Abstract
The objective of the workshop was to examine what Cancer Innovation in Europe means and what it should be standing for in the future. The panel discussion brought together patients, researchers, politicians and industry in order to examine what cancer innovation represents to them, what the challenges are to innovation, and how innovation in this research area can be encouraged and developed in the EU.
Keywords: Workshop, Novartis oncology, European Health Forum Gastein, Innovation in Europe, Cancer
1. Introduction
Cancer is the second largest killer in Europe. Incidence rates are increasing with more than 2 million new cases and more than 1.1 million cancer deaths in Europe each year. Every minute that goes by, 60 Europeans have learned they suffer from cancer while 30 Europeans have died from it.
Cancer research and innovation are keys to preventing these deaths and to eliminate the devastating consequences of this disease which affects the lives of millions of patients and their families.
Europe's traditional role as the home to health innovation is nonetheless being seriously challenged by a series of factors (brain drain of European researchers, the fragmentation of the scientific community, attractiveness of other markets, etc). Cancer research is no exception to this trend.
The panelists of this workshop (Figure 1) discussed some of these challenges to cancer innovation and research in Europe according to their personal and professional perspectives and sought to put forward, together with the participants to the event, some solutions.
Figure 1.

The panelists. Back row from left to right: Richard Sullivan, Roger Wilson, Alojz Peterle. Front row from left to right: Luisa Strani (event organiser), Guido Guidi, Georgs Andrejevs, and Julio E. Celis. Courtesy of the European Health Forum Gastein.
2. Workshop
Prof. Julio E. Celis, Chairman of the Policy Committee of the European CanCer Organisation (ECCO) started the workshop with a brief overview of the organisation and description of ECCO's added value in combating cancer. ECCO, which represents over 50,000 professionals in oncology, is a patient centred organisation that strives for the achievement of optimal treatment for each cancer patient (tailored treatments and, potentially in the future, personalised cancer medicines) based on the understanding of the biology underlying the diseases.
Prof. Celis underlined that one of the main challenges to cancer innovation is that while today's pace of research continues to improve our understanding of the biology that underlies cancer, advances that can save lives, extend survival and enhance quality of life, happens only very slowly.This is because “the pathways through which basic discoveries are translated into clinical applications are convoluted and difficult to manage, and in general Europe's effort to fight cancer are fragmented, divided and in many cases ineffective”.
The challenge, therefore, calls for a change in the attitude towards cancer research in Europe (Ringborg, 2008). ECCO together with other organisations believe that in order to bypass this fragmentation, it is necessary to move from regional or national efforts into EU‐wide collaborations by joining forces through the creation of a Platform for Translational Cancer Research, which would bring together Comprehensive Cancer Centres (CCCs) – a facility in which care and prevention is integrated with research and education – and Basic/Pre‐clinical Cancer Centres. These centres would form part of an integrated network with shared resources so as to optimise translational processes, and to support the cancer “Dream Teams” of the future (Brown, 2009).
Such a platform is, according to Prof. Celis (Figure 2), the only possible way to reach the critical mass that is necessary to innovate and deliver in all areas of cancer research. The platform would aim to:
-
■
Harmonise and share infrastructures and competence
-
■
Define and coordinate specific areas for research collaboration
-
■
Improve training and mobility of researchers
-
■
Attract young researchers from all over the world and retain talent in Europe
-
■
Provide the pharmaceutical and biotechnology industries with strategic partnership
-
■
Accelerate translational research and drive innovation in Europe for the benefit of the patient
Figure 2.

Prof. Julio E. Celis, ECCO spoke about the creation of a European platform for translational research that could support the cancer research ‘Dream Teams’ of the future. Courtesy of the European Health Forum Gastein.
Prof. Celis ended his presentation by highlighting specific areas where the EU could contribute to boost cancer research, including:
-
■
Providing world‐class infrastructure to perform state‐of‐the‐art discovery driven translational research
-
■
Ring‐fence support for translational research
-
■
Ensure the long‐term sustainability of programmes
-
■
Provide a legal framework that stimulates innovation and protects IPRs
Dr. Guido Guidi, Head of Region Europe – Novartis Oncology, spoke of the challenges faced by industry in medicine discovery and of the need to ensure that industry's endeavours are affordable and accessible.
Dr. Guidi (Figure 3) stressed the challenges of drug discovery and underlined that only 1/10,000 new molecules make it to the market. All this work implies huge costs for the pharmaceutical industry, which sees its R&D efforts growing, but these being matched or exceeded in revenue only in 3 out of 10 cases (Grabowski et al., 2002).
Figure 3.

Dr. Guido Guidi, Head of Region Europe‐ Novartis Oncology. Courtesy of the European Health Forum Gastein.
After emphasizing industry's challenges as regards to innovation, the speaker also underlined some of the potential ways for Europe to continue boosting innovation and R&D (Figure 4).
Figure 4.
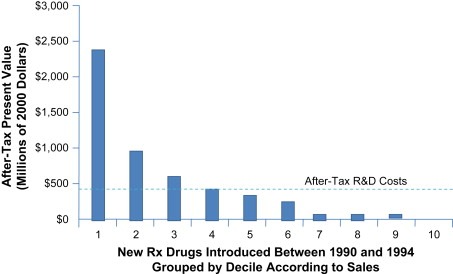
Returns on Research and Development for 1990s New Drug Introductions. Reproduced from “Returns on Research and Development for 1990s New Drug Introductions”. Pharmacoeconomics 2002; 20(Supplement 3): 11–29 with permission of the publisher.
First of all, Dr. Guidi suggested that national regulatory agencies should be involved at an earlier stage in the clinical trials process in order to reduce costs and ensure maximum effectiveness of results and thus focusing pharmaceutical R&D efforts.
Secondly, he underlined that targeted therapies – with Glivec being an example of a success story – represent a major step towards more effective cancer care in the future, but also stressed that access to cancer care should be ensured to all European patients by a favourable political and regulatory framework.
Thirdly, he also acknowledged that the pharmaceutical industry should be more open to work in partnership with other stakeholders. Partnerships are indeed key in order to develop effective cancer medicines.
Dr. Guidi concluded that the EU's role is indeed to boost these partnerships and ensure that, by turning ‘Dream Teams’ to reality, patients are truly at the core of pharmaceutical research efforts.
Mr. Roger Wilson, Founder and Director of Sarcoma UK started his presentation by underlining that cancer patients want innovation in medicine development, in surgery and in radio and chemotherapy (Figure 5).
Figure 5.

Roger Wilson, Sarcoma UK. Courtesy of the European Health Forum Gastein.
He underlined that cancer treatment and new innovation drugs are truly changing, for the better, the lives of cancer patients. In this respect, the role of clinical trials is the key to ensure the development of these new medicines.
Regulators require clinical trials to involve large number of patients, but often such trials are not possible for the rarer cancers. According to the speaker, regulators need to be open to “innovative” approaches to clinical trials and move the focus onto smaller trials which build “a rounded picture of effectiveness in a cumulative way, rather than big trials testing a hypothesis”. He, therefore, called on policy‐makers, regulators and healthcare funders to encourage new thinking in order to get new effective treatments into use more quickly.
Mr Wilson concluded that “each treatment decision is a clinical decision which should be made in the clinic by doctors and patients together, not made in some distant office by politicians, judges or bureaucrats using computer spreadsheets”.
Prof. Georgs Andrejevs, Member of the European Parliament (MEP) and leading member of the informal group ‘MEPs Against Cancer’ (MAC) detailed the European Parliament's role in putting cancer and innovation on the EU agenda. Although healthcare remains the responsibility of member states, the European Parliament has since 2004, urged the European Commission and the Council to put cancer and innovation on their political agendas.
In spite of the general tendency of the EU in recent years to focus on health determinants, rather than on specific diseases, the European Parliament has led the EU in the fight against cancer and finally brought this issue into the EU political focus. This European Parliament's effort concluded in April 2008 with the Resolution of the European Parliament on “combating cancer in an enlarged EU”. The Council picked up on this and issued Conclusions which called, inter alia, upon the EU to boost and coordinate its research efforts against cancer.
Prof. Andrejevs (Figure 6) concluded that thanks to the European Parliament involvement, cancer is now part of the EU health political agenda and the next step will be the adoption of a Cancer Action Plan and the establishment of a Cancer Task Force.
Figure 6.

MEP Prof. Georgs Andrejevs. Courtesy of the European Health Forum Gastein.
Mr. Alojz Peterle, MEP and Co‐Chair of MAC, started his presentation by explaining that, as a cancer survivor, he knows the importance which research and innovation have in the fight against this terrible disease and for the life of millions of patients.
He continued by stressing political innovation is also needed in order to maintain the current momentum and ensure that this disease remains on the agenda of decision‐makers. And indeed the European Parliament is a great place to come up with innovative ideas, because this institution has political weight to urge the EU to act in a certain area.
In light of the above, Mr. Peterle (Figure 7), therefore, called for:
-
■
Political leadership
-
■
Increased collaboration between academia, industry and political and civil society; and
-
■
More efficient frameworks to facilitate the lives of the cancer community.
Figure 7.

Co‐Chair of ‘MEPs Against Cancer’, MEP Alojz Peterle. Courtesy of the European Health Forum Gastein.
3. Debate and conclusions
The debate focused on the role of the EU in the fight against cancer as a whole, how to ensure that political will is turned into action, and how to ensure in practice that all stakeholders engage in boosting innovation and fighting this disease. Some of the issues outlined during the debate are included below.
Alistair Kent from the Genetic Interest Group underlined the patchy record of the EU in general and the European Parliament in particular, in the development of legislation which would boost innovation as shown during the discussions of the dossiers on Advanced Therapies, Clinical trials and animal experimentation.
In response, both Peterle and Andrejevs stressed that the EP is one of the most active institutions where many ideas and initiatives can take form. Unfortunately progressive and innovative ideas are often hampered by the Member States and at the Council level.
As far as practical steps towards boosting innovation in the EU are concerned, Prof. Celis outlined that the EU should take the following steps:
-
■
incentivise and reward researchers adequately;
-
■
improve the grants system (e.g. often limited to a short period of time and without adequate follow‐up);
-
■
improve co‐ordination of activities between the cancer community (take into consideration the multi‐disciplinary aspect of the disease; put together a common vision/strategy; disseminate information, etc).
Some questions were also directed to Prof. Celis relating to practical aspects of the envisaged Platform of Comprehensive Cancer Centres. Prof. Celis replied that today's priority is to, first of all, assess the cancer community's willingness to embark in such a project as well as to identify the right instrument to finance the initiative in a sustainable way.
In response to some comments regarding the “emptiness of political words and lack of effective EU action in terms of boosting innovation and fighting cancer”, Hildrun Sundseth from the European Cancer Patient Coalition highlighted that thanks to EU action, now Member States have guidelines on cancer screening, which did not exist before. In this respect, the role of the EU in leveraging initiatives across all Member States and encouraging the development of best practice cannot be underestimated.
Karl Freese, from the European Commission, also highlighted the positive actions of the EU in combating cancer and boosting innovation but underlined that this is a slow process, whereby results cannot be measured immediately. He described the implementation of cancer screening programmes in Germany as a direct spin‐off of EU's policy and added that the Commission will continue to take cancer and innovation very seriously. He also underlined the need for all stakeholders to work together towards common objectives. This aspect was also reiterated by Mr. Wilson, who stressed the importance of the ‘partnership model’ to boost innovation and fight cancer in the EU.
Richard Sullivan concluded that in promoting cancer research and innovation, collaboration was the key, but that the patients need to be at the centre of future initiatives. The political momentum is right for all stakeholders involved to embark together in “Dream Teams” which will deliver effective and innovative solutions to the fight against cancer.
Andrejevs Georgs, Celis Julio E., Guidi Guido, Peterle Alojz, Sullivan Richard, Wilson Roger, (2009), ‘Tackling cancer in the EU: The role of innovation’, Molecular Oncology, 3, doi: 10.1016/j.molonc.2008.12.003.
This workshop which was organised and sponsored by Novartis Oncology took place on October 2nd, 2008 at the European Health Forum Gastein.
References
- Brown, H. , 2009. Turning the Stockholm Declaration into reality: creating a world-class infrastructure for cancer research in Europe. Mol. Oncol.. 3, 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski, H. , Vernon, J. , DiMasi, J.A. , 2002. Returns on Research and Development for 1990s New Drug Introductions. Pharmacoeconomics. 20, (Supplement 3) 11–29. [DOI] [PubMed] [Google Scholar]
- Ringborg, U. , 2008. The Stockholm Declaration. Mol. Oncol.. 2, 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


