Abstract
Neuroglobin is a recently identified globin molecule that is expressed predominantly in the vertebrate brain. Neuroglobin expression increases in oxygen‐deprived neurons, suggesting it protects neurons from ischemic cell death. We report that neuroglobin transcript and protein are expressed in human glioblastoma cells, and that this expression increases in hypoxia in vitro. We also show that neuroglobin is up‐regulated in hypoxic microregions of glioblastoma tumor xenografts. Our finding of hypoxic up‐regulation of neuroglobin in human glioblast oma cells may provide insight into how tumor cells adapt to and survive in hypoxic microenvironments.
Keywords: Neuroglobin, Malignant glioma, Tumor hypoxia
Abbreviations
- Ngb
neuroglobin
- GBM
glioblastoma multiforme
- RT
reverse transcription
- qRT‐PCR
quantitative real‐time polymerase chain reaction
- SDS‐PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- HRP
horseradish peroxidase
- DAB
3,3′ diamino benzidine tetrahydrochloride
- GFAP
glial fibrillary acidic protein
- HIF‐1
hypoxia‐inducible factor‐1
- VEGFR2
vascular endothelial growth factor receptor 2
1. Introduction
Neuroglobin (Ngb), a recently discovered member of the vertebrate globin family, is a small monomeric heme protein with oxygen binding properties (Burmester et al., 2000; Giuffre et al., 2008). Although Ngb's amino acid sequence shows little similarity to that of hemoglobin or myoglobin, amino acids that confer hemoglobin and myoglobin function are conserved as are all characteristics of the globin fold (reviewed in Brunori and Vallone, 2007). Insertion/deletion events are rare, and approximately half of the positions are strictly conserved (Burmester et al., 2004).
As its name suggests, Ngb is expressed primarily in neuronal tissues, with highest concentrations (∼100μM) found in the retina, localized near mitochondria (Geuens et al., 2003; Pesce et al., 2002). High Ngb levels are also found in the brain in regions that show greatest oxygen consumption (Hankeln et al., 2004). Lower levels of Ngb are reported in pancreas, adrenal gland and testes, but Ngb is absent from liver, heart, striated muscle, lung, small bowel, kidney and vasculature (Hankeln et al., 2004; Mammen et al., 2002).
Although 8years have passed since the discovery of Ngb, its physiological function remains elusive (Greenberg et al., 2008). Ngb binds oxygen, however, its concentration in tissues other than retina is too low to suggest it might function as an oxygen delivery molecule (Schmidt‐Kastner et al., 2006). Based on its regional (greatest in brain areas with high oxygen consumption) and subcellular (near mitochondria) distributions, Ngb may play an important role in scavenging reactive oxygen or nitrosative species, or by activation of yet unknown protective mechanisms (Brunori and Vallone, 2007, 2004, 2006, 2007, 2008, 2008). Studies that have sought evidence for a neuroprotective function in vivo have produced conflicting results (Fraser et al., 2006, 2005, 2006, 2001, 2003) that may reflect differences in the duration of hypoxia (acute vs. chronic) or in the methods used to detect Ngb (in situ hybridization vs. immunohistochemistry) (Hankeln et al., 2004). However, in vitro studies with neuronal cell lines have shown that hypoxia (<1% O2) significantly increases Ngb transcript (Fordel et al., 2004; Rayner et al., 2006; Schmidt‐Kastner et al., 2006; Sun et al., 2001) and that these increases are associated with protection from hypoxic damage (Greenberg et al., 2008, 2001, 2003).
Glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults. Despite aggressive multimodality therapy, the 2‐year survival rate remains only 10–25% (Stupp et al., 2005). The presence of necrosis, and by inference hypoxia, is a pathognomonic feature of human GBM tumors (Wilden and Moore, 1987). Hypoxic tumor cells constitute a source of local treatment failure and disease progression (Dachs and Chaplin, 1998). To survive in hypoxic microenvironments, cells must sense changes in [O2] and activate defense and adaptation mechanisms (Hochachka et al., 1996). Previous work suggests that up‐regulation of Ngb expression may be part of the repertoire of hypoxia defense mechanisms in normal brain. Although most studies have shown that in normal brain, Ngb expression is restricted to neurons, and absent from glia, Chen et al. (2005) reported that Ngb transcript is expressed in cultured astrocytes from newborn mouse brain, and that Ngb protein co‐localizes with glial fibrillary acid protein (GFAP) in cultured astrocytes. Further, microarray data publicly available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress; accession number E‐GEOD‐4209) show that Ngb is variably expressed in human GBM tumor samples. Based on these results, we examined whether human GBM cell lines that vary in their ability to survive under hypoxic stress condition also express Ngb, and whether physiologically relevant levels of hypoxia can moderate Ngb expression in these cells.
2. Results
2.1. Ngb transcript and protein are expressed in GBM cell in vitro
Ngb mRNA was detected in all GBM cell lines incubated under standard laboratory conditions (controls). When incubated under hypoxic conditions (O.6% O2), Ngb mRNA was significantly (P<0.05) up‐regulated at 24h (M006x and M006xLo) and 48h (M010B, M006x, M006xLo, M059K, U87R and U87T) (Figure 1A–G). The single exception was the hypoxia‐sensitive cell line, M059J (Figure 1B), in which Ngb mRNA expression was increased ∼4‐fold, however, this difference was not significant. In accordance with qRT‐PCR results, Ngb protein was detected in all GBM cell lines, with variable expression among cells cultured under standard laboratory conditions. Following incubation under hypoxic conditions, Ngb protein was significantly increased at 24h (M010B and M059K) and 48h (M010B, M059J, M006x, M006xLo, M059K, U87R and U87T) (Figure 2A–G). However, there was no correlation between the magnitude of Ngb protein increase after hypoxia and the respective basal levels of each cell line.
Figure 1.
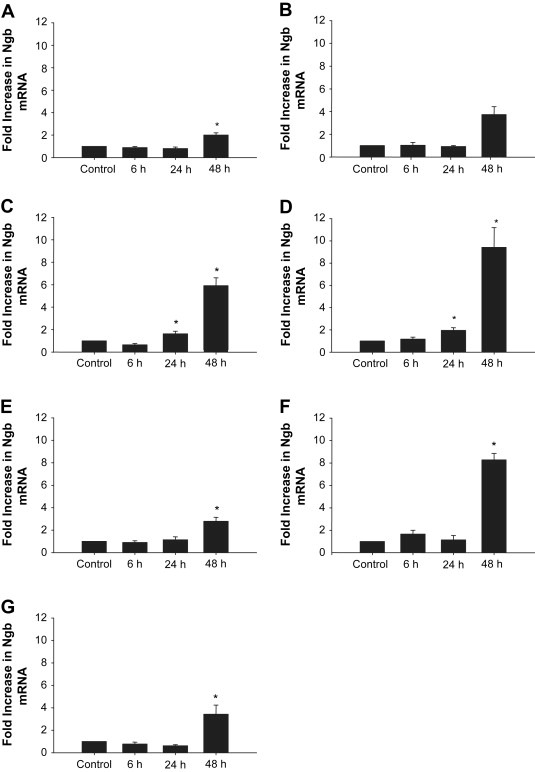
Effect of hypoxia on Ngb mRNA expression in human GBM cells. (A) M010B; (B) M059J; (C) M006x: (D) M006xLo; (E) M059K; (F) U87R; (G) U87T. Ngb mRNA expression was assessed by qRT‐PCR after exposure to hypoxia (0.6% O2) for 0, 6, 24 and 48h. Data are expressed as fold increase relative to aerobic control. (n=4). *P<0.05 (ANOVA).
Figure 2.
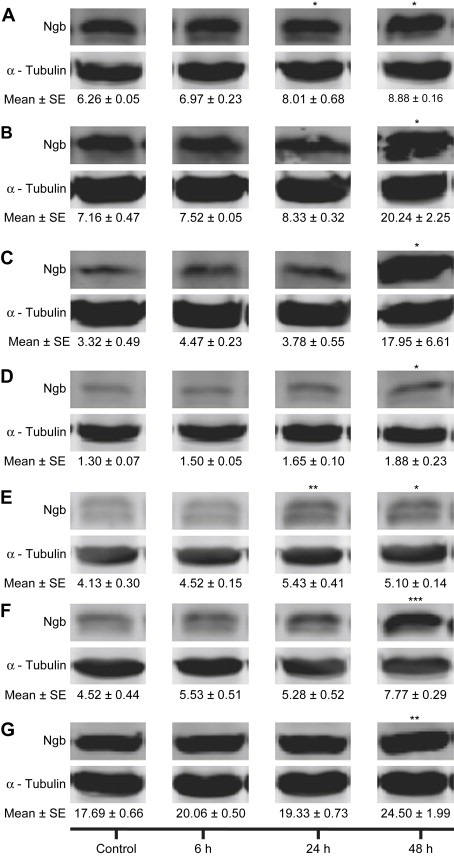
Ngb protein expression in human GBM cells. (A) M010B; (B) M059J; (C) M006x; (D) M006xLo; (E) M059K; (F) U87R; (G) U87T. Ngb expression was assessed by Western blot analyses after exposure to hypoxia (0.6% O2) for 0, 6, 24 and 48h. The integrated intensities of Ngb and α‐tubulin (control) bands were determined and expressed in arbitrary units (AU), and representative blots are shown. (n=4) *P<0.05; **P<0.01; ***P<0.001 (ANOVA).
2.2. Ngb protein is expressed in GBM xenografts
To test whether Ngb expression is up‐regulated under physiologically relevant hypoxic conditions, M006xLo cells were grown as tumor xenografts in NOD/SCID mice. Tumor sections were immunostained with antibodies to Ngb and the hypoxia marker, pimonidazole. Visually distinct regions of necrosis present in serial tumor sections were used as landmarks to compare adjacent tumor sections (Figure 3A). As expected, tumor cells in regions adjacent to necrotic tissue were hypoxic as shown by positive pimonidazole staining (Figure 3B). Strong Ngb immunoreactivity was also detected in the matched tumor regions adjacent to necrosis as well as in scattered foci throughout the tissue section (Figure 3B). Weak Ngb staining was observed throughout the tumor sections, however, these cells lacked pimonidazole immunoreactivity and are thus presumed to have been well oxygenated (Figure 3C).
Figure 3.
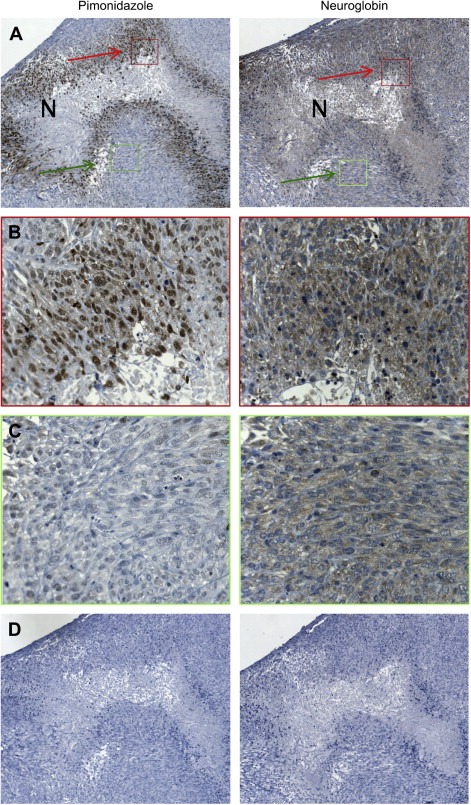
Ngb expression in a M006xLo tumor xenograft. (A) Low‐power (10×) view of adjacent serial sections stained with antibodies to pimonidazole or Ngb. Regions of necrosis (N) were used as tissue landmarks. (B) High‐power views (40×) of regions indicated by the red boxes in panel A. Hypoxic cells identified by pimonidazole staining also show strong positive staining for Ngb. (C) High‐power views (40×) of regions indicated by the green boxes in panel A. Weakly positive Ngb staining was also observed throughout the tumor section, however, these cells showed no pimonidazole immunoreactivity. (D) Low power view (10×) of control tumor sections from which primary antibodies were omitted.
3. Discussion
The objectives of this study were to determine whether Ngb is expressed in human GBM cells and if so, whether its expression could be modified by physiologically relevant levels of hypoxia. Ngb expression in neuronal tissues is well described, however, there are conflicting reports regarding Ngb expression in non‐neuronal brain cells. Double immunostaining of either hippocampal cell cultures or tissue sections of mouse brain and canine retina with antibodies to Ngb and the glial marker GFAP showed no co‐localization, suggesting Ngb is absent in cells of glial origin (Laufs et al., 2004; Ostojic et al., 2006). However, using RT‐PCR and double immunostaining with Ngb and GFAP antibodies, Chen et al. (2005) reported the presence of Ngb mRNA and protein in cultured astrocytes isolated from mouse brain. Brain sections from a Ngb‐overexpressing transgenic mouse showed positive Ngb expression in neuronal cells as well as in cells expressing GFAP (Khan et al., 2007). In the brains of elderly individuals, Ngb has been detected in activated microglial cells and in deep subcortical lesions where it was co‐expressed with known hypoxia markers (i.e., HIF1α, HIF2α, VEGFR2) (Fernando et al., 2006). Ngb transcript has also been quantified in a microarray analysis of human primary GBM tumor samples (http://www.ebi.ac.uk/arrayexpress; accession number E‐GEOD‐4209). Together, these results indicate that under certain conditions (i.e., hypoxia in normal brain, or metabolically active tumor tissue) Ngb may be expressed in cells that also express glial markers.
Here, we report Ngb mRNA and protein are expressed in seven human GBM cell lines. The cell lines with the “M0xx” designation have been characterized previously for their ability to adapt to and survive under conditions of low oxygen availability, and in contrast to GBM cell lines available from cell repositories, are used at relatively low passage numbers in an attempt to avoid “drift” from the cellular and genetic characteristics of the tumor from which they were isolated. The two U87 variant cell lines used here provide examples of GBM cells that differ in their invasiveness properties in vivo.
Although GBM are classified as astrocytic tumors, the cellular origin of GBM is not known. GBM cells lines co‐express both neural and glial markers (reviewed in Rebetz et al., 2008) and there is evidence that malignant transformation of neuronal stem cells may lead to the development of GBM tumors (Sanai et al., 2005, 2004, 2004), but this remains controversial (Stiles and Rowitch, 2008). Thus, it is not entirely unexpected that Ngb, a protein originally identified in neurons, may be present in GBM cells.
The cellular response to hypoxia has been characterized in five of the cell lines: M006x, M006xLo, M059K are hypoxia‐tolerant whereas M010B and M059J are hypoxia‐sensitive (Allalunis‐Turner et al., 1999; Parliament et al., 2000; Turcotte et al., 2002). M006xLo cells are of particular interest as they were selected from the M006x parental line following culture for 2weeks under reduced oxygen and glucose conditions, and accordingly, are exceptionally tolerant of low oxygen conditions. Both Ngb transcript and protein were detected in all GBM cell lines tested, although basal levels of expression varied. The M006xLo cell line was shown overall to have the lowest protein expression, and the two hypoxia‐sensitive cell lines, the highest. If Ngb functions to protect cells from hypoxia/oxidative stress, then these results may seem counterintuitive. However, the repertoire of cellular defense mechanisms involves multiple pathways, and tumor cells that have acquired the ability to survive under low oxygen conditions have likely up‐regulated a variety of adaptive mechanisms. Thus, while Ngb may function as a protective molecule during hypoxia or ischemia/reperfusion, and may show robust expression in hypoxia‐sensitive cell types such as normal neurons and M010B and M059J tumor cells, its expression may be less critical in cells that have constitutively up‐regulated adequate hypoxia defense mechanisms.
Both Ngb transcript and protein were significantly increased in GBM cells cultured under hypoxic conditions. For these experiments, cells were incubated in atmospheres of 0.6% O2, a concentration of oxygen that is within the range of concentrations that have been measured in the hypoxic regions of human tumors (Olive et al., 1996; Vaupel et al., 1991). These results are similar to those previously reported for neuronal cell lines and cultures in which increases in Ngb mRNA were observed following exposure to hypoxia (Schmidt‐Kastner et al., 2006; Sun et al., 2001) or the hypoxia‐mimetic agent, cobalt chloride (Sun et al., 2001). However, the 2‐ to 9‐fold increase in Ngb mRNA levels observed in GBM cells after 48h of hypoxia is higher than previously reported for neuronal cells (Fordel et al., 2004; Rayner et al., 2006; Schmidt‐Kastner et al., 2006; Sun et al., 2001). The use of different cell types and experimental conditions to induce hypoxia may explain differences in the magnitude of the Ngb response. In our work and the studies noted above, no attempt was made to control for differences in glucose consumption rate or changes in pH that might have existed between control and hypoxic cultures. Fordel et al. (2007a) have reported that Ngb mRNA and protein are up‐regulated in a human neuroblastoma cell line after combined oxygen–glucose deprivation, but not after hypoxia (1% O2) alone or glucose deprivation alone.
Among the cell lines in this study, there was no obvious relationship between basal Ngb protein levels and the extent to which its expression was up‐regulated by hypoxia, nor was there a direct relationship between hypoxia‐induced increases in Ngb transcript and protein. Analysis of relationships between transcript and protein expression in human lung cancer and hematopoietic stem cells has shown that only a subset of mRNAs show significant positive correlation between transcript–protein expression levels (Chen et al., 2002; Unwin et al., 2006). The transcriptional and/or translational control of Ngb expression is incompletely understood, as are factors that regulate Ngb protein stability or turnover. Using in silico techniques, Wystub et al. (2004) showed, somewhat unexpectedly, that conserved hypoxia‐response elements, including HIF‐1 consensus binding motifs, are absent from the upstream region of human NGB. However, the NGB promoter region does contain AP‐1 and NFkB binding motifs, both of which have been reported to be activated by hypoxia.
In addition to differences in hypoxia sensitivity, there are other notable phenotypic differences among these GBM cells. The intrinsic radiosensitity of the M0xx‐designated cells varies widely (Allalunis‐Turner et al., 1992). Also of note, the M059J is the only example of a human tumor cell line that lacks expression of DNA‐PKcs, a critical component of the non‐homologous end‐joining pathway responsible for repair of DNA double strand breaks (Lees‐Miller et al., 1995). The absence of DNA‐PKcs renders M059J cells exquisitely radiosensitive (Allalunis‐Turner et al., 1993). Although the rank order of cellular radiosensitivities does parallel the differences in basal Ngb protein expression, at present there is no evidence for a direct role for Ngb in the repair of DNA double strand breaks, the mechanism believed to underlie differences in cellular radiosensitivity.
To test whether physiologically relevant hypoxic conditions could increase Ngb expression, we examined the distribution of Ngb in tissue sections of a GBM xenograft. Pimonidazole is a bioreductive hypoxia marker that irreversibly binds to hypoxic cells at oxygen tensions <10mm Hg (Yaromina et al., 2006), and is a well accepted marker of tumor hypoxia in tumor specimens (Ljungkvist et al., 2007). Interestingly, we observed enhanced pimonidazole and Ngb staining in tumor regions adjacent to necrosis. The presence of hypoxic cells adjacent to necrotic tissue conforms to the classic model of tumor hypoxia (Brown and Wilson, 2004). Thus, it is reasonable to postulate that Ngb expression in vivo also may be controlled by oxygen‐dependent regulatory mechanisms.
Previous studies that examined Ngb expression in vivo reported increases in Ngb protein in brain neuronal cells after hypoxia or ischemic injury (Fordel et al., 2004, 2007, 2006, 2006, 2006, 2006, 2001), suggesting it functions as an endogenous neuroprotectant (Greenberg et al., 2008, 2001, 2003). Consistent with this, Chen et al. (2005) observed that transfection of cultured astrocytes with anti‐sense Ngb oligonucleotide led to a 2.5‐fold increase in apoptotic cells after 5h of ischemic treatment. The report of Ngb overexpression in the brain of an individual with hereditary ferritinopathy also is consistent with Ngb's putative function as a radical scavenger (Powers, 2006). Hereditary ferritinopathy is a rare neuro‐genetic disorder caused by mutations in the l‐ferritin gene, resulting in an accumulation of iron that leads to severe oxidative stress. Peroxynitrite and products of lipid peroxidation are believed to play a pathogenic role in this disorder (Mancuso et al., 2005). In this patient, Ngb overexpression was localized to swollen neuronal and glial nuclei, the same cells that also showed over expression of ferritin and iron accumulation. Powers (2006) suggested that Ngb expression may be an initial protective response against iron‐initiated oxidative damage. However, if the protective response is ineffective or overwhelmed, as would be the case in hereditary ferritinopathy, then Ngb release from dead cells could accelerate iron stress as Ngb heme is converted to iron by heme‐oxygenase. A possible association between ferritin and Ngb expression in human GBM has yet to be explored. Ferritin is an iron binding protein that protects cells against iron‐catalyzed oxidative stress, and elevated ferritin levels have been reported in the cerebral spinal fluid of patients with GBM (Sato et al., 1998). In human GBM tumor sections, elevated ferritin expression has also been observed in tumor cells within the invasive edge and in microglia, but ferritin was absent from neurons and reactive astrocytes (Holtkamp et al., 2005). Additional studies will be required to determine whether ferritin and Ngb are co‐expressed in human GBM and if these proteins function in tandem to increase tumor cell resistance to oxidative stress.
Mechanisms that allow tumor cells to adapt to and survive in hypoxic microenvironments include both metabolic adaptation and transcriptional activation of hypoxia rescue genes. Our findings that Ngb expression increases in GBM cells in response to hypoxia, both in vitro and in vivo, suggest Ngb may be part of the repertoire of defense strategies that GBM cells utilize to survive in hypoxic microenvironments. Human tumors frequently contain hypoxic cells and their presence is a poor prognostic indicator (Brown and Wilson, 2004). For example, compared to well oxygenated tumor cells, hypoxic tumor cells are approximately 3‐fold resistant to ionizing radiation and certain chemotherapeutic agents, are genetically unstable and metastasize frequently (Dachs and Chaplin, 1998). With regard to treatment outcome, patients whose tumors contain a significant proportion of hypoxic cells have worse local–regional control and progression‐free survival following surgery alone or surgery plus radiotherapy (Hockel et al., 1993, 1998, 2000). There is also evidence that hypoxia per se selects for a more aggressive tumor phenotype (Bristow and Hill, 2008). The identification of Ngb expression in human glioblastoma cells, and its up‐regulation by hypoxia, provides additional insight into the pro‐survival mechanisms utilized by these aggressive cancers. Strategies to interfere with Ngb expression and/or function may ultimately result in therapeutic benefit.
4. Experimental procedures
4.1. Cell lines and in vitro culture conditions
The origin and characterization of the following GBM cell lines have been published previously: M059J (ATCC number CRL2366), M010b – hypoxia‐sensitive; M059K (ATCC number CRL2365), M006x, M006xLo – hypoxia‐tolerant (Allalunis‐Turner et al., 1992, 1999, 2000). The U87T and U87R GBM cell lines were established following serial in vivo selection in a glioma mouse model (Johnston et al., 2007). U87T cells were isolated from the actively growing tumor mass of an intra‐cerebral U87 tumor; invasive U87R cells were isolated from the contra‐lateral brain. Both cell lines were kindly provided by Dr Donna Senger (University of Calgary). Cells were maintained as monolayer cultures in DMEM/F12 media supplemented with 10% fetal calf serum, 1mM l‐glutamine, and 100U penicillin G/ml in a humidified atmosphere of 5% CO2 in air at 37°C. All tissue culture supplies were purchased from GIBCO.
4.2. Hypoxia labeling of tumor xenografts
Animal experiments were performed according to guidelines for the use of animals in research established by the Canadian Council for Animal Care and were approved by the Animal Care Committee at the Cross Cancer Institute. M006xLo tumor xenografts were initiated in NOD/SCID mice by intra‐dermal injection of 106 to107 cells in both flanks. When the tumor volumes equaled 100–300mm3, mice received an single injection of the hypoxia marker, pimonidazole HCl (100mg/kg i.p., Natural Pharmacia International). Ninety minutes later, mice were euthanized, and the tumors excised, fixed in buffered formalin, embedded in paraffin and then sectioned at 5μm intervals.
4.3. Generation of hypoxia in vitro
A de‐gassing manifold designed by Dr Cameron Koch was used to generate hypoxia in vitro (Koch et al., 1979). Exponential phase cells (∼2×105) were seeded onto 60‐mm glass plates and then incubated under standard laboratory culture conditions (5% CO2 in air) for 4days. The medium was then replenished and the plates were transferred to aluminum chambers from which the air was evacuated and then replaced with 5% CO2/balance N2 until an oxygen tension of 0.6% was achieved. The sealed, air‐tight aluminum chambers were then incubated at 37°C for 6– 48h. At the end of each incubation interval, the aluminum chambers were unsealed, the tissue culture plates removed and RNA and protein isolated from the cells as described in Section 4.4.
4.4. Quantitative real‐time reverse transcription‐PCR
Total RNA from cultured cell lines was isolated using the RNeasy mini kit (QIAGEN). Reverse transcription (RT) was carried out with 1μg total RNA per 20μl reaction volume using QuantiTect reverse transcription kit (QIAGEN). RT experiments were performed with GeneAmp PCR system 9700 (Applied Biosystems). Quantitative real‐time PCR (qRT‐PCR) analysis was performed with a 7900 HT Fast Real‐Time PCR System (Applied Biosystems) using TaqMan fast universal PCR master mix and a validated TaqMan Gene Expression assay (Applied Biosystems) for the human NGB gene (assay ID: Hs00222034_ml). Human 18S rRNA gene was used as endogenous control. Amplification data were analyzed with SDS RQ Manager 1.2. Relative quantities of Ngb transcripts were normalized against relative quantities of the 18S rRNA transcripts, and fold‐expression changes calculated using the equation 2− ΔΔCT.
4.5. Western blotting
Whole‐cell lysates were prepared using complete Lysis‐M buffer (Roche Diagnostics) and protein content determined using a protein assay kit (Pierce). Equal amounts of protein (50μg) were resolved using 13% SDS‐PAGE under reducing condition and electro‐transferred to nitrocellulose membranes. Membranes were blocked with 5% donkey serum then incubated with Ngb antibody (1:1000 rabbit anti‐human polyclonal antibody, Sigma) or α‐tubulin antibody (1:5000, mouse anti‐human α‐tubulin monoclonal antibody, Sigma) as loading control. Membranes were incubated with IRDye® 800 donkey anti‐rabbit (1:5000, Rockland Immunochemicals for Research) or Alexa Fluor® 680 donkey anti‐mouse (1:5000, Molecular Probes, Invitrogen) and scanned using the Odyssey Infrared Imaging System (Li‐COR Biosciences). Bands were analyzed using Odyssey application software version 2.1 and the integrated areas of bands were determined and expressed in arbitrary units (AU).
4.6. Tumor tissue immunostaining
Detection of pimonidazole adducts was as previously described (Kleiter et al., 2006) with modifications. Tissue endogenous peroxidase was quenched by incubation with 3% H2O2 for 15min. Sections were processed for antigen retrieval, incubated with blocking buffer (0.5M glycine in PBS‐0.2% brij35) and then with antibodies to Ngb (1:150, mouse anti‐human Ngb monoclonal antibody, BioVendor Laboratory Medicine Inc., Czech Republic) or pimonidazole (1:500, rabbit anti‐pimonidazole polyclonal antibody, Natural Pharmacia International). Tissue sections were incubated with horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit or anti‐mouse antibodies (DakoCytomation) and positive staining visualized by the chromogenic reaction of HRP with DAB (DakoCytomation).
4.7. Statistical analyses
Data from four replicate experiments were expressed as mean±standard error. Statistical analyses were performed using SigmaPlot 11 software (Systat Software, Inc). Differences between groups were compared using one‐way ANOVA or ANOVA on ranks (Kruskal–Wallis) based on the normality and equal variance tests. To determine exactly which groups are different and the size of the difference, all pairwise multiple comparison procedures were carried out using Fisher Least Significant Difference, Dunn's method or Student–Newman–Keuls method as post‐hoc tests.
Acknowledgements
We thank Dr Jason Derry for assistance with the qRT‐PCR analyses, Darryl Glubrecht for assistance with immunohistochemistry, Brenda Wolokoff for preparing the tumor sections and Bonnie Andrais for tissue culture. This study was supported by an award from the National Cancer Institute of Canada with funds provided by the Canadian Cancer Society.
Emara Marwan, Salloum Nicole, Allalunis‐Turner Joan, (2009), Expression and hypoxic up‐regulation of neuroglobin in human glioblastoma cells, Molecular Oncology, 3, doi: 10.1016/j.molonc.2008.11.002.
References
- Allalunis-Turner, M.J. , Barron, G.M. , Day, R.S.D. , Fulton, D.S. , Urtasun, R.C. , 1992. Radiosensitivity testing of human primary brain tumor specimens. Int. J. Radiat. Oncol. Biol. Phys. 23, 339–343. [DOI] [PubMed] [Google Scholar]
- Allalunis-Turner, M.J. , Barron, G.M. , Day, R.S.D. , Dobler, K.D. , Mirzayans, R. , 1993. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat. Res. 134, 349–354. [PubMed] [Google Scholar]
- Allalunis-Turner, M.J. , Franko, A.J. , Parliament, M.B. , 1999. Modulation of oxygen consumption rate and vascular endothelial growth factor mRNA expression in human malignant glioma cells by hypoxia. Br. J. Cancer. 80, 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow, R.G. , Hill, R.P. , 2008. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer. 8, 180–192. [DOI] [PubMed] [Google Scholar]
- Brown, J.M. , Wilson, W.R. , 2004. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 4, 437–447. [DOI] [PubMed] [Google Scholar]
- Brunori, M. , Vallone, B. , 2007. Neuroglobin, seven years after. Cell. Mol. Life Sci. 64, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester, T. , Weich, B. , Reinhardt, S. , Hankeln, T. , 2000. A vertebrate globin expressed in the brain. Nature. 407, 520–523. [DOI] [PubMed] [Google Scholar]
- Burmester, T. , Haberkamp, M. , Mitz, S. , Roesner, A. , Schmidt, M. , Ebner, B. , Gerlach, F. , Fuchs, C. , Hankeln, T. , 2004. Neuroglobin and cytoglobin: genes, proteins and evolution. IUBMB Life. 56, 703–707. [DOI] [PubMed] [Google Scholar]
- Burmester, T. , Hankeln, T. , 2004. Neuroglobin: a respiratory protein of the nervous system. News Physiol. Sci. 19, 110–113. [DOI] [PubMed] [Google Scholar]
- Chen, G. , Gharib, T.G. , Huang, C.C. , Taylor, J.M. , Misek, D.E. , Kardia, S.L. , Giordano, T.J. , Iannettoni, M.D. , Orringer, M.B. , Hanash, S.M. , Beer, D.G. , 2002. Discordant protein and mRNA expression in lung adenocarcinomas. Mol. Cell. Proteomics. 1, 304–313. [DOI] [PubMed] [Google Scholar]
- Chen, X.Q. , Qin, L.Y. , Zhang, C.G. , Yang, L.T. , Gao, Z. , Liu, S. , Lau, L.T. , Fung, Y.W. , Greenberg, D.A. , Yu, A.C. , 2005. Presence of neuroglobin in cultured astrocytes. Glia. 50, 182–186. [DOI] [PubMed] [Google Scholar]
- Dachs, G.U. , Chaplin, D.J. , 1998. Microenvironmental control of gene expression: implications for tumor angiogenesis, progression, and metastasis. Semin. Radiat. Oncol. 8, 208–216. [DOI] [PubMed] [Google Scholar]
- Fernando, M.S. , Simpson, J.E. , Matthews, F. , Brayne, C. , Lewis, C.E. , Barber, R. , Kalaria, R.N. , Forster, G. , Esteves, F. , Wharton, S.B. , 2006. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 37, 1391–1398. [DOI] [PubMed] [Google Scholar]
- Fordel, E. , Geuens, E. , Dewilde, S. , Rottiers, P. , Carmeliet, P. , Grooten, J. , Moens, L. , 2004. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 319, 342–348. [DOI] [PubMed] [Google Scholar]
- Fordel, E. , Thijs, L. , Martinet, W. , Lenjou, M. , Laufs, T. , Van Bockstaele, D. , Moens, L. , Dewilde, S. , 2006. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci. Lett. 410, 146–151. [DOI] [PubMed] [Google Scholar]
- Fordel, E. , Thijs, L. , Martinet, W. , Schrijvers, D. , Moens, L. , Dewilde, S. , 2007. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 398, 114–122. [DOI] [PubMed] [Google Scholar]
- Fordel, E. , Thijs, L. , Moens, L. , Dewilde, S. , 2007. Neuroglobin and cytoglobin expression in mice. Evidence for a correlation with reactive oxygen species scavenging. FEBS J. 274, 1312–1317. [DOI] [PubMed] [Google Scholar]
- Fraser, J. , de Mello, L.V. , Ward, D. , Rees, H.H. , Williams, D.R. , Fang, Y. , Brass, A. , Gracey, A.Y. , Cossins, A.R. , 2006. Hypoxia-inducible myoglobin expression in nonmuscle tissues. Proc. Natl. Acad. Sci. USA. 103, 2977–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens, E. , Brouns, I. , Flamez, D. , Dewilde, S. , Timmermans, J.P. , Moens, L. , 2003. A globin in the nucleus!. J. Biol. Chem. 278, 30417–30420. [DOI] [PubMed] [Google Scholar]
- Giuffre, A. , Moschetti, T. , Vallone, B. , Brunori, M. , 2008. Neuroglobin: enzymatic reduction and oxygen affinity. Biochem. Biophys. Res. Commun. 367, 893–898. [DOI] [PubMed] [Google Scholar]
- Greenberg, D.A. , Jin, K. , Khan, A.A. , 2008. Neuroglobin: an endogenous neuroprotectant. Curr. Opin. Pharmacol. 8, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankeln, T. , Wystub, S. , Laufs, T. , Schmidt, M. , Gerlach, F. , Saaler-Reinhardt, S. , Reuss, S. , Burmester, T. , 2004. The cellular and subcellular localization of neuroglobin and cytoglobin – a clue to their function?. IUBMB Life. 56, 671–679. [DOI] [PubMed] [Google Scholar]
- Hochachka, P.W. , Buck, L.T. , Doll, C.J. , Land, S.C. , 1996. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA. 93, 9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockel, M. , Knoop, C. , Schlenger, K. , Vorndran, B. , Baussmann, E. , Mitze, M. , Knapstein, P.G. , Vaupel, P. , 1993. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 26, 45–50. [DOI] [PubMed] [Google Scholar]
- Hockel, M. , Schlenger, K. , Hockel, S. , Aral, B. , Schaffer, U. , Vaupel, P. , 1998. Tumor hypoxia in pelvic recurrences of cervical cancer. Int. J. Cancer. 79, 365–369. [DOI] [PubMed] [Google Scholar]
- Holtkamp, N. , Afanasieva, A. , Elstner, A. , van Landeghem, F.K. , Konneker, M. , Kuhn, S.A. , Kettenmann, H. , von Deimling, A. , 2005. Brain slice invasion model reveals genes differentially regulated in glioma invasion. Biochem. Biophys. Res. Commun. 336, 1227–1233. [DOI] [PubMed] [Google Scholar]
- Hundahl, C. , Stoltenberg, M. , Fago, A. , Weber, R.E. , Dewilde, S. , Fordel, E. , Danscher, G. , 2005. Effects of short-term hypoxia on neuroglobin levels and localization in mouse brain tissues. Neuropathol. Appl. Neurobiol. 31, 610–617. [DOI] [PubMed] [Google Scholar]
- Hundahl, C. , Fago, A. , Dewilde, S. , Moens, L. , Hankeln, T. , Burmester, T. , Weber, R.E. , 2006. Oxygen binding properties of non-mammalian nerve globins. FEBS J. 273, 1323–1329. [DOI] [PubMed] [Google Scholar]
- Hundahl, C. , Kelsen, J. , Kjaer, K. , Ronn, L.C. , Weber, R.E. , Geuens, E. , Hay-Schmidt, A. , Nyengaard, J.R. , 2006. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats. Brain Res. 1085, 19–27. [DOI] [PubMed] [Google Scholar]
- Jin, K. , Mao, X.O. , Xie, L. , Khan, A.A. , Greenberg, D.A. , 2008. Neuroglobin protects against nitric oxide toxicity. Neurosci. Lett. 430, 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, A.L. , Lun, X. , Rahn, J.J. , Liacini, A. , Wang, L. , Hamilton, M.G. , Parney, I.F. , Hempstead, B.L. , Robbins, S.M. , Forsyth, P.A. , Senger, D.L. , 2007. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 5, e212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A.A. , Sun, Y. , Jin, K. , Mao, X.O. , Chen, S. , Ellerby, L.M. , Greenberg, D.A. , 2007. A neuroglobin-overexpressing transgenic mouse. Gene. 398, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter, M.M. , Thrall, D.E. , Malarkey, D.E. , Ji, X. , Lee, D.Y. , Chou, S.C. , Raleigh, J.A. , 2006. A comparison of oral and intravenous pimonidazole in canine tumors using intravenous CCI-103F as a control hypoxia marker. Int. J. Radiat. Oncol. Biol. Phys. 64, 592–602. [DOI] [PubMed] [Google Scholar]
- Koch, C.J. , Howell, R.L. , Biaglow, J.E. , 1979. Ascorbate anion potentiates cytotoxicity of nitro-aromatic compounds under hypoxic and anoxic conditions. Br. J. Cancer. 39, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs, T.L. , Wystub, S. , Reuss, S. , Burmester, T. , Saaler-Reinhardt, S. , Hankeln, T. , 2004. Neuron-specific expression of neuroglobin in mammals. Neurosci. Lett. 362, 83–86. [DOI] [PubMed] [Google Scholar]
- Lees-Miller, S.P. , Godbout, R. , Chan, D.W. , Weinfeld, M. , Day, R.S. , Barron, G.M. , Allalunis-Turner, J. , 1995. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 267, 1183–1185. [DOI] [PubMed] [Google Scholar]
- Li, R.C. , Lee, S.K. , Pouranfar, F. , Brittian, K.R. , Clair, H.B. , Row, B.W. , Wang, Y. , Gozal, D. , 2006. Hypoxia differentially regulates the expression of neuroglobin and cytoglobin in rat brain. Brain Res. 1096, 173–179. [DOI] [PubMed] [Google Scholar]
- Ljungkvist, A.S. , Bussink, J. , Kaanders, J.H. , van der Kogel, A.J. , 2007. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat. Res. 167, 127–145. [DOI] [PubMed] [Google Scholar]
- Mammen, P.P. , Shelton, J.M. , Goetsch, S.C. , Williams, S.C. , Richardson, J.A. , Garry, M.G. , Garry, D.J. , 2002. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J. Histochem. Cytochem. 50, 1591–1598. [DOI] [PubMed] [Google Scholar]
- Mancuso, M. , Davidzon, G. , Kurlan, R.M. , Tawil, R. , Bonilla, E. , Di Mauro, S. , Powers, J.M. , 2005. Hereditary ferritinopathy: a novel mutation, its cellular pathology, and pathogenetic insights. J. Neuropathol. Exp. Neurol. 64, 280–294. [DOI] [PubMed] [Google Scholar]
- Nordsmark, M. , Overgaard, J. , 2000. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother. Oncol. 57, 39–43. [DOI] [PubMed] [Google Scholar]
- Olive, P.L. , Trotter, T. , Banath, J.P. , Jackson, S.M. , Le Riche, J. , 1996. Heterogeneity in human tumour hypoxic fraction using the comet assay. Br. J. Cancer. Suppl. 27, S191–S195. [PMC free article] [PubMed] [Google Scholar]
- Ostojic, J. , Sakaguchi, D.S. , de Lathouder, Y. , Hargrove, M.S. , Trent, J.T. , Kwon, Y.H. , Kardon, R.H. , Kuehn, M.H. , Betts, D.M. , Grozdanic, S. , 2006. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest. Ophthalmol. Vis. Sci. 47, 1016–1023. [DOI] [PubMed] [Google Scholar]
- Parliament, M.B. , Allalunis-Turner, M.J. , Franko, A.J. , Olive, P.L. , Mandyam, R. , Santos, C. , Wolokoff, B. , 2000. Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br. J. Cancer. 82, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce, A. , Bolognesi, M. , Bocedi, A. , Ascenzi, P. , Dewilde, S. , Moens, L. , Hankeln, T. , Burmester, T. , 2002. Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep. 3, 1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, J.M. , 2006. p53-mediated apoptosis, neuroglobin overexpression, and globin deposits in a patient with hereditary ferritinopathy. J. Neuropathol. Exp. Neurol. 65, 716–721. [DOI] [PubMed] [Google Scholar]
- Rayner, B.S. , Duong, T.T. , Myers, S.J. , Witting, P.K. , 2006. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J. Neurochem. 97, 211–221. [DOI] [PubMed] [Google Scholar]
- Rebetz, J. , Tian, D. , Persson, A. , Widegren, B. , Salford, L.G. , Englund, E. , Gisselsson, D. , Fan, X. , 2008. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS ONE. 3, e1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai, N. , Alvarez-Buylla, A. , Berger, M.S. , 2005. Neural stem cells and the origin of gliomas. N. Engl. J. Med. 353, 811–822. [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Honda, Y. , Asoh, T. , Oizumi, K. , Ohshima, Y. , Honda, E. , 1998. Cerebrospinal fluid ferritin in glioblastoma: evidence for tumor synthesis. J. Neurooncol. 40, 47–50. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner, R. , Haberkamp, M. , Schmitz, C. , Hankeln, T. , Burmester, T. , 2006. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 1103, 173–180. [DOI] [PubMed] [Google Scholar]
- Singh, S.K. , Clarke, I.D. , Hide, T. , Dirks, P.B. , 2004. Cancer stem cells in nervous system tumors. Oncogene. 23, 7267–7273. [DOI] [PubMed] [Google Scholar]
- Singh, S.K. , Hawkins, C. , Clarke, I.D. , Squire, J.A. , Bayani, J. , Hide, T. , Henkelman, R.M. , Cusimano, M.D. , Dirks, P.B. , 2004. Identification of human brain tumour initiating cells. Nature. 432, 396–401. [DOI] [PubMed] [Google Scholar]
- Stiles, C.D. , Rowitch, D.H. , 2008. Glioma stem cells: a midterm exam. Neuron. 58, 832–846. [DOI] [PubMed] [Google Scholar]
- Stupp, R. , Mason, W.P. , van den Bent, M.J. , Weller, M. , Fisher, B. , Taphoorn, M.J. , Belanger, K. , Brandes, A.A. , Marosi, C. , Bogdahn, U. , 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Jin, K. , Mao, X.O. , Zhu, Y. , Greenberg, D.A. , 2001. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. U.S.A. 98, 15306–15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Jin, K. , Peel, A. , Mao, X.O. , Xie, L. , Greenberg, D.A. , 2003. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. U.S.A. 100, 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte, M.L. , Parliament, M. , Franko, A. , Allalunis-Turner, J. , 2002. Variation in mitochondrial function in hypoxia-sensitive and hypoxia-tolerant human glioma cells. Br. J. Cancer. 86, 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin, R.D. , Smith, D.L. , Blinco, D. , Wilson, C.L. , Miller, C.J. , Evans, C.A. , Jaworska, E. , Baldwin, S.A. , Barnes, K. , Pierce, A. , 2006. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood. 107, 4687–4694. [DOI] [PubMed] [Google Scholar]
- Vaupel, P. , Schlenger, K. , Knoop, C. , Hockel, M. , 1991. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 51, 3316–3322. [PubMed] [Google Scholar]
- Wilden, J. , Moore, I. , 1987. Histological Factors in the Prognosis of Malignant Glioma. Brain Oncology: Biology, Diagnosis and Therapy Martinus Nijhoff; Dordrecht: pp. 243-247 [Google Scholar]
- Wystub, S. , Ebner, B. , Fuchs, C. , Weich, B. , Burmester, T. , Hankeln, T. , 2004. Interspecies comparison of neuroglobin, cytoglobin and myoglobin: sequence evolution and candidate regulatory elements. Cytogenet. Genome Res. 105, 65–78. [DOI] [PubMed] [Google Scholar]
- Yaromina, A. , Zips, D. , Thames, H.D. , Eicheler, W. , Krause, M. , Rosner, A. , Haase, M. , Petersen, C. , Raleigh, J.A. , Quennet, V. , 2006. Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: the need for a multivariate approach in biomarker studies. Radiother. Oncol. 81, 122–129. [DOI] [PubMed] [Google Scholar]


