts of the immunogen as ligand. Three protein fragments were separately cloned, recombinantly expressed and affinity purified as described above. The fragments, corresponding to the N‐terminal (5.1), middle (5.2) and C‐terminal (5.3) part of Immunogen 5 respectively (Figure 4A), were coupled to chromatography matrices to allow for affinity purification using the protein fragments as capture agents. The rabbit polyclonal serum was used for sequential affinity purification as outlined in Figure 4A. A fourth affinity column was added with the intact Immunogen 5 to capture antigen specific antibodies not binding to the sub‐fragments. The columns were separately eluted yielding antibodies binding to the C‐terminal, middle and N‐terminal part of Immunogen 5. The results obtained (Table 1) show that most (39%) of these antibodies were captured using the 37 aa C‐terminal fragment. Similar amounts (35%, 17 aa) for the middle fragment and relatively low amounts (18%, 73 aa) for the N‐terminal fragment. Interestingly, only 8% of the antibodies were captured using the complete Immunogen 5 suggesting very few conformational epitopes present only on the complete immunogen, not available on any of the sub‐fragments.
Figure 4.
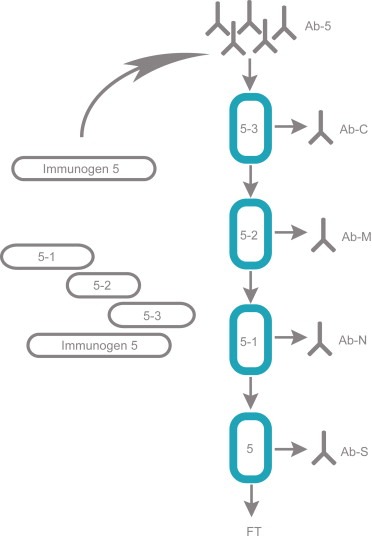
Fractionation of antibodies towards Immunogen 5. Four separate affinity columns were employed to facilitate a sequential purification of antibodies. According to this outline, four antibody fractions, an N‐terminal, middle, C‐terminal and a rest fraction were obtained after separate elution. The relative antibody yields from the various columns are shown in Table 1.
Table 1.
‐ Characteristics of fractionated antibodies.
| Antibody ID | Target residues | EC‐50 (nM) | Relative amount (%) a | Peptide 5.1 (aa 236–363) (%) b | Peptide 5.2 (aa 347–492) (%) b | Peptide 5.3 (aa 364–530) (%) b |
|---|---|---|---|---|---|---|
| Ab‐5 | 274–400 | 1.9 | 100 | 66 | 29 | 24 |
| Ab‐N | 274–346 | 4.5 | 18 | 86 | 1 | 1 |
| Ab‐M | 347–363 | 0.7 | 35 | 20 | 18 | 1 |
| Ab‐C | 364–400 | 1.2 | 39 | 1 | 94 | 87 |
| Ab‐S | 274–400 | N/A | 8 | 1 | 2 | 2 |
Polyclonal antibodies raised against Immunogen 5 were analyzed in terms of composition, (relative amounts after fractionation) apparent affinities (EC‐50) and specificity (reactivity towards peptide 5.1–5.3).
Numbers are percentages of respective antibody eluted, relative the total amount of Immunogen 5 specific antibodies.
Numbers are percentages of binding intensities values for respective antibody relative binding to Immunogen 5.
2.6. Multiplexed specificity determination and affinity ranking
In order to assess cross‐reactivity and to evaluate binding specificity, all fractions were tested in a multiplexed antigen array format. Besides the protein fragments used for immunization and fractionation, more than 90 different PrEST proteins were immobilized on color‐coded beads, combined to form a 100‐plex bead mixture to incubated with the antibodies (Supplementary Figure 1A). All eluted antibody fractions showed to bind to Immunogen 5 or respective proteins overlapping Immunogen 5 as summarized in Table 1. While the C‐terminal peptide 5.3 was only recognized by Ab–5 and Ab–C, peptide 5.2, located in the middle of Immunogen 5, was detected by Ab–5, Ab–C and Ab–M. The binders Ab–N, Ab–M and Ab–5 bound to the N‐terminal peptide 5.1 and antibodies eluted from the final full‐length column (Ab–S) showed no binding other than to Immunogen 5 (Supplementary Figure 1a). No potential cross‐reactivity towards unrelated proteins, classified by a signal intensity ≥10% of Immunogen 5, was observed (data not shown). Fractions obtained were tested using Western blot of BT474 cell lysate showing specific recognition of a major band with the expected size of HER2 for all antibody fractions except Ab–M (Supplementary Figure 1b). To obtain apparent affinities for the antibodies with the aim to rank the binding quality of different antibody fractions, competition assays with multiplexed read‐out were performed. Based on the observation made in the specificity studies, Immunogen 5 was utilized as soluble competitor and EC‐50 values were calculated. For Immunogen 5 apparent affinities in the lower nanomolar range were obtained (Table 1) and binders could be ranked with decreasing binding affinities as Ab–M<Ab–C<Ab–5<Ab–N.
2.7. Binding of fractionated antibodies to live cells
The fractionated antibodies were tested for binding to live cells by flow cytometric analysis using human breast tumor cell line BT474 cells. Interestingly, the middle fraction (Ab–M) did not bind to the native HER2, while both the antibody fractions recognizing the C‐ and N‐terminal parts of Immunogen 5 showed specific binding (Figure 5A). As shown, the applied monoclonal antibodies bound stronger as compared to the polyclonal fractions.
Figure 5.
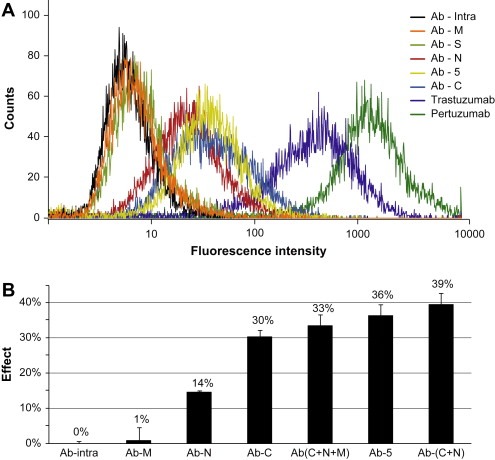
Performance of antibody sub‐fractions in cell binding and cell growth inhibition. (A) Purified fractions Ab‐C and Ab‐N together with Ab‐5 showed moderate cell binding to the HER2 expressing human BT474 cell line using flow cytometry while fractions Ab‐M and Ab‐S along with the negative control Ab‐Intra showed not to bind. (B) Growth inhibition was studied with human BT474 cells treated with 500 ng/ml of sub‐fractions Ab‐N and Ab‐C as well as for combinations of these. The growth inhibition effect was calculated as a relative measure of live cells over cells treated with the negative control Ab‐intra.
2.8. Cell growth inhibition with fractionated antibodies
The growth inhibition of the various antibody fractions was tested using the human breast tumor cell line BT474 as described above. All fractions were tested in triplicates using the same concentration of antibody (500ng/ml). A clear growth inhibiting effect was obtained for both the N‐ and the C‐terminal antibodies, although a stronger effect was observed for the C‐terminal fraction (30%). The inhibition with C‐terminal antibodies was only slightly weaker than the positive control Ab–5 (36%). In an attempt to reconstitute the original composition in Ab–5 and to test potential synergistic effects, cocktails of Ab–N, Ab–M and Ab–C were made based on their original occurrence in Ab–5 (Table 1) while maintaining the same concentration of antibody (500ng/ml). Combining all three fractions of antibodies (Ab–N, Ab–M, Ab–C) yielded nearly the same inhibitory effect (33%) as Ab–5, while the mixture of only N‐ and C‐terminal antibodies yielded a slightly higher growth inhibition (39%) as compared to Ab–5. This suggests a synergistic effects by mixing the N‐ and C‐terminal antibodies, demonstrates the potency of the antibody fraction directed to the C‐terminal part of Immunogen 5.
2.9. In‐depth epitope mapping
In order to map the epitopes of the polyclonal antibody fractions directed towards the N‐ and C‐terminal regions of Immunogen 5 in further detail an epitope mapping approach using a bacterial surface display assay based on Staphylocoocus carnosus (Rockberg et al., 2008) was employed. The gene corresponding to HER2‐ecd was fragmented and cloned into a surface display vector and transformed into S. carnosus to create a library size of 3×105 members, each displaying one unique part of the HER2‐ecd on its surface. The cells were treated with the different antibody fractions (Ab–C and Ab–N), labeled with secondary fluorescent antibodies and analyzed using fluorescently activated cell sorting. Antibody binding cells were captured and their DNA inserts were sequenced using pyrosequencing (Ronaghi et al., 1998). In this way, fragments with binding epitopes were mapped in a systematic manner despite the fact that a polyclonal serum was used. The resulting plentitude of clones were sequenced based on sorting for both the N‐ and C‐terminal antibody fractions and aligned back to the HER‐2 sequence (see Supplementary Figure 2). The results were used to map the specific epitopes of the two antibody fractions and in both cases a limited number of epitopes were observed (Figure 6). Three distinct epitopes (N1:YNTDTFES, N2:NPEGRYTFGA and N3:VGSCTLVCPLHNQEVT), were obtained for the antibodies recognizing the N‐terminal part of Immunogen 5 (Ab–N), while only two separate epitopes (C1:LPESFDGD and C2:LQVF) were mapped for the antibodies towards the C‐terminal part (Ab–C). All five linear epitopes are exposed on the surface of the native HER2 receptor (Figure 7). Interestingly, the epitope N3 on domain II overlaps with the conformational epitope recognized by the therapeutic antibody pertuzumab. The epitopes on the domain III is part of an alpha‐helical structure (epitope C2) while the other is part of a loop structure in proximity of a beta‐pleated sheet structure (epitope C1).
Figure 6.
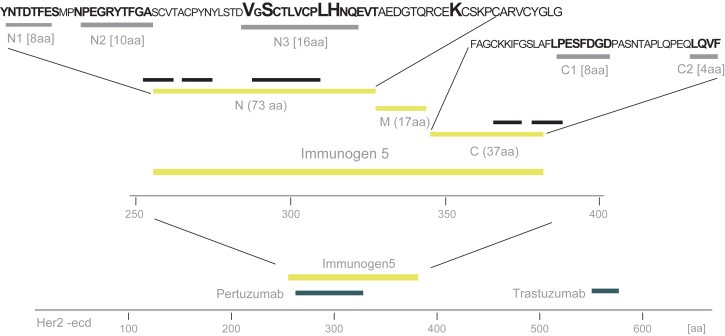
In‐depth epitope mapping of antibody sub‐fractions. Ab–N and Ab–C were mapped using a bacterial cell display system (Rockberg et al., 2008). The extracellular domain is shown with the approximate binding regions of the two monoclonal antibodies pertuzumab and trastuzumab. Immunogen 5 and its three sub regions (N, M and C) cover a stretch of 127 aa and the epitope mapping revealed consensus sequence depicted as N1, N2, N3 on the N‐terminal region and C1 and C2 on the C‐terminal region (see also Supplementary Figure 2). Four of the residues in epitope N3 (emphasized) showed to be residues in the previously published (Franklin et al., 2004) pertuzumab epitope.
Figure 7.
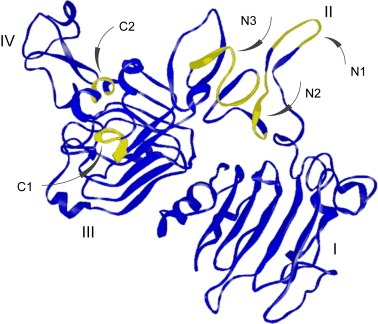
Location of the mapped epitopes on the HER2 structure. The locations of the five epitopes mapped (see Figure 5) are shown on the structure of HER2 (Franklin et al., 2004). The epitopes of the C‐terminal region is located on domain III and the epitopes are part of an alpha‐helical structure (epitope C2) or a loop structure in proximity of a beta‐pleated sheet structure (epitope C1), whereas the N‐terminal epitopes span over the HER2 dimerization arm (N1–N2) and residues involved in the pertuzumab binding site (N3).
3. Discussion
Here we demonstrate a strategy based on polyclonal antibodies to identify new functional epitopes, as exemplified by the generation of antibodies to linear epitopes of the human HER2 that effect cell growth. The functional antibodies with growth inhibition properties could be divided into two different parts located on domain II and III of the receptor, respectively. One of the epitopes mapped to domain II overlapped with the conformational epitope recognized by pertuzumab, an antibody in clinical testing, while the two new epitopes on the surface of the domain III were in close proximity to each other and encompassed a linear stretch of 25 amino acids (LPESFDGDPASNTAPLQPEQLQVF). It can be suggested that this information can be used to pursue the generation of novel humanized monoclonal antibodies (Carter et al., 1992) based on the Immunogen 5 as antigen. In this case, the various antibody candidates can be implemented into the same assays, including flow sorting and growth inhibition of BT474 cells and epitope mapping using the bacterial cell display, to generate antibody molecules of clinical value.
Finding accessible epitopes on HER2 is challenging for several reasons. Structurally, the extracellular domain of HER2 both has a high level of cysteines, known to generate specific intramolecular disulphide bonds complicating in vitro re‐folding of recombinant antigens expressed in E. coli, and is also subjected to glycosylation, masking potential epitopes. An interesting observation is that the immunogens containing whole structural domains I–IV did not generate polyclonal antibodies able to recognize the native structure (Figure 2). This is particularly interesting for Immunogen 3, which contains the 25 amino acid residues comprising epitopes C1 and C2, which did not result in antibodies binding native HER2 in the flow cytometric experiment. These epitopes are thus recognized by the animal immunized with Immunogen 5 but not the animals immunized with Immunogen 3. This might be explained by an effectively denaturated Immunogen 5 exposing only linear epitopes, while structural elements of Immunogen 3 could mask these linear epitopes for the animal's immune system. In this particular case, it is tempting to speculate that complete or partially unfolded protein fragment (Immunogen 5) directed the antibody response to linear epitopes. Thus, the results suggest that antibodies with linear epitopes are generated using Immunogen 5 of which some recognize the native HER2 and thereby contributes to the growth inhibition.
In vivo, the effect of monoclonal antibody as therapeutic agents mediated growth inhibition through mechanisms such as antibody dependent cellular cytotoxicity through Fc receptors on the effector cells, complement‐dependent cytotoxicity, and interception of oncogenic pathways (Ben‐Kasus et al., 2007). As the presented antibodies have so far only been studied ex‐vivo, their effective mechanism remains to be determined in studies subsequent of the described epitope discovery strategy, when representative monoclonal binders have been raised, validated and selected.
In summary, we present a rapid and simple method for exploring the epitope space of proteins. The use of monospecific antibodies generated by affinity‐purification of polyclonal antisera using recombinant protein fragments as ligands (Agaton et al., 2004) allow for the epitope space of the protein target to be explored in a rapid and simple manner. The combination of sub‐fractioning again using affinity purification and subsequent epitope mapping using a bacterial surface display approach allows linear epitopes to be precisely mapped. If this new approach is combined with a functional assay, as exemplified by the cell inhibition assay here, it holds the great potential to map epitopes precisely and suitable for protein targeting. Such epitopes can subsequently be used to guide a path to further experiments aiming in the generation of therapeutically adapted antibodies for clinical applications.
4. Experimental procedures
4.1. Molecular biology and immunotechnology
cDNA corresponding to the following positions on the HER2 protein was generated using reverse transcription PCR with specific primers for Immunogen 1 (aa 42–186), Immunogen 2 (aa 236–363), Immunogen 3 (aa 364–530), Immunogen 4 (aa 531–626), Immunogen 5 (aa 274–400) and Immunogen intra (aa 1120–1243). Experiments used for generation of cDNA, cloning, protein expression, immunization of rabbits and affinity purification of the immunogens were performed in concordance with previously published methods (Agaton et al., 2004; Nilsson et al., 2005). Antibody sub‐fractions were obtained by sequential affinity purification using four serially connected Hi‐trap columns (GE‐Healthcare AB, Uppsala, Sweden) coupled with protein fragments overlapping Immunogen 5 (Figure 7). In brief, 10ml serum from the Immunogen 5 immunization was buffered in PBS, sterile filtered and depleted from Tag‐specific His6‐ABP antibodies using same protocol as previous authors (Larsson et al., 2006). Depleted flow‐through antibodies were affinity purified using a Äkta Explorer system (GE Health Care AB). Relevant fractions were immediately pooled after elution and pH adjusted to 7.25 using 1M Tris–HCl and 10× PBS. Monoclonal antibodies trastuzumab (Roche, Switzerland) and pertuzumab (Genentech, San Francisco, CA) were purified by size‐exclusion chromatography using MilliQ‐water as eluent on a PD‐10 column (GE Healthcare, Uppsala, Sweden) (Persson et al., 2007). The monoclonal antibodies were subsequently diluted to 1mg/ml using PBS pH 7.2 before usage. Immunohistochemistry was performed on breast ductal carcinoma as well as normal tissues using Ab–5 antibody in same manner as previously described (Agaton et al., 2004; Nilsson et al., 2005).
4.2. Bead coupling for suspension arrays
Proteins were immobilized on carboxylated beads (COOH Micorspheres, Luminex‐Corp.) in accordance with the manufacturer's protocol. Coupling of up to 106 beads was performed in a filter membrane bottomed microtiter plate (MultiScreen‐HTS, Millipore) (Schwenk et al., 2007). Hereby, beads were activated using 1‐Ethyl‐3‐(3‐dimethylaminopropyl)carbodi‐imide and N‐Hydroxysuccinimide. PrEST proteins and His6ABP were diluted in MES buffer to a concentration of 40μg/ml and added to the beads and incubated over 120min under constant mixing (Thermomixer, Eppendorf). Beads were finally washed, re‐suspended and transferred to micro‐centrifuge tubes (Starlab) for storage in a protein‐containing buffer (Blocking Reagent for ELISA, Roche) with NaN3. All coupled beads were treated with sonication in an ultrasonic cleaner (Branson Ultrasonic Corporation) for 5min prior to final storage at 4°C.
4.3. Determination of binding specificity
Multiplexed analysis of binding specificities was performed as previously described (Schwenk et al., 2007). In short, antibody dilutions and a bead mixture of 100 bead IDs that corresponded to 98 PrEST antigens, one HisABP and an anti‐rabbit IgG antibody were prepared in PBST. 45μl of msAb dilutions were added to 5μl of bead and incubated for 60min under constant mixing in a 96 well plate (Corning). Subsequently, 25μl of R‐Phycoerythrin labeled anti‐rabbit IgG antibody (0.5μg/ml, Jackson ImmunoResearch) were added for a final incubation of 60min.
4.4. Multiplexed competition assays
Serial dilutions of competitor PrEST proteins were prepared in PBST and mixed at a 1:1 ratio with solutions Ab–5, Ab–N, Ab–M and Ab–C respectively. Incubation took place in a total volume of 50μl for 60min under constant mixing. Subsequently, the antibody‐competitor solutions were transferred to a second plate that contained 5μl of bead mixtures per well. After 60min 25μl of R‐Phycoerythrin labeled anti‐rabbit IgG antibody (0.5μg/ml, Jackson ImmunoResearch) were added incubated for another 60min. Three independent replicates were performed and average values of those were used for data analysis. A four parameter logistic model was chosen for fitting competition curves to calculate EC50 values and to compare relative binding qualities. As a measure for competition, resulting curves were observed upon their shape and their estimated EC50 values that had to be of a greater number value than the standard error.
4.5. Suspension array read‐out and data analysis
Measurements were performed using Luminex LX200 instrumentation with Luminex IS 2.3 software. For each experiment 100 events per bead ID were counted and the median fluorescence intensity (MFI) of was chosen to display interactions. Data analysis and graphical representations were performed with R, a language and environment for statistical computing and graphics (Ihaka and Gentleman, 1996).
4.6. Cell culture and dose‐response studies
BT474 breast cancer cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA), maintained in RPMI (supplemented with 10% FBS and 1% bovine insulin) and kept in 37°C at 5% CO2 humidified atmosphere. BT474 cells were seeded at 5×104cells/well in 24‐well dishes. After 24h, cells were treated in triplicate dilutions Ab–5 in concentrations ranging from 1 to 1000ng/ml. PBS was used as control yielding growth rates of 16×104cells/well after day 5. After 5days, cells were trypsinized and counted in triplicates. Growth inhibition was calculated as the percentage of cells compared with untreated cultures (Figure 2B). The same protocol was used for the growth study using a total antibody concentration of 500ng/ml. In brief cells were seeded at 5×104cells/well in 24‐well dishes and treated in triplicates using Ab–5, Ab–N, Ab–M, Ab–C and antibody cocktails (Ab–N, Ab–M and Ab–C) and (Ab–N and Ab–C). Antibody cocktails were composed to reflect the molar ratios of antibodies present in the Ab–5 serum (Table 1). Effect was calculated as percentage of cells present in cultures compared with cultures treated with negative control (Ab–intra).
4.7. Flow sorting analysis
Cells were released from the culture dish by trypzination, centrifuged and re‐suspended in PBS pH 7.2 supplemented with 1% human serum albumin (HSA), and counted. A total of 150,000 cells were labeled for 45min with 0.35mg primary antibody in a reaction volume of 75μl in a 96‐well plate in room temperature. Unbound antibodies were washed away using 2×100μl PBS:HSA as washing agent. This was followed by antibody labeling using 0.35mg secondary goat anti‐rabbit monoclonal antibody (Invitrogen, Carlsbad, CA) conjugated to Alexa 488 in a reaction volume of 75μl for 45min at room temperature. Cells were washed in PBS:HSA 2×100μl and re‐suspended in a sample tube to a final volume of 150μl. Ability for antibodies to bind BT474 cells was evaluated by fluorescence‐activated cell sorting a flow cytometer (BD FACS Vantage SE, BD Biosciences) measuring fluorescence emission at FL‐1 (excitation at 488nm). Trastuzumab (Genentech, San Francisco, CA) and Pertuzumab (Genentech, San Francisco, CA) were used as positive cell labeling controls using Alexa 488 goat anti‐human monoclonal antibodies (Invitrogen, Carlsbad, CA) as secondary reagent.
4.8. Epitope mapping
Epitope mappings of antibodies Ab–N and Ab–C were performed using cell surface display (Rockberg et al., 2008). In brief DNA corresponding to HER2‐ecd was amplified by PCR using vector pAY593 as a template (kindly provided by Affibody AB, Bromma, Sweden). Amplified DNA was fragmentized by sonication to pieces of approximately 50–350bp, cloned into staphylococcal display vector (pSCEM1) and transformed into Staphylococcus carnosus yielding around 30,000 transformants. In‐frame cloned fragments were displayed as peptides on the staphylococcal surface. Cell aliquots corresponding to about a 10 fold coverage of the library was incubated with 4μg of Ab–N and Ab–C respectively in reaction volumes of 70ml. Cells were washed and fluorescently labeled with 4μg secondary goat antibodies (Invitrogen, Carlsbad, CA) and washed again before subjected to FACS. Cells expressing recognized epitopes were gated as positive and sequenced by pyrosequencing (Biotage, Uppsala Sweden) and finally aligned back to the HER2 sequence.
Supporting information
Supplementary data
Supplementary data
Acknowledgements
We are grateful to John Löfblom and Per‐Åke Nygren for assistance and useful comments and advices. The entire staff of the Human Proteome Resource (HPR) center in Stockholm and Uppsala, Sweden and in Mumbai, India, are acknowledged for their tremendous efforts. Pertuzumab (Omnitarg) was a kind gift from Genentech (South San Francisco, CA). This work was supported by grants from the Knut and Alice Wallenberg Foundation.
Appendix A. Supplemental material 1.
Supplementary information for this manuscript can be downloaded at doi: 10.1016/j.molonc.2009.01.003.
Rockberg Johan, Schwenk Jochen M., Uhlén Mathias, (2009), Discovery of epitopes for targeting the human epidermal growth factor receptor 2 (HER2) with antibodies, Molecular Oncology, 3, doi: 10.1016/j.molonc.2009.01.003.
References
- Agaton, C. , Falk, R. , Hoiden Guthenberg, I. , Gostring, L. , Uhlen, M. , Hober, S. , 2004. Selective enrichment of monospecific polyclonal antibodies for antibody-based proteomics efforts. J. Chromatogr. A. 1043, 33–40. [DOI] [PubMed] [Google Scholar]
- Agus, D.B. , Akita, R.W. , Fox, W.D. , Lewis, G.D. , Higgins, B. , Pisacane, P.I. , Lofgren, J.A. , Tindell, C. , Evans, D.P. , Maiese, K. , 2002. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2, 127–137. [DOI] [PubMed] [Google Scholar]
- Ben-Kasus, T. , Schechter, B. , Sela, M. , Yarden, Y. , 2007. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol. Oncol. 1, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, P. , Presta, L. , Gorman, C.M. , Ridgway, J.B. , Henner, D. , Wong, W.L. , Rowland, A.M. , Kotts, C. , Carver, M.E. , Shepard, H.M. , 1992. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA. 89, 4285–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H.S. , Mason, K. , Ramyar, K.X. , Stanley, A.M. , Gabelli, S.B. , Denney, D.W. , Leahy, D.J. , 2003. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 421, 756–760. [DOI] [PubMed] [Google Scholar]
- Franklin, M.C. , Carey, K.D. , Vajdos, F.F. , Leahy, D.J. , de Vos, A.M. , Sliwkowski, M.X. , 2004. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 5, 317–328. [DOI] [PubMed] [Google Scholar]
- Gelmon, K., Fumoleau, P., Verma, S., Wardley, A., Conte, P.F., Miles, D., Gianni, L., McNally, V.A., Ross, G.A., Baselga, J., 2008. Results of a Phase II trial of trastuzumab (H) and pertuzumab (P) in patients (pts) with HER2-positive metastatic breast cancer (MBC) who had progressed during trastuzumab therapy. Proceedings from ASCO 2008, Abs TBC.
- Ihaka, R. , Gentleman, R. , 1996. R: a language for data analysis and graphics. J. Computat. Graphical Statist.. 5, 299–314. [Google Scholar]
- Larsson, K. , Wester, K. , Nilsson, P. , Uhlen, M. , Hober, S. , Wernerus, H. , 2006. Multiplexed PrEST immunization for high-throughput affinity proteomics. J. Immunol. Methods. 315, 110–120. [DOI] [PubMed] [Google Scholar]
- Lindskog, M. , Rockberg, J. , Uhlen, M. , Sterky, F. , 2005. Selection of protein epitopes for antibody production. Biotechniques. 38, 723–727. [DOI] [PubMed] [Google Scholar]
- Logtenberg, T. , 2007. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 25, 390–394. [DOI] [PubMed] [Google Scholar]
- Menard, S. , Tagliabue, E. , Campiglio, M. , Pupa, S.M. , 2000. Role of HER2 gene overexpression in breast carcinoma. J. Cell Physiol. 182, 150–162. [DOI] [PubMed] [Google Scholar]
- Nahta, R. , Hung, M.C. , Esteva, F.J. , 2004. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res.. 64, 2343–2346. [DOI] [PubMed] [Google Scholar]
- Nahta, R. , Yu, D. , Hung, M.C. , Hortobagyi, G.N. , Esteva, F.J. , 2006. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat. Clin. Pract. Oncol. 3, 269–280. [DOI] [PubMed] [Google Scholar]
- Nilsson, P. , Paavilainen, L. , Larsson, K. , Odling, J. , Sundberg, M. , Andersson, A.C. , Kampf, C. , Persson, A. , Al-Khalili Szigyarto, C. , Ottosson, J. , 2005. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics. 5, 4327–4337. [DOI] [PubMed] [Google Scholar]
- Pal, S.K. , Pegram, M. , 2006. Targeting HER2 epitopes. Semin. Oncol. 33, 386–391. [DOI] [PubMed] [Google Scholar]
- Pal, S.K. , Pegram, M. , 2007. HER2 targeted therapy in breast cancer … beyond Herceptin. Rev. Endocr. Metab. Disord. 8, 269–277. [DOI] [PubMed] [Google Scholar]
- Persson, M. , Gedda, L. , Lundqvist, H. , Tolmachev, V. , Nordgren, H. , Malmstrom, P.U. , Carlsson, J. , 2007. [177Lu]pertuzumab: experimental therapy of HER-2-expressing xenografts. Cancer Res.. 67, 326–331. [DOI] [PubMed] [Google Scholar]
- Popat, S. , Smith, I.E. , 2008. Therapy Insight: anthracyclines and trastuzumab—the optimal management of cardiotoxic side effects. Nat. Clin. Pract. Oncol. 5, 324–335. [DOI] [PubMed] [Google Scholar]
- Portera, C.C. , Walshe, J.M. , Rosing, D.R. , Denduluri, N. , Berman, A.W. , Vatas, U. , Velarde, M. , Chow, C.K. , Steinberg, S.M. , Nguyen, D. , 2008. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin. Cancer Res.. 14, 2710–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockberg, J. , Lofblom, J. , Hjelm, B. , Uhlen, M. , Stahl, S. , 2008. Epitope mapping of antibodies using bacterial surface display. Nat. Methods. 5, 1039–1045. [DOI] [PubMed] [Google Scholar]
- Ronaghi, M. , Uhlen, M. , Nyren, P. , 1998. A sequencing method based on real-time pyrophosphate. Science. 281, 363–365. [DOI] [PubMed] [Google Scholar]
- Schechter, A.L. , Hung, M.C. , Vaidyanathan, L. , Weinberg, R.A. , Yang-Feng, T.L. , Francke, U. , Ullrich, A. , Coussens, L. , 1985. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 229, 976–978. [DOI] [PubMed] [Google Scholar]
- Schwenk, J.M. , Lindberg, J. , Sundberg, M. , Uhlen, M. , Nilsson, P. , 2007. Determination of binding specificities in highly multiplexed bead-based assays for antibody proteomics. Mol. Cell. Proteomics. 6, 125–132. [DOI] [PubMed] [Google Scholar]
- Sharon, J. , Liebman, M.A. , Williams, B.R. , 2005. Recombinant polyclonal antibodies for cancer therapy. J. Cell Biochem. 96, 305–313. [DOI] [PubMed] [Google Scholar]
- Slamon, D.J. , Clark, G.M. , Wong, S.G. , Levin, W.J. , Ullrich, A. , McGuire, W.L. , 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 235, 177–182. [DOI] [PubMed] [Google Scholar]
- Spiridon, C.I. , Ghetie, M.A. , Uhr, J. , Marches, R. , Li, J.L. , Shen, G.L. , Vitetta, E.S. , 2002. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin. Cancer Res.. 8, 1720–1730. [PubMed] [Google Scholar]
- Yarden, Y. , Sliwkowski, M.X. , 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol.. 2, 127–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data


