Abstract
Cancer in the 21st century has become the number one cause of death in developed countries. Although much progress has been made in improving patient survival, tumour relapse is one of the important causes of cancer treatment failure. An early observation in the study of cancer was the heterogeneity of tumours. Traditionally, this was explained by a combination of genomic instability of tumours and micro environmental factors leading to diverse phenotypical characteristics. It was assumed that cells in a tumour have an equal capacity to propagate the cancer. This model is currently known as the stochastic model. Recently, the Cancer stem cell model has been proposed to explain the heterogeneity of a tumour and its progression. According to this model, the heterogeneity of tumours is the result of aberrant differentiation of tumour cells into the cells of the tissue the tumour originated from. Tumours were suggested to contain stem cell‐like cells, the cancer stem cells or tumour‐initiating cells, which are uniquely capable of propagating a tumour much like normal stem cells fuel proliferation and differentiation in normal tissue.
In this review we discuss the normal stem cell biology of the stomach and intestine followed by both the stochastic and cancer stem cell models in light of recent findings in the gastric and intestinal systems. The molecular pathways underlying normal and tumourigenic growth have been well studied, and recently the stem cells of the stomach and intestine have been identified. Furthermore, intestinal stem cells were identified as the cells‐of‐origin of colon cancer upon loss of the tumour suppressor APC. Lastly, several studies have proposed the positive identification of a cancer stem cell of human colon cancer.
At the end we compare the cancer stem cell model and the stochastic model. We conclude that clonal evolution of tumour cells resulting from genetic mutations underlies tumour initiation and progression in both cancer models. This implies that at any point during tumour development any tumour cell can revert to a cancer stem cell after having gained a clonal advantage over the original cancer stem cell. Therefore, these models represent two sides of the same coin.
Keywords: Cancer stem cells, Stochastic model, Gastrointestinal cancer, Stem cell, WNT
1. Introduction
Multicellular organisms develop from a single pluripotent embryonic stem cell. This cell has the capacity to differentiate into all cells of the organism while maintaining and expanding a pool of the primitive cell types of the three germ layers. With time, the stem cells loose part of their differentiation potential and become specialized stem cells of the adult organs. Despite the enormous complexity of the process, a fine‐tuned regulatory system keeps the development of the organism in check. However, a lifetime of exposure of the genetic program to internal and external factors leads to the accumulation of mutations. In adult mammals, tissue homeostasis and repair of injured organs depends on small reservoirs of tissue‐specific stem cells. Adult stem cells are defined by their ability for self renewing cell divisions. These divisions defined by two characteristics. First, the cell divisions gives rise to two daughter cells, of which one or both have the exact same proliferative capacity as the parent cell. Secondly, at least one of the daughter cells has the exact same differentiating potential as the parent cell giving rise to cells that can differentiate into one or more specialized cells of the organ (He et al., 2009). To maintain and repair the tissues during the lifespan of the animal, adult stem cells use three different types of cell division: 1) asymmetric divisions, generating one stem cell and one progenitor cell, 2) self‐renewing symmetric division, generating two daughter stem cells to expand the stem cell population, or 3) non self‐renewing symmetric division, resulting in the generation of two progenitors.
The hierarchical organization of adult tissue consisting of stem cells, progenitors and terminally differentiated cells is suggested to have developed to help the organism recover from injury and to slow aging, while on the other hand protecting the cells from accumulating damage that would ultimately lead to cancer. Adult stem cells have evolved a mechanism to lower this risk by restricting their expansion, whereas their immediate offspring, the progenitor cells, strongly expand in number but their limited life span protects them from accumulating mutations (Lobo et al., 2007). Rapidly self‐renewing tissues that harbour actively cycling stem cell populations to maintain tissue homeostasis, such as small intestine and stomach, offer the opportunity to study adult stem cells as well as their relationship with cancer initiation and progression.
Since ancient times, human beings have tried to understand the mechanisms underlying the carcinogenic transformation of a tissue. Indeed, it was the Greek physician Hippocrates (460 BC–370 BC) who named cancer “karkinos”, because, as documented by Galean 400 years later, this disease “has the veins stretched on all sides, as the crab has its feet” (Galen, 1968). Two thousand years later we are still trying to explain the heterogeneity of tumours. Recently, this has lead to the formulation of a new model for tumour development and maintenance, the cancer stem cell or hierarchical model, which challenges the old model that is now described as the stochastic model. In this review, we will discuss these two models together with recent advances in Stem Cells and Cancer of the gastrointestinal system.
2. Stem cells in the gastrointestinal tract
2.1. The intestine
The intestinal tract is responsible for digestion and the uptake of food, and is anatomically subdivided into the small intestine and colon. The inner lining of the intestine is a single layer of epithelium. The intestinal epithelium is the most rapidly renewing tissue in our body (Heath, 1996). The epithelium of the small intestine is shaped into small pits called Crypts, which are located in between Villi protruding into the lumen of the small intestine. The colon comprises crypts similar to the small intestine but the differentiated epithelium forms a flat surface epithelium (Figure 1).
Figure 1.
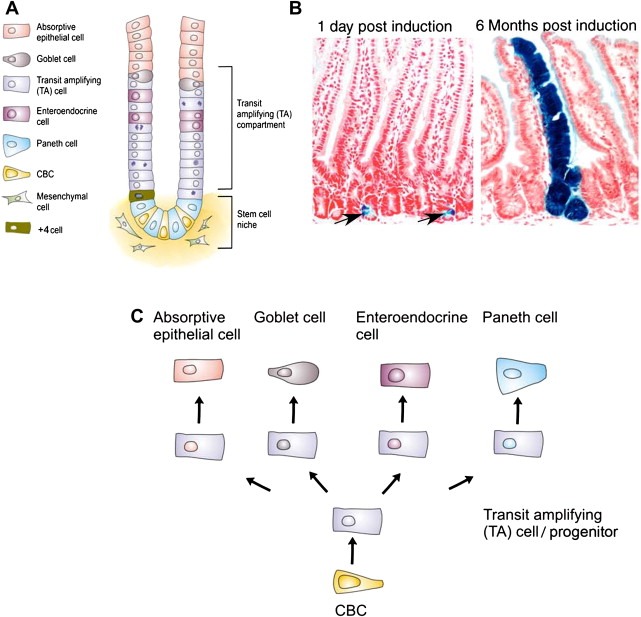
Anatomy of the intestine. A) Schematic representation of the crypt‐villus axis. B) Lineage tracing experiments demonstrating that LGR5 cells are the stem cells of the intestine. Blue ribbons show the expression of LacZ from the Rosa26LacZ locus upon induction of CRE enzyme with tamoxifen, on the LGR5EGFP‐IRES‐CREert/Rosa26R mouse. After one day single CBC cells are induced. Six months later, entire blue ribbons indicate that the CBC cells that had an activated Rosa26LacZ are still producing all the cells of the intestinal epithelium. C) Differentiation hierarchy of the small intestine.
Cell renewal is fuelled by stem cells residing in the crypts. The stem cells produce proliferative progeny called transient amplifying (TA) cells or progenitors. The dividing TA cells concomitantly migrate up the crypts. When they reach the villi they undergo terminal differentiation to form the various functional epithelial cell types. These cells, enterocytes, goblet cells, and enteroendocrine cells, continue migrating upwards along the villus until they reach the villus tip, where they undergo apoptosis and are shed into the lumen of the intestine. A fourth cell‐type, called the Paneth cell, evades this upward migration and instead, differentiates as it migrates to the very bottom of the crypt. With the exception of the Paneth cells, which can live for up to 6 weeks, the entire epithelium of the small intestine is completely renewed every 5 days (Figure 1A). The developmental hierarchy of the colon essentially resembles that of the small intestine, with the most notable exception being the absence of the Paneth cells.
2.1.1. Intestinal self‐renewal: the Wnt pathway
The Wnt pathway is one of the main pathways regulating epithelial self‐renewal in the intestine (Clevers, 2006). Loss of Wnt signalling in vivo effectively blocks cell proliferation in the intestinal crypts, destroying the epithelium (Korinek et al., 1998). The β‐catenin/TCF transcription factor complex is the molecular effector of the Wnt pathway. In the absence of a Wnt signal, a protein complex comprising Axin, Adenomatous Polyposis Coli (APC) and Glycogen Synthase Kinase 3B (GSK3B) is activated and binds β‐catenin. Subsequently, β‐catenin is phosphorylated at several N‐terminal serine/threonine residues. Once phosphorylated, β‐catenin is targeted for degradation by the proteosome. In the absence of β‐catenin, TCF transcription factors bound to target gene enhancers/promoters in the nucleus form a transcriptional repressor complex with Groucho proteins and silence the Wnt target genes. In the presence of the Wnt signal, the destruction complex is deactivated, β‐catenin consequently accumulates in the cytoplasm and translocates to the nucleus, where it forms an active transcription complex with the TCF/LEF transcription factor family.
The activity of the Wnt pathway, in conjunction with other pathways such as Notch and BMP, is vital for the proper organisation of the epithelium as well as proliferation and stem cell self‐renewal. A Wnt signalling gradient exists along the crypt‐villus axis. When cells migrate away from the Wnt source at the base of the crypt, they progressively loose their proliferative capacity and differentiate. Wnt is also required for the expression of cell surface receptors and their ligands involved in the organisation of the epithelium as exemplified by the EphB/EphrinB receptors and ligands, which dictate the position of the different cells along the crypt‐villus axis of the epithelium (Batlle et al., 2002).
2.1.2. Stem cells of the intestine
The identity of the intestinal stem cells has been fiercely debated over the last 50 years, with two competing models dominating the literature. Historically, the crypts were thought to comprise a population of terminally differentiated Paneth cells at the base. At the boundary of the Paneth cells and the TA cell compartment, a stem cell was assumed to reside at the so called +4 position. The stem cell candidate at the +4 position was subsequently found to be both radiosensitive and label‐retaining, two qualifications ascribed to stem cells (Potten et al., 1974). Furthermore, the expression of Bmi1, a gene thought to be involved in stem cell maintenance, was shown to be elevated in the +4 cell (Sangiorgi and Capecchi, 2008) (Figure 1A). Alternatively, Leblond identified small cycling cells, interspersed with the Paneth cells at the crypt base, which were named Crypt Base Columnar (CBC) cells (Bjerknes and Cheng, 1981; Cheng and Leblond, 1974). Because of technical constraints there has never been conclusive evidence concerning the true identity of the stem cell. The major obstacle for the identification of the stem cell has been the lack of unique molecular markers. Therefore, assumed functional properties, which may or may not apply to stem cells, such as label‐retention, have been used for their identification.
Because the Wnt pathway was shown to be the driving force behind proliferation in the crypts, we studied the transcriptional targets of the pathway with the aim of identifying potential stem cell‐specific genes (van de Wetering et al., 2002). Most Wnt targets were expressed throughout the crypt, reflecting the role of Wnt signalling in both regulation of cell proliferation in the TA compartment as well as driving Paneth cell differentiation at the crypt base. However, a small group of genes were expressed in a more restricted fashion at the crypt base (Van der Flier et al., 2007). One of these genes, Lgr5 (Leucine‐rich repeat containing G protein‐coupled receptor), encoding an orphan G‐protein coupled receptor, was uniquely expressed in the CBC cells, previously suggested to be stem cells.
In order to determine if these Lgr5 expressing cells are stem cells we used knock‐in mouse models to document endogenous Lgr5 expression and functionally evaluate their stem cell potential in‐vivo. First, the expression of Lgr5 in the CBC was confirmed using a knock in allele of LacZ (Lgr5LacZ) as well as knock in of an EGFP (Lgr5EGFP‐ires‐CreERT2) expressing construct in the Lgr5 locus. Indeed Lgr5 was specifically expressed in the CBC cells (Figure 1A). Subsequently, the LGR5EGFP‐ires‐CreERT2 mouse was crossed with a CRE‐activatable Rosa26LacZ reporter mouse strain. From the Lgr5 locus, this mouse expressed both EGFP as well as a tamoxifen inducible form of CRE. Activation of CRE by tamoxifen results in the removal of a roadblock from the Rosa locus resulting in the irreversible activation of LacZ in Lgr5 expressing cells as well as their offspring. This lineage trace experiment demonstrated that indeed LGR5 is expressed in the stem cell of the intestine. The cells identified retain their ability to generate all cell types of the intestine for the lifetime of the mouse, while dividing approximately once a day (Barker et al., 2007) (Figure 1B and C). Recently, CD133 was suggested to be expressed on the CBC cells as well, and lineage trace experiments with CD133 also indicated that CBC cells are stem cells (Zhu et al., 2009).
2.2. The stomach
The stomach is divided into two anatomically distinct areas: the corpus (main body of the stomach) and the pyloric‐antrum (area close to the intestine). The corpus is responsible for the digestive functions of the stomach, by secreting hydrochloric acid and zymogens, whereas the pyloric‐antrum mainly produces mucus and gastric hormones. In both areas, the epithelium is comprised of tubular‐shaped mucosal invaginations known as gastric units. Each gastric unit is divided into two parts: 1) the pit, lined by mucus‐ secreting cells and 2) the gland, composed of various cell‐types located within three distinct regions denoted the isthmus, the neck and the base (Figure 2A).
Figure 2.
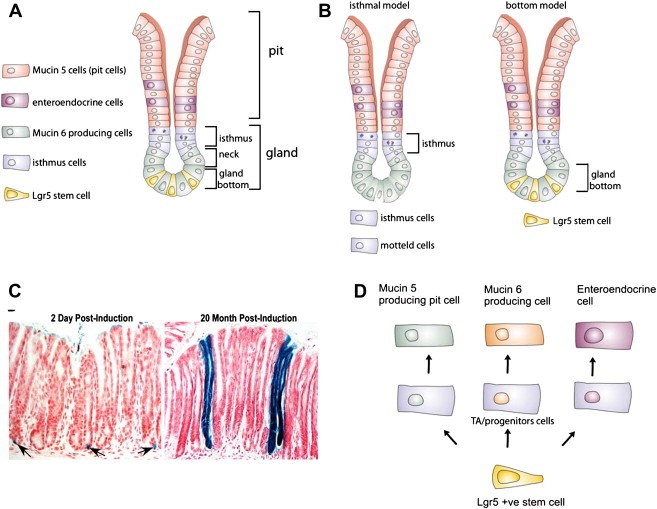
Anatomy of the stomach. A) Schematic representation of a gastric unit. B) Position of the proposed stem cells in a gastric unit. C) Lineage tracing experiments demonstrating that the LGR5 cells are stem cells of the stomach antrum. Blue ribbons show the expression of LacZ from the Rosa26LacZ locus upon induction of CRE enzyme with tamoxifen, on the LGR5EGFP‐IRES‐CREert/Rosa26R mouse. After two days single LGR5 cells are induced. Twenty months later entire blue ribbons are still present, indicating that the LGR5 stem cells are still producing all the cells of the intestinal epithelium. C) Differentiation hierarchy of the small intestine.
The cellular composition of these gastric units varies depending on the anatomical region of the stomach (Lee et al., 1982). In the corpus, the gastric units are formed by (1) surface mucous cells also known as pit cells (2) Parietal cells or acid secreting cells (3) chief cells, which contain zymogen granules and secrete the enzyme pepsinogen and (4) endocrine or hormone producing cells (e.g. Somatostatin, Histamine, and Leptin) (Karam and Leblond, 1993). In contrast, in the pyloric‐antrum, the gastric unit composition is more simple, mainly consisting of mucous cells, secreting protective gastric mucin (MUC5AC), enteroendocrine cells (Gastrin and Somatostatin), and occasional Parietal cells (Lee and Leblond, 1985) (Figure 2A).
2.2.1. The gastric stem cell, two schools of thought
The gastric epithelium, like the intestinal epithelium, is continuously undergoing cell renewal. The study of adult mouse gastric epithelial turnover using tritiated thymidine labelling showed that, with the exception of the parietal and chief cells, the label‐retaining cells in the gland of the gastric units migrate towards the surface of the epithelium (Creamer et al., 1961) (Karam and Leblond, 1993). At birth, gastric units in both the corpus and pylorus are polyclonal. In contrast, X‐chromosome inactivation and chemical mutagenesis studies have shown that 90–95% of the gastric units in the pyloric and corpus become monoclonal during adulthood. This indicates that the gastric units are derived from a single multipotent stem cell (McDonald et al., 2008; Nomura et al., 1998a; Tatematsu et al., 1994; Thompson et al., 1990).
The first functional evidence for the existence of these multipotent stem cells in the adult mouse gastric epithelium was provided by Bjerknes and Cheng in 2002 (Bjerknes and Cheng, 2002). The authors took advantage of the ubiquitous expression of the LacZ allele in the ROSA26LacZ mice to induce, by chemical random mutagenesis, a loss of gene expression at low frequency in the gastric epithelium of adult hemizygous mice. At later time points, LacZ negative clones within the epithelium were found to contain all four major gastric cell lineages, consistent with the notion that they derived from a common precursor, the multipotent adult stomach stem cell. However, since the initial mutation event leading to loss of reporter gene activity in this model occurred at random, the identity of the stem cell was not revealed.
The identification and localization of the adult gastric stem cell has been hampered by the lack of specific markers. The use of surrogate markers and indirect lineage tracing approaches has led to the evolution of two opposing schools of thought on the identity of the adult stem cells. Historically, electron microscopy and 3H‐thymidine radio autography revealed the existence of an undifferentiated, granule‐free cell located in the isthmus region of the corpus units. It was shown that in the corpus, these cells are ultrastructurally characterized by a high nucleus‐to‐cytoplasm ratio, a lack of secretory granules, few small mitochondria and many free ribosomes (Karam and Leblond, 1993). These granule‐free cells were proposed to act as multipotent stem cells giving rise to progenitors of pit, parietal, and neck cells, which in turn, differentiate into their respective cell lineages.
Along the same line, in the isthmus of the antro‐pyloric units, 3H‐thymidine radio autography revealed the existence of undifferentiated mottled‐granule cells. It was proposed that these mottled‐granule cells would undergo clonal expansion, giving rise to a group of cells that, following several divisions, become committed to differentiate into dense‐granule cells (pit progenitor) and core granule cells (gland progenitors), the precursors of the corresponding lineages (Figure 2B). According to this model, the stem cells located in the isthmus would proliferate and their immediate progeny then differentiate within the isthmus while migrating bi‐directionally apically and basally towards the pit and the gland respectively (Karam, 1999; Karam and Leblond, 1993; Lee and Leblond, 1985).
Using these morphological criteria, the corpus granule free cell population has been isolated using Laser Capture microdissection and the genetic profile of these cells analysed. This expression profile, which was markedly different from the differentiated parietal and zymogenic lineages revealed high expression of genes regulating insulin‐like growth factor (IGF) signalling, proteosomal degradation, RNA processing and localization, as well as genes involved in the Wnt and GPCR signalling pathways. Indeed, this genetic profile resembles that of the mouse hematopoietic stem cells and embryonic stem cells, highlighting the immature/progenitor nature of granule‐free cells. In these studies, however, pyloric isthmus cells were not profiled (Giannakis et al., 2006; Mills et al., 2002).
More recently we applied to the stomach, the same strategy used to identify the stem cell of the small intestine (Barker et al., 2010). We showed that Lgr5 is expressed in a specific population of cells located at the very bottom of the pyloric gastric units. Three dimensional reconstruction of isolated gastric glands revealed that 3–4 Lgr5 expressing cell are exclusively located at the very bottom of the gastric units (Figure 2A and B). The use of transmission electron microscopy combined with cryo‐immunogold labelling showed that Lgr5 cells present classical features of immature cells such as limited basal rough endoplasmic reticulum, a large, centrally located nucleus, and apical microvilli. More mature cells with abundant apical granules occupied the positions just above the Lgr5 cell zone. Lgr5 cells were completely absent from the isthmus region, where the mottled‐granule cells are located.
To test the stemness of these Lgr5 expressing cells in the stomach antrum, lineage tracing experiments were conducted in Lgr5EGFP‐ires‐CreERT2/Rosa26R LacZ reporter mice. This demonstrated that LGR5 expressing cells were cycling adult stem cells able to produce the different cell lineages of the antro‐pyloric units, formally proving that Lgr5‐marked cells are multipotent adult stem cells in the stomach antrum (Figure 2C and D). The genetic profile of these cells is characterized by the expression of several Wnt target genes, whereas differentiated endocrine or mucin expressing genes are absent. Furthermore, in our recently developed gastric culture system, only Lgr5 cell derived cultures are able to self renew and differentiate in vitro, when grown from single Lgr5‐stem cells. Lgr5 expression is absent from the adult corpus, implying the existence of a Lgr5 negative stem cell population in this region. However, neonatal Lgr5 stem cells are responsible for setting up the mature corpus epithelium, including the Lgr5 negative stem cells responsible for maintaining the homeostasis of the adult corpus.
Deborah Gumucio and co‐workers have described the presence of rare villin expressing stem/progenitor cells in the pyloric region of a villin transgenic mouse (Qiao et al., 2007). Although villin is normally not expressed in the stomach, the 12.4‐Kb villin‐1 promoter/enhancer fragment used in their study marks a rare population of Gastric Progenitor cells at or below the isthmus of antral glands. While normally quiescent, these cells are able to divide in response to inflammatory signals. In these mice, the administration of the cytokine INFγ, reactivates these gastric stem cells, generating entire gastric units harbouring all the epithelial lineages of the antral glands (Qiao et al., 2007).
These studies suggest that the antral glands posses two different stem cells: a self‐renewing Lgr5 stem cell, that is continuously dividing and contributing to the daily renewal of the antral gastric epithelia, and a quiescent stem cell, that can contribute to the expansion of the gastric units following inflammatory injury. In our lineage tracing experiments using the Lgr5EGFP‐ires‐CreERT2/Rosa26R‐LacZ reporter mice, the induction of LacZ expression was detected in all the cells of the glands, including the quiescent stem cell reported by D. Gumucio and colleagues. This suggests that the quiescent stem cell is a descendent of the Lgr5 stem cell that has lost Lgr5 expression and cycling potential while retaining its stemness. Consequently, we can speculate that upon injury, the quiescent stem cell replaces the damaged tissue by forming Lgr5‐expressing cycling stem cells.
It will be interesting to see whether the Lgr5 stem cells and quiescent stem cells contribute to the tissue under distinct circumstances or whether they contribute to tissue homeostasis together at the same time. In the first situation Lgr5‐marked stem cells form tissue during homeostasis while the quiescent stem cell becomes activated in the tissue after injury. Alternatively, descendents of the Lgr5 stem cell are kept aside as quiescent stem cells to provide healthy new Lgr5 cycling stem cells after injury, but also to replace damaged or old Lgr5 stem cells during normal homeostasis. Of note is that from embryonic development all cells of the stomach are descendants of Lgr5‐expressing stomach stem cells.
3. Cancer initiation and progression
The self‐renewing capacity of stem cells and proliferative capacity of stem cells and progenitors are critical for an organism to heal after injury, for slowing the process of aging, as well as for protecting tissue from damage by the quick turnover of cells. Although this ability is essential to sustain the organism, it also poses serious risks. Genetic and or epigenetic changes can result in cells losing control over their proliferation resulting in tumour growth. In order for a cell to become cancerous it needs to acquire a series of cellular alterations: self‐sufficiency in growth signals, insensitivity to growth‐inhibitory signals, evasion of programmed cell death, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis (Hanahan and Weinberg, 2000). Observations of human cancers and animal models argue that tumour development proceeds via a process where each successive genetic change confers one or another type of growth advantage to the cells of the tumour. This genetic evolution of the cancer cell will cause the tumour to progress through different stages.
Important new insights have developed recently regarding two fundamental questions about tumour development: 1) which cell acquires the genetic alterations and becomes the first tumour cell (cell of origin)? 2) Which cells are responsible for propagating the tumour after its initiation? Two models have been suggested to explain the tumour progression, the stochastic model and the Cancer Stem Cell (CSC) model or hierarchical model (Lobo et al., 2007) (Figure 3). It is important to distinguish the cell of origin from the CSC. The cell of origin could be any cell type, e.g. a stem cell, a progenitor, or a differentiated cell (Figure 4). Once a cell acquires a mutation that affects its normal lifecycle and becomes the cell of origin, its cancerous development would follow according to the CSC or stochastic model. In other words even if the stem cell is the starting point of the tumour (cell of origin), it can form a tumour that adheres to the properties of the CSC or the stochastic model.
Figure 3.
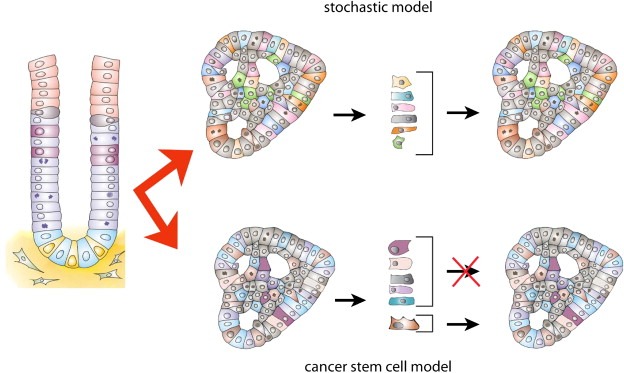
Comparison of the stochastic and the cancer stem cell model. In the stochastic model all cells have an equal probability to propagate the tumour. In the cancer stem cell model a unique population of CSC has the potential to propagate the tumour.
Figure 4.
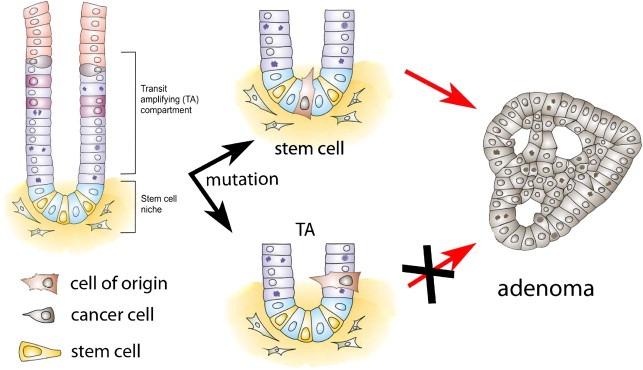
Tumour initiation by the cell of origin. Recently, it was shown that adenomas induced by loss of APC originate from the normal stem cells of the intestine, not the progenitors or differentiated cells. LGR5 expressing stem cells are the cells of origin.
Until approximately 15 years ago, tumours were viewed according to the principles of the stochastic model. The most important characteristic of this model is that all cells of the tumour have equal ability to propagate the tumour by means of proliferation (Figure 3). The morphological heterogeneity observed when studying tumours is explained by genetic instability of the tumour cells, causing tumour cells to follow different aberrant differentiation pathways as well as cell intrinsic and extrinsic processes associated with tumour development, such as angiogenesis or Epithelial Mesenchymal Transitions (EMT). The main therapeutic consequence of this model is that in order to successfully treat a tumour, all of its cells need to be removed because all will be equally able to cause a relapse after therapy.
In the last two decades the CSC model has put tumour heterogeneity in a new light. According to the CSC model, the tumour is viewed as an entity that can be studied applying the principles of stem cell biology. Thus, tumour cells maintain a hierarchical organization consisting of a population of self renewing cells at the bottom of the hierarchy which will give rise to its progeny: 1) More CSC through self renewing cell divisions 2) Progenitor cells with limited proliferative potential 3) (Aberrantly) differentiated cells with no proliferative potential (Figure 3). The clinical implication of this model is that by removing the CSC the tumour will no longer be capable of growing. So, instead of focussing on the reduction of the bulk of the tumour, specific therapies targeting the CSC will be required to eradicate the tumour (He et al., 2009). Here we will summarize the evidence regarding these models in the gastrointestinal system.
3.1. Cancer of the intestine
Colorectal cancer is one of the leading cancer related deaths in the world (Jemal et al., 2006). Progression of colorectal cancer has been extensively described. It starts with the formation of small lesions called aberrant crypt foci (ACF), which expand giving rise to adenomas that progress into carcinoma in situ to finally end up as an invasive adenocarcinoma (Sancho et al., 2004).
The vast majority of colorectal cancer is caused by mutations in key components of the Wnt pathway. The mutations are either inactivating mutations of the tumour suppressor genes APC or Axin2, or activating mutations of the oncogene β‐Catenin. Both cases result in nuclear localization of β‐Catenin and the constitutive activation of the Wnt target genes (Fodde and Brabletz, 2007). The progression of adenomas into carcinoma in situ is suggested to depend on a strict sequence of events, where the mutations in the Wnt pathway are followed by mutations in KRAS, SMAD4 and lastly p53 (Fearon and Vogelstein, 1990).
Genomic instability is one of the important characteristics of late stage colon cancer. The role of genomic instability in tumour progression has been the subject of many studies, but at which stage of tumour progression it is playing a role remains largely elusive. On the one hand, it was proposed that the progression of a carcinoma to an invasive cancer is a consequence of increasing genomic instability (Soreide et al., 2006). Alternatively, it has been suggested that genomic instability could play a role at an initial stage of tumourigenesis and be involved in tumour progression immediately as a consequence of the initiating APC mutation (Fodde et al., 2001).
Additional genetic alterations that are thought to be involved in the progression of colon cancer include the loss of positional clues. In the normal epithelium the Wnt gradient establishes a border between the crypt and villus. This gradient also controls the expression of EphB/EphrinB receptors and ligands responsible for positional migration of the intestinal cells. Progression of colon carcinogenesis is accompanied by EphB2 down regulation required for adenomas to progress to carcinomas by freeing themselves of their positional constrains (Batlle et al., 2005).
3.1.1. Cell of origin of colon cancer
One of the important questions of cancer development concerns the identity of the cell of origin, which sustains the mutation that will lead to the first step in the formation of a cancerous cell. Stem cells and their dividing progeny appear to be the most likely candidates as they already have the proliferative capacity seen in a tumour. However, on the basis of histology, it was suggested that colon cancer might arise from late progenitors or even an early differentiated cell (Shih et al., 2001). Of note, in the hematopoietic system both the HSC as well as early progenitors were shown to be capable of initiating tumours (Bonnet and Dick, 1997; Perez‐Caro et al., 2009).
The identification of specific genes expressed in the stem cells of the intestine has recently allowed our lab and others to formally show that the cell of origin of adenomas, induced by constituently active Wnt signaling, is the stem cell of the small intestine (Barker et al., 2009; Zhu et al., 2009). Both studies made use of CRE inducible activation of the Wnt pathway, with the CRE being expressed from either the endogenous LGR5 or the CD133 locus of these stem cell marker genes. It was demonstrated that both LGR5 and CD133 induced activation of the Wnt pathway leads to efficient tumour formation. Importantly, Barker and colleagues also showed that tumour formation does not occur when the loss of APC is induced in progenitors or differentiated cells. These results show that the cell of origin of adenomas in the small intestine is the stem cell. It remains to be seen whether LGR5‐expressing cells or CD133‐expressing cells of the tumour are exclusively capable of causing tumour progression and therefore are markers of the intestinal CSC.
3.1.2. CSCs of the intestine
As had been shown for other tumour models, in 2007, several groups proposed the cell marker CD133 as a marker of the cells that were uniquely capable of initiating tumour growth in immunodeficient NOD/SCID mice (O'Brien et al., 2007; Ricci‐Vitiani et al., 2007). Later, CD44+/CD166+ cells that do not express the CD133 marker were also identified as a subgroup of human colorectal cancer cells uniquely capable of inducing tumours in mice (Dalerba et al., 2007). In both cases, the tumours generated in mice phenocopied the colorectal cancers isolated from human patients.
Additional support for the cancer stem cell model came from the observation that CSC are present in metastasis of human colon cancer. Genetic analysis demonstrated that these cells had clonally derived from the parental tumour after engrafting in mice. Additional mutations distinguished them from the original cancer cells, indicating that the tumour cells that propagated the tumour had undergone further mutagenesis compared to the parental tumour (Odoux et al., 2008). These data suggest that human colon cancer indeed harbours a population of cells with cancer stem cell characteristics.
The frequency of these cells differs significantly between studies. One reason might be that, at least for CD133, different antibodies recognize different forms of glycosylated CD133 and therefore different cells are isolated. Whereas CD133 was initially suggested to be a specific marker of stem cells (Zhu et al., 2009), it was recently shown that, at least in the intestine, CD133 is expressed on all stem cells and progenitors, suggesting it does not represent a small subpopulation of cells (Shmelkov et al., 2008; Snippert et al., 2009).
Recently, another interesting observation on CSC was their regulation by the microenvironment. In this study, a group of cells was identified that had CSC properties that depended on their surrounding microenvironment, just as normal stem cells. More importantly, cells without CSC properties were demonstrated to gain CSC properties when subjected to a specific set of factors (Vermeulen et al., 2010). It had previous been shown that not all cells within an APC induced tumour have equal Wnt pathway activity (Brabletz et al., 2001; Fodde and Brabletz, 2007). Taken together these studies demonstrate that tumours do not only possess great heterogeneity, but that CSC properties within a tumour can be gained or lost depending on the microenvironment.
3.2. Gastric cancer
Gastric cancer is the fourth most common cancer and the second highest cause of cancer‐related mortality (1 million deaths per year) worldwide (Parkin et al., 2001). Histological analysis has classified gastric cancer as intestinal and diffuse gastric cancer. Macroscopically, the intestinal‐type cancer is characterized by the presence of intestinal metaplastic lesions most commonly arising at the antrum region (Teir, 1961). In addition to intestinal metaplasia, spasmolytic peptide metaplasia (SPEM), characterized by the presence of antral‐type glands within the corpus, has also been described as a precursor lesion of gastric cancer (Correa and Houghton, 2007). The dynamics of the precancerous process has been very well defined, involving a transition from normal mucosa to chronic superficial gastritis, atrophic gastritis, and intestinal metaplasia which then leads to final dysplasia and gastric adenocarcinoma. Relatively little is known about the key genetic events involved. It has been shown that a gastric cancer evolves as a polyclonal lesion probably due to genomic instability, p53 deletion and telomerase activation (Nomura et al., 1998b).
Gastric cancer has been clearly associated with chronic inflammation (Taketo, 2006). The expression of Cyclooxygenase‐2 (COX‐2), a rate‐limiting enzyme for prostaglandin biosynthesis, is induced during inflammation and triggers the induction of proinflammatory prostaglandin, PGE(2). The COX‐2/PGE(2) pathway plays a key role in gastric tumourigenesis. Transgenic mice expressing COX‐2 and mPGES‐1 in gastric epithelial cells develop hyperplastic lesions in the glandular stomach (Oshima et al., 2004). Also, TNFα dependent inflammation has been associated with SPEM development in mouse models (Oshima et al., 2005).
One of the major causes of gastric chronic inflammation in humans is Helicobacter Pylori infection. Despite the fact that Helicobacter pylori colonizes the human stomach for many decades without adverse consequences, the presence of H. pylori is associated with an increased risk of several diseases, including peptic ulcers, noncardia gastric adenocarcinoma, and gastric mucosa associated lymphoid tissue (MALT) lymphoma (Suerbaum and Michetti, 2002). It has been shown that H. Pylori induces COX2 and microsomal prostaglandin E synthase (mPGES)‐1 in the gastric mucosa (Oshima et al., 2004; Sung et al., 2000). Furthermore, Giannakis et al. have identified H. pylori located in the surface of human gastric progenitor cells (Giannakis et al., 2008).
3.2.1. Gastric cancer stem cell and gastric cancer cell of origin
The canonical Wnt/beta‐catenin signalling pathway plays an important role in development and maintenance of the gastric epithelia, mainly balancing the ratio between stemness, proliferation, and differentiation (Katoh, 2007; Lickert et al., 2001). Aberrant Wnt signalling has been implicated in human and mouse gastric adenocarcinoma. In humans, gastric cancer risk is 10 times increased in patients carrying APC germ‐line mutations (Offerhaus et al., 1992). Similarly, human gastric cancer patients show nuclear β‐Catenin accumulation (Clements et al., 2002; Nakatsuru et al., 1992; Park et al., 1999) as well as aberrant methylation of key components of the Wnt signalling pathway regulation. Furthermore, in the mouse model for Familial Adenomatous Polyposis (FAP) that carries a germ‐line mutation on Apc (ApcMin/+ mouse), mice spontaneously develop multiple tumours in the stomach (Tomita et al., 2007). Additionally, it has been shown that the induction of Wnt pathway cooperates with inflammatory signalling pathways to contribute to tumour development in the mouse gastric mucosa (Oshima et al., 2006). Recent studies indicate that Helicobacter infection would trigger the activated macrophages to express TNFα, which stimulates the surrounding epithelial cells to promote Wnt signalling (Oguma et al., 2008). Similarly to the intestine, an important unanswered question was which cell is responsible for the initiation (cell of origin) of the gastric tumour. We recently reported that deletion of APC, specifically in the gastric Lgr5 stem cells, leads to efficient adenoma formation in the stomach, suggesting that, as in the small intestine, the Lgr5 expressing stem cell is the cell of origin of APC driven adenomas in the stomach. The Lgr5 stem cell marker is expressed on a subpopulation of the cells throughout the resulting adenomas, however, the tumour load in the intestine prevented further analysis of tumour progression (Barker et al., 2010). These results demonstrate that gastric stem cells, when acquiring a mutation, initiate tumourigenesis in the gastric epithelia, and may be the explanation for the increased risk of gastric cancer in FAP patients carrying APC germ‐line mutations.
On the other hand, an alternative source for gastric cancer has been described. Chronic Helicobacter infection following complete destruction of the gastric epithelia by lethal irradiation induces repopulation of the stomach with bone marrow‐derived cells (BMDC). Subsequently, these cells progress through metaplasia and dysplasia to intraepithelial cancer. This progression, though, does not occur upon acute injury, acute inflammation or transient parietal cell loss (Houghton et al., 2004), indicating that BMDC could be a source of gastric cancer cells under situations of chronic severe injury, where tissue stem cells might be unable to regenerate.
As for other cancers, studies have indicated that gastric cancer might contain CSCs. The gastric CSC was identified using the CD44 cellular marker (Takaishi et al., 2009). In this study, CD44 positive gastric cancer cell lines, implanted into the stomach and skin of immunodeficient mice, induced the formation of tumours harbouring CD44+ cancer cells as well as a population of cells that no longer expressed CD44, suggesting the existence of CSC in the gastric cancer.
4. Discussion
Great progress has been made in last half century in our understanding of the molecular mechanisms that lead to cancer. However, cancer still remains a major cause of death in persons under 85 years in the USA (Jemal et al., 2006). Current therapy often results in a strong reduction of overall tumour size but, with time, most tumours relapse and become unresponsive to the therapies. The CSC theory developed from the observation that tumour cells are organized in subpopulations with specific phenotypical characteristics. This observation suggested that a tumour has retained much more of the normal hierarchical organization of its tissue of origin than previously assumed. Because normal tissue growth is fuelled by stem cells, it was postulated that tumours have a similar organization in which a cancer stem cell is responsible for tumour expansion. A consequence of this idea is that in order to eradicate a tumour it is necessary to target the cancer stem cell. The presence of CSCs would be an explanation for why drug or radiation therapy often does not result in curing the disease. While therapy might efficiently remove the bulk of the tumour, a small remaining subgroup of CSC will relapse and the cancer will return. In the last 15 years this idea has been shown to possess much merit, however, questions have recently arisen regarding how applicable this model is.
4.1. Interpretation of the xenograft assay
The first concern with the xenograft assay arose on how cells are defined as CSC. The proof for the existence and identification of cancer stem cell relies heavily on xenotransplantation assays. There are, however, several serious biological constraints which make the interpretation of these experiments difficult. Firstly, for the transplantation of human cancer cells, immunodeficient mice are used. Therefore, the cancer stem cell is the cell that grows best in the absence of an immune system. Secondly, for growing, cancer cells rely on the interaction with soluble and membrane factors of their surrounding tissue. Human cells do not necessarily respond to the same growth factors as mouse cells, and human cells might not have the required receptors to interact in the mouse environment. When these issues (the immune system and relevant growth factors) were addressed, it was observed that the frequency of CSCs greatly increases to possibly more than 25% of all tumour cells (Kelly et al., 2007; Quintana et al., 2008; Shmelkov et al., 2008). Nevertheless, these studies show that only a subset of these cells were capable of propagating the tumour.
4.2. Clonal evolution of tumour cells
An important characteristic of biological expansion of genes, cells, and organisms is the gain of a growth advantage by competing with rivals by genetic mutations. Cancer is a disease that is caused by this fundamental characteristic of genetic mutations. When we compare the stochastic model, where all cells have equal potential to propagate a tumour, to the CSC model, the principle of clonal evolution in cancer initiation as well as progression holds true for both the stochastic as well as the CSC models. In both models, a normal cell gained the ability to proliferate with disregard for its normal surroundings and developmental role in the tissue. The cell that sustained the genetic ability for tumourigenic growth did so without losing, or by gaining, the ability for unlimited proliferative capacity (Shackleton et al., 2009).
Both, data and intuition suggest that cells that resemble normal stem cells already possess many of the above mentioned traits. Therefore, it is likely that these cells will more readily be the target of successful tumourigenic mutations than committed progenitors or differentiated cells. As shown by several groups and described in this review, the cell of origin indeed appears to be a normal stem cell or early progenitor. Furthermore, studies on cancer stem cells have definitively shown that the mutated cells that form a tumour still adhere to a differentiation hierarchy resembling that of the normal tissue, explaining, at least in part, the heterogeneity of tumours. It follows that, at any given time, a tumour contains cell populations with a different potential to proliferate or self renew. When isolated for study, some cells will therefore proliferate more readily than others. However, at the same time, all cells in the tumour are subject to both intrinsic mutagenesis, primarily due to increasing genomic instability, as well as extrinsic mutagens. Within a tumour, all cells that receive additional mutations can therefore serve as the next cell of origin in tumour propagation. This implies that what may be the CSC that propagated the tumour at the start, might no longer be the CSC after subsequent rounds of mutagenic assaults. Of important relevance in this regard is the clinical treatment of cancer, which by selectively destroying responsive tumour cells, generates a selective pressure for a resistant cell to arise. As a result, the cell that initially had the upper hand in tumour progression might be at a disadvantage, whereas a cell previously unlikely to grow in the presence of its competitors, now develops a growth advantage. In this context it is remarkable that, with only minor but specific genetic alterations, differentiated cells are reshaped into induced pluripotent cells (Okita et al., 2007; Takahashi et al., 2007).
Comparing the two models in the context of continuing genetic mutations and selective pressure, one can reach the conclusion that the two models may not be very different. Study of the CSC has shown that, within a tumour at any given time, significant heterogeneity exists in terms of the capacity of subpopulations of cells to propagate the tumour. However, in terms of clinical treatment, the power of clonal selection might make the distinction between the different populations less useful. We would first need to identify the cancerous cells that are still capable of undergoing mutations, or the cell of origin within a tumour, before we identify a population of cells that is uniquely capable of progressing tumour growth. All of these cells need to be eradicated to prevent a tumour from relapsing after treatment.
Acknowledgements
We would like to thank T. Mahmoudi and N. Barker for carefully reading the manuscript and S. Bartfeld for her help with making the figures.
Vries Robert G.J., Huch Meritxell, Clevers Hans, (2010), Stem cells and cancer of the stomach and intestine, Molecular Oncology, 4, doi: 10.1016/j.molonc.2010.05.001.
References
- Barker, N. , Huch, M. , Kujala, P. , van de Wetering, M. , Snippert, H.J. , van Es, J.H. , Sato, T. , Stange, D.E. , Begthel, H. , van den Born, M. , Danenberg, E. , van den Brink, S. , Korving, J. , Abo, A. , Peters, P.J. , Wright, N. , Poulsom, R. , Clevers, H. , 2010. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6, 25–36. [DOI] [PubMed] [Google Scholar]
- Barker, N. , Ridgway, R.A. , van Es, J.H. , van de Wetering, M. , Begthel, H. , van den Born, M. , Danenberg, E. , Clarke, A.R. , Sansom, O.J. , Clevers, H. , 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 457, 608–611. [DOI] [PubMed] [Google Scholar]
- Barker, N. , van Es, J.H. , Kuipers, J. , Kujala, P. , van den Born, M. , Cozijnsen, M. , Haegebarth, A. , Korving, J. , Begthel, H. , Peters, P.J. , Clevers, H. , 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Batlle, E. , Bacani, J. , Begthel, H. , Jonkheer, S. , Gregorieff, A. , van de Born, M. , Malats, N. , Sancho, E. , Boon, E. , Pawson, T. , Gallinger, S. , Pals, S. , Clevers, H. , 2005. EphB receptor activity suppresses colorectal cancer progression. Nature. 435, 1126–1130. [DOI] [PubMed] [Google Scholar]
- Batlle, E. , Henderson, J.T. , Beghtel, H. , van den Born, M.M. , Sancho, E. , Huls, G. , Meeldijk, J. , Robertson, J. , van de Wetering, M. , Pawson, T. , Clevers, H. , 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 111, 251–263. [DOI] [PubMed] [Google Scholar]
- Bjerknes, M. , Cheng, H. , 1981. The stem-cell zone of the small intestinal epithelium I to V. Am. J. Anat. 160, 51–63. [DOI] [PubMed] [Google Scholar]
- Bjerknes, M. , Cheng, H. , 2002. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G767–G777. [DOI] [PubMed] [Google Scholar]
- Bonnet, D. , Dick, J.E. , 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737. [DOI] [PubMed] [Google Scholar]
- Brabletz, T. , Jung, A. , Reu, S. , Porzner, M. , Hlubek, F. , Kunz-Schughart, L.A. , Knuechel, R. , Kirchner, T. , 2001. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. U.S.A. 98, 10356–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. , Leblond, C.P. , 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561. [DOI] [PubMed] [Google Scholar]
- Clements, W.M. , Wang, J. , Sarnaik, A. , Kim, O.J. , MacDonald, J. , Fenoglio-Preiser, C. , Groden, J. , Lowy, A.M. , 2002. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 62, 3503–3506. [PubMed] [Google Scholar]
- Clevers, H. , 2006. Wnt/beta-catenin signaling in development and disease. Cell. 127, 469–480. [DOI] [PubMed] [Google Scholar]
- Correa, P. , Houghton, J. , 2007. Carcinogenesis of Helicobacter pylori . Gastroenterology. 133, 659–672. [DOI] [PubMed] [Google Scholar]
- Creamer, B. , Shorter, R.G. , Bamforth, J. , 1961. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 2, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba, P. , Dylla, S.J. , Park, I.K. , Liu, R. , Wang, X. , Cho, R.W. , Hoey, T. , Gurney, A. , Huang, E.H. , Simeone, D.M. , Shelton, A.A. , Parmiani, G. , Castelli, C. , Clarke, M.F. , 2007. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. U.S.A. 104, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon, E.R. , Vogelstein, B. , 1990. A genetic model for colorectal tumorigenesis. Cell. 61, 759–767. [DOI] [PubMed] [Google Scholar]
- Fodde, R. , Brabletz, T. , 2007. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 19, 150–158. [DOI] [PubMed] [Google Scholar]
- Fodde, R. , Kuipers, J. , Rosenberg, C. , Smits, R. , Kielman, M. , Gaspar, C. , van Es, J.H. , Breukel, C. , Wiegant, J. , Giles, R.H. , Clevers, H. , 2001. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 3, 433–438. [DOI] [PubMed] [Google Scholar]
- Galen, 1968. De tumoribus praeter naturam, a critical edition with translation and indices. The University of Michigan, University Microfilms #68-13,387, 29.
- Giannakis, M. , Chen, S.L. , Karam, S.M. , Engstrand, L. , Gordon, J.I. , 2008. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc. Natl. Acad. Sci. U.S.A. 105, 4358–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis, M. , Stappenbeck, T.S. , Mills, J.C. , Leip, D.G. , Lovett, M. , Clifton, S.W. , Ippolito, J.E. , Glasscock, J.I. , Arumugam, M. , Brent, M.R. , Gordon, J.I. , 2006. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J. Biol. Chem. 281, 11292–11300. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , Weinberg, R.A. , 2000. The hallmarks of cancer. Cell. 100, 57–70. [DOI] [PubMed] [Google Scholar]
- He, S. , Nakada, D. , Morrison, S.J. , 2009. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 25, 377–406. [DOI] [PubMed] [Google Scholar]
- Heath, J.P. , 1996. Epithelial cell migration in the intestine. Cell Biol. Int. 20, 139–146. [DOI] [PubMed] [Google Scholar]
- Houghton, J. , Stoicov, C. , Nomura, S. , Rogers, A.B. , Carlson, J. , Li, H. , Cai, X. , Fox, J.G. , Goldenring, J.R. , Wang, T.C. , 2004. Gastric cancer originating from bone marrow-derived cells. Science. 306, 1568–1571. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Siegel, R. , Ward, E. , Murray, T. , Xu, J. , Smigal, C. , Thun, M.J. , 2006. Cancer statistics, 2006. CA Cancer J. Clin. 56, 106–130. [DOI] [PubMed] [Google Scholar]
- Karam, S.M. , 1999. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 4, D286–D298. [DOI] [PubMed] [Google Scholar]
- Karam, S.M. , Leblond, C.P. , 1993. Dynamics of epithelial cells in the corpus of the mouse stomach. Anat. Rec. 236, 259–279. [DOI] [PubMed] [Google Scholar]
- Katoh, M. , 2007. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 3, 30–38. [DOI] [PubMed] [Google Scholar]
- Kelly, P.N. , Dakic, A. , Adams, J.M. , Nutt, S.L. , Strasser, A. , 2007. Tumor growth need not be driven by rare cancer stem cells. Science. 317, 337 [DOI] [PubMed] [Google Scholar]
- Korinek, V. , Barker, N. , Moerer, P. , van Donselaar, E. , Huls, G. , Peters, P.J. , Clevers, H. , 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383. [DOI] [PubMed] [Google Scholar]
- Lee, E.R. , Leblond, C.P. , 1985. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am. J. Anat. 172, 205–224. [DOI] [PubMed] [Google Scholar]
- Lee, E.R. , Trasler, J. , Dwivedi, S. , Leblond, C.P. , 1982. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am. J. Anat. 164, 187–207. [DOI] [PubMed] [Google Scholar]
- Lickert, H. , Kispert, A. , Kutsch, S. , Kemler, R. , 2001. Expression patterns of Wnt genes in mouse gut development. Mech. Dev. 105, 181–184. [DOI] [PubMed] [Google Scholar]
- Lobo, N.A. , Shimono, Y. , Qian, D. , Clarke, M.F. , 2007. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699. [DOI] [PubMed] [Google Scholar]
- McDonald, S.A. , Greaves, L.C. , Gutierrez-Gonzalez, L. , Rodriguez-Justo, M. , Deheragoda, M. , Leedham, S.J. , Taylor, R.W. , Lee, C.Y. , Preston, S.L. , Lovell, M. , Hunt, T. , Elia, G. , Oukrif, D. , Harrison, R. , Novelli, M.R. , Mitchell, I. , Stoker, D.L. , Turnbull, D.M. , Jankowski, J.A. , Wright, N.A. , 2008. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 134, 500–510. [DOI] [PubMed] [Google Scholar]
- Mills, J.C. , Andersson, N. , Hong, C.V. , Stappenbeck, T.S. , Gordon, J.I. , 2002. Molecular characterization of mouse gastric epithelial progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 99, 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuru, S. , Yanagisawa, A. , Ichii, S. , Tahara, E. , Kato, Y. , Nakamura, Y. , Horii, A. , 1992. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum. Mol. Genet. 1, 559–563. [DOI] [PubMed] [Google Scholar]
- Nomura, S. , Esumi, H. , Job, C. , Tan, S.S. , 1998. Lineage and clonal development of gastric glands. Dev. Biol. 204, 124–135. [DOI] [PubMed] [Google Scholar]
- Nomura, S. , Kaminishi, M. , Sugiyama, K. , Oohara, T. , Esumi, H. , 1998. Clonal analysis of isolated intestinal metaplastic glands of stomach using X linked polymorphism. Gut. 42, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, C.A. , Pollett, A. , Gallinger, S. , Dick, J.E. , 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 445, 106–110. [DOI] [PubMed] [Google Scholar]
- Odoux, C. , Fohrer, H. , Hoppo, T. , Guzik, L. , Stolz, D.B. , Lewis, D.W. , Gollin, S.M. , Gamblin, T.C. , Geller, D.A. , Lagasse, E. , 2008. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 68, 6932–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offerhaus, G.J. , Giardiello, F.M. , Krush, A.J. , Booker, S.V. , Tersmette, A.C. , Kelley, N.C. , Hamilton, S.R. , 1992. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 102, 1980–1982. [DOI] [PubMed] [Google Scholar]
- Oguma, K. , Oshima, H. , Aoki, M. , Uchio, R. , Naka, K. , Nakamura, S. , Hirao, A. , Saya, H. , Taketo, M.M. , Oshima, M. , 2008. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 27, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita, K. , Ichisaka, T. , Yamanaka, S. , 2007. Generation of germline-competent induced pluripotent stem cells. Nature. 448, 313–317. [DOI] [PubMed] [Google Scholar]
- Oshima, H. , Matsunaga, A. , Fujimura, T. , Tsukamoto, T. , Taketo, M.M. , Oshima, M. , 2006. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 131, 1086–1095. [DOI] [PubMed] [Google Scholar]
- Oshima, H. , Oshima, M. , Inaba, K. , Taketo, M.M. , 2004. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 23, 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, M. , Oshima, H. , Matsunaga, A. , Taketo, M.M. , 2005. Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-alpha-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res. 65, 9147–9151. [DOI] [PubMed] [Google Scholar]
- Park, W.S. , Oh, R.R. , Park, J.Y. , Lee, S.H. , Shin, M.S. , Kim, Y.S. , Kim, S.Y. , Lee, H.K. , Kim, P.J. , Oh, S.T. , Yoo, N.J. , Lee, J.Y. , 1999. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 59, 4257–4260. [PubMed] [Google Scholar]
- Parkin, D.M. , Bray, F. , Ferlay, J. , Pisani, P. , 2001. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 94, 153–156. [DOI] [PubMed] [Google Scholar]
- Perez-Caro, M. , Cobaleda, C. , Gonzalez-Herrero, I. , Vicente-Duenas, C. , Bermejo-Rodriguez, C. , Sanchez-Beato, M. , Orfao, A. , Pintado, B. , Flores, T. , Sanchez-Martin, M. , Jimenez, R. , Piris, M.A. , Sanchez-Garcia, I. , 2009. Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO J. 28, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten, C.S. , Kovacs, L. , Hamilton, E. , 1974. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 7, 271–283. [DOI] [PubMed] [Google Scholar]
- Qiao, X.T. , Ziel, J.W. , McKimpson, W. , Madison, B.B. , Todisco, A. , Merchant, J.L. , Samuelson, L.C. , Gumucio, D.L. , 2007. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 133, 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana, E. , Shackleton, M. , Sabel, M.S. , Fullen, D.R. , Johnson, T.M. , Morrison, S.J. , 2008. Efficient tumour formation by single human melanoma cells. Nature. 456, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani, L. , Lombardi, D.G. , Pilozzi, E. , Biffoni, M. , Todaro, M. , Peschle, C. , De Maria, R. , 2007. Identification and expansion of human colon-cancer-initiating cells. Nature. 445, 111–115. [DOI] [PubMed] [Google Scholar]
- Sancho, E. , Batlle, E. , Clevers, H. , 2004. Signaling pathways in intestinal development and cancer. Annu. Rev. Cell Dev. Biol. 20, 695–723. [DOI] [PubMed] [Google Scholar]
- Sangiorgi, E. , Capecchi, M.R. , 2008. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton, M. , Quintana, E. , Fearon, E.R. , Morrison, S.J. , 2009. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 138, 822–829. [DOI] [PubMed] [Google Scholar]
- Shih, I.M. , Wang, T.L. , Traverso, G. , Romans, K. , Hamilton, S.R. , Ben-Sasson, S. , Kinzler, K.W. , Vogelstein, B. , 2001. Top-down morphogenesis of colorectal tumors. Proc. Natl. Acad. Sci. U.S.A. 98, 2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelkov, S.V. , Butler, J.M. , Hooper, A.T. , Hormigo, A. , Kushner, J. , Milde, T. , St Clair, R. , Baljevic, M. , White, I. , Jin, D.K. , Chadburn, A. , Murphy, A.J. , Valenzuela, D.M. , Gale, N.W. , Thurston, G. , Yancopoulos, G.D. , D'Angelica, M. , Kemeny, N. , Lyden, D. , Rafii, S. , 2008. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J. Clin. Invest. 118, 2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert, H.J. , van Es, J.H. , van den Born, M. , Begthel, H. , Stange, D.E. , Barker, N. , Clevers, H. , 2009. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 136, 2187–2194. e2181 [DOI] [PubMed] [Google Scholar]
- Soreide, K. , Janssen, E.A. , Soiland, H. , Korner, H. , Baak, J.P. , 2006. Microsatellite instability in colorectal cancer. Br. J. Surg. 93, 395–406. [DOI] [PubMed] [Google Scholar]
- Suerbaum, S. , Michetti, P. , 2002. Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Sung, J.J. , Leung, W.K. , Go, M.Y. , To, K.F. , Cheng, A.S. , Ng, E.K. , Chan, F.K. , 2000. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am. J. Pathol. 157, 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Tanabe, K. , Ohnuki, M. , Narita, M. , Ichisaka, T. , Tomoda, K. , Yamanaka, S. , 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takaishi, S. , Okumura, T. , Tu, S. , Wang, S.S. , Shibata, W. , Vigneshwaran, R. , Gordon, S.A. , Shimada, Y. , Wang, T.C. , 2009. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 27, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo, M.M. , 2006. Mouse models of gastrointestinal tumors. Cancer Sci. 97, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu, M. , Fukami, H. , Yamamoto, M. , Nakanishi, H. , Masui, T. , Kusakabe, N. , Sakakura, T. , 1994. Clonal analysis of glandular stomach carcinogenesis in C3H/HeN<==>BALB/c chimeric mice treated with N-methyl-N-nitrosourea. Cancer Lett. 83, 37–42. [DOI] [PubMed] [Google Scholar]
- Teir, S. , 1961. Natl. Cancer Inst. 1, (27) 949–971. [PubMed] [Google Scholar]
- Thompson, M. , Fleming, K.A. , Evans, D.J. , Fundele, R. , Surani, M.A. , Wright, N.A. , 1990. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 110, 477–481. [DOI] [PubMed] [Google Scholar]
- Tomita, H. , Yamada, Y. , Oyama, T. , Hata, K. , Hirose, Y. , Hara, A. , Kunisada, T. , Sugiyama, Y. , Adachi, Y. , Linhart, H. , Mori, H. , 2007. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 67, 4079–4087. [DOI] [PubMed] [Google Scholar]
- van de Wetering, M. , Sancho, E. , Verweij, C. , de Lau, W. , Oving, I. , Hurlstone, A. , van der Horn, K. , Batlle, E. , Coudreuse, D. , Haramis, A.P. , Tjon-Pon-Fong, M. , Moerer, P. , van den Born, M. , Soete, G. , Pals, S. , Eilers, M. , Medema, R. , Clevers, H. , 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 111, 241–250. [DOI] [PubMed] [Google Scholar]
- Van der Flier, L.G. , Sabates-Bellver, J. , Oving, I. , Haegebarth, A. , De Palo, M. , Anti, M. , Van Gijn, M.E. , Suijkerbuijk, S. , Van de Wetering, M. , Marra, G. , Clevers, H. , 2007. The Intestinal Wnt/TCF Signature. Gastroenterology. 132, 628–632. [DOI] [PubMed] [Google Scholar]
- Vermeulen, L. , De Sousa, E.M.F. , van der Heijden, M. , Cameron, K. , de Jong, J.H. , Borovski, T. , Tuynman, J.B. , Todaro, M. , Merz, C. , Rodermond, H. , Sprick, M.R. , Kemper, K. , Richel, D.J. , Stassi, G. , Medema, J.P. , 2010. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476. [DOI] [PubMed] [Google Scholar]
- Zhu, L. , Gibson, P. , Currle, D.S. , Tong, Y. , Richardson, R.J. , Bayazitov, I.T. , Poppleton, H. , Zakharenko, S. , Ellison, D.W. , Gilbertson, R.J. , 2009. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 457, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]


