Abstract
We investigated the pro‐inflammatory response mediated by TNFα in glioblastoma and whether treatment with organoselenium Ebselen (2‐phenyl‐1,2‐benzisoselenazol‐3[2H]one) can affect TNFα induced inflammatory response. Exposure to TNFα increased the expression of pro‐inflammatory mediator interleukin IL‐6, IL‐8, monocyte chemoattractant protein‐1 (MCP‐1) and cyclooxygenase (COX‐2). Treatment with Ebselen abrogated TNFα induced increase in pro‐inflammatory mediators. Ebselen not only abrogated TNFα induced enhanced invasiveness of glioma cells by down‐regulating matrix metallo proteinase (MMP‐9) and urokinase plasminogen (uPa) activity, but also inhibited glioma cell migration. Treatment with Ebselen also down‐regulated the enhanced ROS production of TNFα treated glioma cells. In addition, Ebselen induced DNA damage repair signaling response in glioma cells both in the presence and absence of TNFα. These studies indicate that together with its known ability to sensitize glioma cell to TNFα induced apoptosis, Ebselen can overcome TNFα induced pro‐inflammatory mediators to prevent a build up of a deleterious pro‐inflammatory tumor microenvironment.
Keywords: Glioblastoma, TNFα, Ebselen, Pro-inflammatory cytokines
1. Introduction
The increased presence of inflammatory cytokines in the tumor microenvironment plays a major factor in inducing malignancy (Coussens and Werb, 2002). Pro‐inflammatory cytokines act as a tumor promoter by regulating cascade of cytokines, chemokines, MMPs and pro‐angiogenic activities (Kopp and Ghosh, 1995). TNFα is a pro‐inflammatory cytokine whose signaling pathways are linked to both proapoptotic and antiapoptotic responses (Aggarwal, 2003; Wallach et al., 1999). Despite TNFα's ability to induce apoptosis, several tumors are resistant to TNFα mediated apoptosis (Tsujimoto et al., 1985). The resistance to TNFα induced apoptosis in various cancer cells has been attributed to the activation of NF‐κB; and GBM are resistant to cytotoxic effect of TNFα.
Studies have indicated that some anticancer chemotherapeutic drugs and TNFα can kill some resistant tumor cells (Safrit and Bonavida, 1992). We have recently documented that Ebselen – a selenoorganic compound, sensitizes glioma cells to TNFα mediated apoptosis by abrogating NF‐κB activation (Sharma et al., 2008). TNFα induced activation of NF‐κB is crucial for tumor progression as it induces the expression of the pro‐inflammatory cytokines IL‐6 and IL‐8 (Kopp and Ghosh, 1995), that plays an important role in tumor progression. Moreover, increased IL‐8 levels contribute to glial tumor neovascularity and progression (Brat et al., 2005). Increased expression of pro‐inflammatory mediator has been implicated as a possible prognostic indicator in glioblastoma and to play a role in angiogenesis in GBM (Chang et al., 2005). As Ebselen is a potential chemopreventive agent in inflammation‐associated carcinogenesis (Nakamura et al., 2002) and since the causal relationship between inflammation and tumor progression is well documented, we investigated whether Ebselen could abrogate TNFα induced pro‐inflammatory response in the tumor microenvironment while sensitizing cells to TNFα induced apoptosis at the same time.
2. Materials and methods
2.1. Cell culture and treatment
Glioblastoma cell lines A172 and T98G were obtained from American Type Culture Collection and cultured in DMEM supplemented with 10% fetal bovine serum. On attaining semi‐confluence, cells were switched to serum free media and after 12h cells were treated with 10μM of Ebselen (in dimethyl sulphoxide, DMSO) in the presence or absence of TNFα (50ng/ml) in serum free media for another 24h. Following treatment, the conditioned medium from cells were collected and stored at −80°C for cytokine bead array, gelatin zymography and urokinase plasminogen activator assay, whereas cells were processed for Western blot and FACS analysis. All reagents were purchased from Sigma unless otherwise stated. DMSO treated cells were used as controls.
2.2. Cytokine bead array
Cytometric bead array kit (CBA kit; BD Biosciences) was used to quantitatively measure cytokine and chemokine levels in the supernatant collected from control and glioma cells treated with different combinations of TNFα and Ebselen. The assay was performed and analyzed on FACS Calibur (Becton Dickinson) as described previously (Sharma et al., 2007).
2.3. Western blot analysis
Proteins from whole cell lysates were isolated as described previously (Sharma et al., 2007). Twenty to 50μg of protein isolated from cells treated with TNFα either in the presence or absence of Ebselen was electrophoresed on 6– 10% polyacrylamide gel and Western blotting performed as described (Tewari et al., 2008). The following antibodies were used – COX‐2 (cell signaling), MSH2 and MLH1 (Santa Cruz Biotechnology). Secondary antibodies were purchased from Vector Laboratories. After addition of chemiluminescence reagent (Amersham), blots were exposed to Chemigenius, Bioimaging System (Syngene) for developing and images were captured using Genesnap software (Syngene). The blots were stripped and reprobed with anti‐β‐actin to determine equivalent loading as described (Sharma et al., 2007).
2.4. Gelatin zymography
MMP‐9 activity in conditioned medium from cells treated with different combinations of TNFα and Ebselen was analyzed by gelatin zymography as described previously (Li et al., 2007). Briefly, the conditioned medium was resolved by 10% SDS‐PAGE in the presence of 1mg/mL gelatin. The resulting gel was washed in 2.5% Triton X‐100 and then incubated for 16h in an enzyme assay buffer [25mmol/L Tris (pH 8.0), 5mmol/L CaCl2, 0.9% NaCl, 0.05% NaN3] at 37°C. After staining with Coomassie brilliant blue R‐250, gelatinases were identifiable as clear bands.
2.5. Urokinase plasminogen activator (uPa) assay
The assay was performed according to the manufacturer's instructions (Chemicon). Culture supernatants (160μl) from glioma cells treated with TNFα in the presence and absence of Ebselen were added to each well of 96‐well plate. Following the addition of assay buffer and chromogenic substrate the samples were incubated for 24h at 37°C, after which absorbance was read at 405nm. Optical density values obtained were compared with known standards to obtain relative activities.
2.6. Wound healing assay
Wound healing was performed as described previously (Sharma et al., 2007). Briefly, cells were grown in serum containing media and upon reaching semi‐confluence, serum was removed and cells grown in serum free media. A plastic pipet tip was drawn across the center of the culture to produce a clean wound area, 12h after serum depletion. Cells were then left untreated or treated with TNFα in the presence and absence of Ebselen for 24h and cell movement in the wound area was examined. The migration distance between the leading edge of the migrating cells and the edge of the wound at the beginning and end of each indicated time point was taken by light microscopy.
2.7. Measurement of ROS
Intracellular ROS generation in cells treated with TNFα in the presence and absence of Ebselen was assessed using 2′,7′‐ dichlorodihydrofluorescein diacetate (CM‐H2DCFDA, Molecular Probes) as described previously (Sharma et al., 2007). Briefly, cells were incubated with H2DCFDA (5μM) for 30min at 37°C, washed twice with PBS, and fluorescent intensity of 5×105 cells and analyzed on FACS Calibur (Becton Dickinson).
2.8. Statistical analysis
All comparisons between groups were performed using two‐tailed paired Student's t‐test. All values of P less than 0.05 were taken as significant.
3. Results
3.1. Ebselen suppresses the release of pro‐inflammatory mediators from glioblastoma cells
As activation of NF‐κB induces the expression of pro‐inflammatory cytokines (Kopp and Ghosh, 1995) that plays an important role in tumor progression, and since Ebselen is known to alter the expression of inflammatory mediators (Haddad et al., 2002), we performed CBA to investigate the cytokine/chemokine profile of TNFα treated glioma in the presence and absence of Ebselen. A general trend toward increase in the release of pro‐inflammatory cytokine IL‐6 and chemokines IL‐8 and MCP‐1 was observed in TNFα treated glioma cells. Treatment of A172 cells with TNFα elevated the levels of IL‐6, IL‐8, and MCP‐1 significantly by 1.8‐, 2.0‐ and 2.5‐fold respectively, as compared to control (Figure 1a). However, treatment of cells with TNFα in the presence of Ebselen abrogated the ability of TNFα to induce pro‐inflammatory cytokine/chemokine release. The levels of pro‐inflammatory mediators released from glioma treated with both Ebselen and TNFα was almost comparable or even less than that of untreated control (Figure 1a). Treatment with Ebselen alone also resulted in a significant reduction of pro‐inflammatory cytokines/chemokines release from glioma cells, as compared to control (Figure 1a). Similar trend was observed in T98G cells (Figure 1a).
Figure 1.
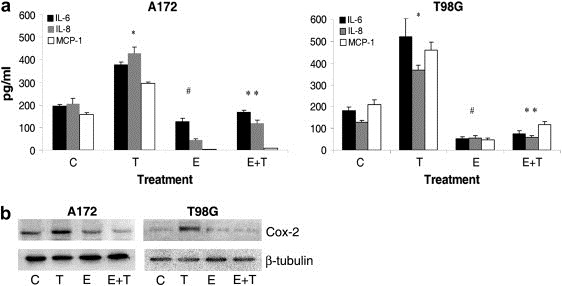
Ebselen alters the release of pro‐inflammatory mediators and decreases invasiveness of TNFa treated glioma cells. (a) Expression of IL‐6, IL‐8 and MCP‐1 in A172 and T98G glioma cells treated with different combinations of TNFα and Ebselen for 24h, as observed by CBA. Increase in pro‐inflammatory cytokine/chemokine observed upon TNFα treatment was significantly suppressed in the presence of Ebselen. Values represent mean±SEM from three individual experiments. * Significant increase from control. # Significant decrease from control. ** Significant decrease from TNFα treated cells (P<0.05). (b) Ebselen decreases the elevated COX‐2 level in TNFα treated A172 and T98G cells. Western blot analysis demonstrates expression of pro‐inflammatory mediator COX‐2 in cells treated with either TNFα or Ebselen or both. A representative blot is shown from three independent experiments with identical results. C, T and E denote control, TNFα and Ebselen respectively.
TNFα induces the expression of pro‐inflammatory mediator COX‐2 (Yamamoto et al., 1995). Since organoselenium reduces the expression of COX‐2 in human lung cancer (El‐Bayoumy et al., 2006), we investigated the effect of Ebselen on the expression of COX‐2 in TNFα treated cells. COX‐2 level was elevated in TNFα treated glioma cells and treatment with Ebselen down‐regulated the elevated COX‐2 expression in TNFα treated cells, to levels comparable to that of control (Figure 1b).
3.2. Ebselen down‐regulates the increased invasiveness of TNFα treated glioma cells
As TNFα induces the expression of MMP‐9 associated with invasion (Esteve et al., 2002), the effect of Ebselen on MMP‐9 activity both in the presence and absence of TNFα was investigated. Treatment with TNFα elevated MMP‐9 activity in T98G cells as compared to control (Figure 2a). This increase in MMP‐9 activity was reduced considerably almost to control levels in the presence of Ebselen. Treatment with Ebselen alone had no effect on MMP‐9 activity (Figure 2a).
Figure 2.
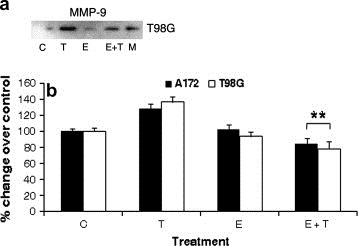
Ebselen decreases invasive potential of glioma cells. (a) Co‐treatment of TNFα treated glioma cells with Ebselen down‐regulates MMP‐9 activity. Gelatin zymography was performed on conditioned medium collected from T98G cells treated with Ebselen in the presence and absence of TNFα. The figure is a representative of two independent experiments with identical results. C, T, E and M denote control, TNFα, Ebselen and marker respectively. (b) Treatment with Ebselen decreases the uPa in TNFα treated glioma cells. uPa activity was performed on conditioned medium obtained from cells treated with TNFα in the presence of Ebselen. Ebselen down‐regulates uPa activity in TNFα treated cells. The graph represents change in uPa activity under different conditions over control which is set to 100%. Values represent the means±SEM from three independent experiments. ** Significant decrease from TNFα treated cells (P<0.05).
The binding of urokinase plasminogen activator (uPA) to its receptor (uPAR) initiates a proteolytic cascade facilitating the activation of MMP, which in turn degrades the extracellular matrix (Lakka et al., 2003). Since Ebselen decreased MMP‐9 activity in TNFα treated glioma cells (Figure 2a), we determined uPA activity in TNFα treated cells in the presence and absence of Ebselen (Figure 2b). The slight but insignificant increase in uPA activity observed in TNFα treated glioma cells was significantly down‐regulated in the presence of Ebselen, to levels even lesser than that observed in control (Figure 2b). Treatment with Ebselen alone had no significant effect on uPA activity (Figure 2b). Thus, treatment with Ebselen abrogated uPA activity of TNFα treated glioma cells below basal levels (Figure 2b).
3.3. Ebselen inhibits glioma cell migration both in the presence and absence of TNFα
As chemokines are crucial regulators of cancer cell migration and IL‐8 and MCP‐1 levels were significantly altered in TNFα treated cells, we investigated the migration ability of glioma cells treated with TNFα or Ebselen or both using wound‐healing assay. There was a decrease in cell migration, as demonstrated by the difference in the wound area covered in Ebselen treated cells as compared to control. Although TNFα treated cells migrated to cover the wound area, co‐treatment with Ebselen inhibited motility of TNFα treated A172 cells considerably (Figure 3). Similar results were obtained with T98G cells (data not shown).
Figure 3.
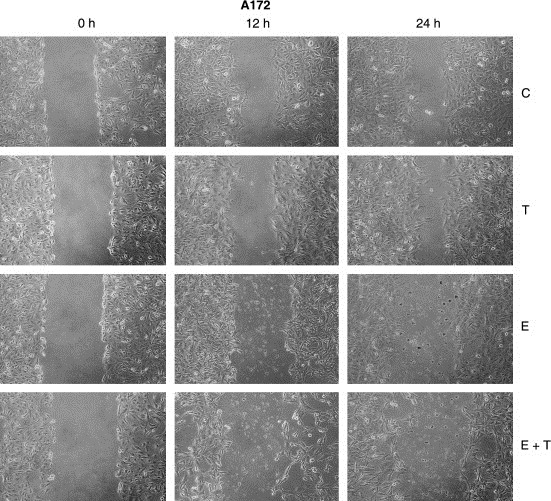
Ebselen inhibits glioma cell migration both in the presence and absence of TNFα. Ability of A172 cells to migrate after scratch wound injury was inhibited by Ebselen. Ebselen greatly inhibited the migration of TNFα treated cells. TNFα or Ebselen or both were added to culture after scratch wound and the treatment lasted for 24h. Micrographs depict cultures with scratch wound that were left untreated or treated with either TNFα or Ebselen or both for 0, 12 and 24h.
3.4. Ebselen reduces ROS generation in TNFα treated glioma cells
Ebselen exhibits antioxidant activities. It not only inhibits phorbol ester‐induced oxidative and inflammatory changes (Nakamura et al., 2002), but also inhibits ROS production in dendritic cells during antigen presentation to modulate cytokine production by T cells (Matsue et al., 2003). TNFα dependent activation of NF‐κB leads to enhanced ROS production and further NF‐κB activation, thereby contributing to sustained oxidant production in chronic inflammation (Gauss et al., 2007). As NF‐κB activity was down‐regulated in cells treated with Ebselen both in the presence and absence of TNFα (Sharma et al., 2008), we performed FACS analysis to determine ROS levels in cells treated with different combinations of TNFα and Ebselen. The increase in ROS production observed in TNFα treated T98G cells was down‐regulated considerably in the presence of Ebselen. Treatment with Ebselen alone also decreased ROS levels as compared to control (Figure 4). Similar results were also observed in A172 cells (data not shown). These results indicate that Ebselen is capable of abrogating the increased ROS levels observed in TNFα treated glioma cells (Figure 4).
Figure 4.
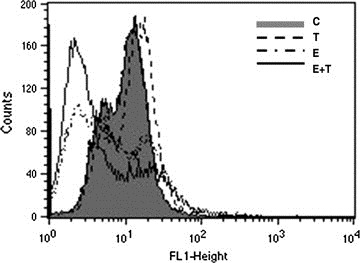
Ebselen decreases ROS generation in TNFα treated glioma cells. The elevation in ROS production observed upon TNFα treatment was decreased in the presence of Ebselen. FACS analysis was performed to determine ROS generation in T98G glioblastoma cells treated with TNFα or Ebselen or both for 24h. The figure is a representative of two independent experiments.
3.5. Ebselen induces increase in DNA damage signaling response
ROS has been shown to induce oxidative damage to DNA directly, and they are capable of damaging proteins involved in DNA repair (Jaiswal et al., 2000). The mismatch repair (MMR) system promotes genomic fidelity by repairing base–base mismatches and insertion‐deletion that are generated during DNA replication and recombination. Inflammation‐induced DNA damage is controlled by the MMR protein MSH2 (Kohonen‐Corish et al., 2002). The loss of MMR protein MLH1 and MLH2 in colorectal adenocarcinomas is accompanied by Cox‐2 over‐expression (Balbinotti et al., 2007). Besides, the efficacies of a number of cancer chemotherapeutics are dependent on MMR system (Bellacosa, 2001). Since the increased release of pro‐inflammatory mediators and ROS from TNFα treated cells was abrogated in the presence of Ebselen, we investigated the expression of MMR protein MLH1 and MSH2 in cells treated with Ebselen in the presence and absence of TNFα. The level of MLH1 and MSH2 observed in control cells was down‐regulated upon TNFα treatment (Figure 5). While treatment with Ebselen had no effect on either MLH1 or MSH2 expression in A172 cells, it was capable of restoring the decreased MLH1 or MSH2 levels in TNFα treated cells to the control levels (Figure 5). Similar trend was observed in the expression of MSH2 in T98G cells (Figure 5).
Figure 5.
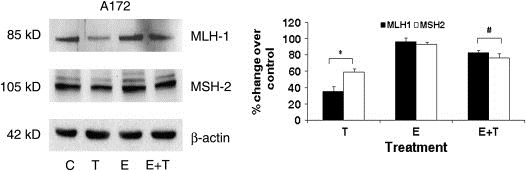
Ebselen positively affects the DNA mismatch repair system in TNFα treated cells. Ability of glioma cells to repair DNA mismatch both in the presence and absence of TNFα was detected by determining the expression of MSH2 and MLH1. Protein was isolated from A172 glioma cells treated with 10μM Ebselen in the presence or absence of TNFα and Western blot analysis was performed to detect the expression of MSH2 and MLH1. A representative blot is shown from three independent experiments with identical results. Blots were reprobed for β‐actin to establish equivalent loading.
4. Discussion
The importance of NF‐κB in controlling proliferation and invasion of cancer has been well documented (Karin and Greten, 2005). The resistance to TNFα induced apoptosis in various cancer cells including glioblastoma has been attributed to the activation of the transcription factor NF‐κB. We have recently reported that Ebselen sensitizes glioma cells to TNFα induced apoptosis by down ‐regulating NF‐κB activation (Sharma et al., 2008), as has been reported in other malignancies (Shimohashi et al., 2000). Ebselen possess anti‐inflammatory properties (Nakamura et al., 2002; Haddad et al., 2002; Shimohashi et al., 2000; Schewe, 1995; Zhang et al., 2002) and NF‐κB activity is crucial for the induction of pro‐inflammatory cytokines (Duan et al., 2004). It is possible that the ability of Ebselen to abrogate TNFα induced release of pro‐inflammatory mediators was achieved through down‐regulation of NF‐κB activity (Haddad et al., 2002;, Jozsef and Filep, 2003).
Increased IL‐6 gene amplification in GBM is associated with decreased patient survival (Tchirkov et al., 2007), and IL‐8 blockade has been shown to inhibit MMP‐9 activity which is associated with invasiveness of tumor cells (Mian et al., 2003). Besides, inhibition of MCP‐1 has been shown to suppress migration of glioma cells (Liang et al., 2008). The ability of Ebselen to down ‐regulate IL‐8 and MCP‐1 in TNFα treated glioma cells could have possibly contributed to the: (i) decreased invasive potential through regulation of MMP‐9 and uPA activity; and (ii) decreased migratory ability. The crucial link between inflammation and neoplastic progression comes from studies suggesting that non‐steroidal anti‐inflammatory drugs (NSAID) inhibit growth of various malignancies by inhibiting COX‐2, involved in inducing inflammatory reactions in damaged tissues (Williams et al., 1999). The ability of Ebselen to abrogate levels of COX‐2 and other pro‐inflammatory cytokines in glioma cells might prevent the establishment of feed forward cycle of inflammation that promotes disease progression.
The ability of Ebselen to elevate the decreased expression of MMR proteins observed in TNFα treated cells suggests that Ebselen positively regulates DNA damage repair response. This together with its ability to reduce oxidative stress might prevent further accumulation of genetic instability. Thus treatment with Ebselen not only: (i) abrogated TNFα induced increased expression of pro‐inflammatory mediators, ROS, migration and invasive potential of glioblastoma cells; but also (ii) positively increased DNA damage repair signaling response in glioma cells. These results raise the possibility of abrogating pro‐inflammatory response by Ebselen as a strategy to regulate the tumor microenvironment of glioma cells. This together with the ability of Ebselen to sensitize glioma cells to TNFα induced apoptosis warrants attention as an effective anti‐glioma therapy.
Acknowledgements
This work was supported by a grant from the Defence Research and Development Organization, Government of India, to ES. The authors thank Mr Uttam Kumar Saini for technical assistance.
Tewari Richa, Sharma Vivek, Koul Nitin, Ghosh Abhishek, Joseph Christy, Hossain Sk Ugir, Sen Ellora, (2009), Ebselen abrogates TNFα induced pro‐inflammatory response in glioblastoma, Molecular Oncology, 3, doi: 10.1016/j.molonc.2008.10.004.
References
- Aggarwal, B.B. , 2003 Sep. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, (9) 745–756. [DOI] [PubMed] [Google Scholar]
- Balbinotti, R.A. , Ribeiro, U. , Sakai, P. , Safatle-Ribeiro, A.V. , Balbinotti, S.S. , Scapulatempo, C. , Alves, V.A. , Corbett, C.E. , Carrilho, F.J. , 2007 Nov–Dec. hMLH1, hMSH2 and cyclooxygenase-2 (cox-2) in sporadic colorectal polyps. Anticancer Res. 27, (6C) 4465–4471. [PubMed] [Google Scholar]
- Bellacosa, A. , 2001 Nov. Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ. 8, (11) 1076–1092. [DOI] [PubMed] [Google Scholar]
- Brat, D.J. , Bellail, A.C. , Van Meir, E.G. , 2005 Apr. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 7, (2) 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.Y. , Li, M.C. , Liao, S.L. , Huang, Y.L. , Shen, C.C. , Pan, H.C. , 2005 Nov. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J. Clin. Neurosci. 12, (8) 930–933. [DOI] [PubMed] [Google Scholar]
- Coussens, L.M. , Werb, Z. , 2002 Dec 19–26. Inflammation and cancer. Nature. 420, (6917) 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, L. , Aoyagi, M. , Tamaki, M. , Yoshino, Y. , Morimoto, T. , Wakimoto, H. , Nagasaka, Y. , Hirakawa, K. , Ohno, K. , Yamamoto, K. , 2004 Jan 1. Impairment of both apoptotic and cytoprotective signalings in glioma cells resistant to the combined use of cisplatin and tumor necrosis factor alpha. Clin. Cancer Res. 10, (1 Pt 1) 234–243. [DOI] [PubMed] [Google Scholar]
- El-Bayoumy, K. , Das, A. , Narayanan, B. , Narayanan, N. , Fiala, E.S. , Desai, D. , Rao, C.V. , Amin, S. , Sinha, R. , 2006 Jul. Molecular targets of the chemopreventive agent 1,4-phenylenebis (methylene)-selenocyanate in human non-small cell lung cancer. Carcinogenesis. 27, (7) 1369–1376. [DOI] [PubMed] [Google Scholar]
- Esteve, P.O. , Chicoine, E. , Robledo, O. , Aoudjit, F. , Descoteaux, A. , Potworowski, E.F. , St-Pierre, Y. , 2002 Sep 20. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B.J. Biol. Chem. 277, (38) 35150–35155. [DOI] [PubMed] [Google Scholar]
- Gauss, K.A. , Nelson-Overton, L.K. , Siemsen, D.W. , Gao, Y. , DeLeo, F.R. , Quinn, M.T. , 2007 Sep. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J. Leukocyte Biol. 82, (3) 729–741. [DOI] [PubMed] [Google Scholar]
- Haddad, el.B. , McCluskie, K. , Birrell, M.A. , Dabrowski, D. , Pecoraro, M. , Underwood, S. , Chen, B. , De Sanctis, G.T. , Webber, S.E. , Foster, M.L. , Belvisi, M.G. , 2002 Jul 15. Differential effects of ebselen on neutrophil recruitment, chemokine, and inflammatory mediator expression in a rat model of lipopolysaccharide-induced pulmonary inflammation. J. Immunol. 169, (2) 974–982. [DOI] [PubMed] [Google Scholar]
- Jaiswal, M. , LaRusso, N.F. , Burgart, L.J. , Gores, G.J. , 2000 Jan 1. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 60, (1) 184–190. [PubMed] [Google Scholar]
- Jozsef, L. , Filep, J.G. , 2003 Nov 1. Selenium-containing compounds attenuate peroxynitrite-mediated NF-kappaB and AP-1 activation and interleukin-8 gene and protein expression in human leukocytes. Free Rad. Biol. Med. 35, (9) 1018–1027. [DOI] [PubMed] [Google Scholar]
- Karin, M. , Greten, F.R. , 2005 Oct. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5, (10) 749–759. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish, M.R. , Daniel, J.J. , te Riele, H. , Buffinton, G.D. , Dahlstrom, J.E. , 2002 Apr 1. Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Res. 62, (7) 2092–2097. [PubMed] [Google Scholar]
- Kopp, E.B. , Ghosh, S. , 1995. NF-kappa B and rel proteins in innate immunity. Adv. Immunol. 58, 1–27. [DOI] [PubMed] [Google Scholar]
- Lakka, S.S. , Gondi, C.S. , Yanamandra, N. , Dinh, D.H. , Olivero, W.C. , Gujrati, M. , Rao, J.S. , 2003 May 15. Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 63, (10) 2454–2461. [PubMed] [Google Scholar]
- Li, L. , Gondi, C.S. , Dinh, D.H. , Olivero, W.C. , Gujrati, M. , Rao, J.S. , 2007 Apr 1. Transfection with anti-p65 intrabody suppresses invasion and angiogenesis in glioma cells by blocking nuclear factor-kappaB transcriptional activity. Clin. Cancer Res. 13, (7) 2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. , Bollen, A.W. , Gupta, N. , 2008 Jan. CC chemokine receptor-2A is frequently overexpressed in glioblastoma. J. Neurooncol. 86, (2) 153–163. [DOI] [PubMed] [Google Scholar]
- Matsue, H. , Edelbaum, D. , Shalhevet, D. , Mizumoto, N. , Yang, C. , Mummert, M.E. , Oeda, J. , Masayasu, H. , Takashima, A. , 2003 Sep 15. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J. Immunol. 171, (6) 3010–3018. [DOI] [PubMed] [Google Scholar]
- Mian, B.M. , Dinney, C.P. , Bermejo, C.E. , Sweeney, P. , Tellez, C. , Yang, X.D. , Gudas, J.M. , McConkey, D.J. , Bar-Eli, M. , 2003 Aug 1. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 9, (8) 3167–3175. [PubMed] [Google Scholar]
- Nakamura, Y. , Feng, Q. , Kumagai, T. , Torikai, K. , Ohigashi, H. , Osawa, T. , Noguchi, N. , Niki, E. , Uchida, K. , 2002 Jan 25. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 277, (4) 2687–2694. [DOI] [PubMed] [Google Scholar]
- Safrit, J.T. , Bonavida, B. , 1992 Dec 1. Sensitivity of resistant human tumor cell lines to tumor necrosis factor and adriamycin used in combination: correlation between down-regulation of tumor necrosis factor-messenger RNA induction and overcoming resistance. Cancer Res. 52, (23) 6630–6637. [PubMed] [Google Scholar]
- Schewe, T. , 1995 Oct. Molecular actions of ebselen—an antiinflammatory antioxidant. Gen. Pharmacol. 26, (6) 1153–1169. [DOI] [PubMed] [Google Scholar]
- Sharma, V. , Joseph, C. , Ghosh, S. , Agarwal, A. , Mishra, M.K. , Sen, E. , 2007 Sep. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Therap. 6, (9) 2544–2553. [DOI] [PubMed] [Google Scholar]
- Sharma, V. , Tewari, R. , Sk, U.H. , Joseph, C. , Sen, E. , 2008 Nov 1. Ebselen sensitizes glioblastoma cells to Tumor Necrosis Factor (TNFalpha)-induced apoptosis through two distinct pathways involving NF-kappaB downregulation and Fas-mediated formation of death inducing signaling complex. Int. J. Cancer. 123, (9) 2204–2212. [DOI] [PubMed] [Google Scholar]
- Shimohashi, N. , Nakamuta, M. , Uchimura, K. , Sugimoto, R. , Iwamoto, H. , Enjoji, M. , Nawata, H. , 2000 Jun 12. Selenoorganic compound, ebselen, inhibits nitric oxide and tumor necrosis factor-alpha production by the modulation of jun-N-terminal kinase and the NF-kappab signaling pathway in rat Kupffer cells. J. Cell Biochem. 78, (4) 595–606. [PubMed] [Google Scholar]
- Tchirkov, A. , Khalil, T. , Chautard, E. , Mokhtari, K. , Veronese, L. , Irthum, B. , Vago, P. , Kemeny, J.L. , Verrelle, P. , 2007 Feb 12. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br. J. Cancer. 96, (3) 474–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari, R. , Sharma, V. , Koul, N. , Sen, E. , 2008 Aug 14. Involvement of Miltefosine mediated ERK activation in glioma cell apoptosis through Fas regulation. J. Neurochem. 107, (3) 616–627. [DOI] [PubMed] [Google Scholar]
- Tsujimoto, M. , Yip, Y.K. , Vilcek, J. , 1985 Nov. Tumor necrosis factor: specific binding and internalization in sensitive and resistant cells. Proc. Natl. Acad. Sci. USA. 82, (22) 7626–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach, D. , Varfolomeev, E.E. , Malinin, N.L. , Goltsev, Y.V. , Kovalenko, A.V. , Boldin, M.P. , 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17, 331–367. [DOI] [PubMed] [Google Scholar]
- Williams, C.S. , Mann, M. , DuBois, R.N. , 1999 Dec 20. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 18, (55) 7908–7916. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K. , Arakawa, T. , Ueda, N. , Yamamoto, S. , 1995 Dec 29. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 270, (52) 31315–31320. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Nomura, A. , Uchida, Y. , Iijima, H. , Sakamoto, T. , Iishii, Y. , Morishima, Y. , Mochizuki, M. , Masuyama, K. , Hirano, K. , Sekizawa, K. , 2002 Mar 1. Ebselen suppresses late airway responses and airway inflammation in guinea pigs. Free Rad. Biol. Med. 32, (5) 454–464. [DOI] [PubMed] [Google Scholar]


