Abstract
Bursting activity of striatal medium spiny neurons results from membrane potential oscillations between a down- and an upstate that could be regulated by G-protein-coupled receptors. Among these, dopamine D2 and adenosine A2A receptors are highly enriched in striatal neurons and exhibit strong interactions whose physiological significance and molecular mechanisms remain partially unclear. More particularly, respective involvements of common intracellular signaling cascades and A2A–D2 receptor heteromerization remain unknown. Here we show, by performing perforated-patch-clamp recordings on brain slices and loading competitive peptides, that D2 and A2A receptors regulate the induction by N-methyl-D-aspartate of a depolarized membrane potential plateau through mechanisms relying upon specific protein–protein interactions. Indeed, D2 receptor activation abolished transitions between a hyperpolarized resting potential and a depolarized plateau potential by regulating the CaV1.3a calcium channel activity through interactions with scaffold proteins Shankl/3. Noticeably, A2A receptor activation had no effect per se but fully reversed the effects of D2 receptor activation through a mechanism in which A2A–D2 receptors heteromerization is strictly mandatory, demonstrating therefore a first direct physiological relevance of these heteromers. Our results show that membrane potential transitions and firing patterns in striatal neurons are tightly controlled by D2 and A2A receptors through specific protein–protein interactions including A2A–D2 receptors heteromerization.
Keywords: basal ganglia, G-protein-coupled receptor, heteromerization, membrane potential oscillation, calcium channel
INTRODUCTION
GABAergic striatal medium spiny neurons (MSNs) display particular passive and active membrane properties that shape their intrinsic excitability and their responsiveness to synaptic inputs mediated by N-methyl-D-aspartate (NMDA) and AMPA receptors activation. In vivo, these neurons, from both the dorsal part and the accumbens nucleus, present a unique type of spontaneous electrical behavior, consisting of oscillations of the membrane potential between two preferred potentials, the ‘upstate’ driving the neuron to firing threshold and the ‘downstate’ near the hyperpolarized potassium equilibrium potential (O’Donnell and Grace, 1995; Wilson and Kawaguchi, 1996; Stern et al, 1998; Goto and O’Donnell, 2001). It is proposed that transitions from down- to upstate are mainly triggered by excitatory glutamatergic synaptic input. Although these transitions depend on glutamatergic NMDA and AMPA receptors, others inward currents that could be strongly regulated, such as L-type Ca2+ channels (Hounsgaard and Kiehn, 1989; Vergara et al, 2003), participate in the maintenance of depolarized plateau potentials.
Several neurotransmitters acting on G-protein-coupled receptors (GPCRs), as dopamine acting at D1 or D2 receptors, modulate the activity of intrinsic conductances in MSNs (Nicola et al, 2000; Surmeier et al, 2007). D2 receptors negatively regulate the activity of L-type Ca2+ channels and hence can modify state transitions and spike firing in these striatal neurons by a mechanism that remains to be fully identified (Hernandez-Lopez et al, 1997, 2000; Olson et al, 2005).
Adenosine is another very important modulator of striatal neurotransmission through its actions on adenosine receptors, and most specifically the A2A receptors, that are highly abundant in the striatum (Schiffmann et al, 1991; Schiffmann and Vanderhaeghen, 1993; Svenningsson et al, 1999). Adenosine is an intrinsic regulatory signal as it is locally produced as a function of the activity of striatal circuits. There are two main sources of extracellular adenosine (for review see Schiffmann et al, 2007). First, extracellular levels of adenosine may increase as a function of the general workload (ie increased firing per unit of time) of the circuit through the dephosphorylation of intracellularly consumed ATP and the transport of adenosine by nucleoside transporters. In parallel, adenosine could also be formed extracellularly through the dephosphorylation by ectonucleotidases of vesicular ATP released upon nerve stimulation. Therefore, under physiological conditions extracellular levels of adenosine increase locally as a function of neuronal firing and synaptic activity.
GABAergic striatopallidal enkephalinergic neurons express predominantly adenosine A2A receptors and dopamine D2 receptors (Schiffmann et al, 1991; Schiffmann and Vanderhaeghen, 1993; Ferré et al, 1997; Svenningsson et al, 1999). A tight interplay between the A2A and D2 receptors, with reciprocal antagonistic interactions, modulates the function of the striatopallidal neuron (Schiffmann and Vanderhaeghen, 1993; Ferré et al, 1993; Stromberg et al, 2000; D’Alcantara et al, 2001). However, the molecular mechanisms of these interactions and the effect of A2A receptor on neuronal excitability and state transitions are still poorly understood. One type of A2A–D2 receptor interaction takes place at the second messenger level, as both receptors can potentially target the same intracellular signaling cascade through their stimulating and inhibiting coupling to adenylyl cyclase activity (Stoof and Kebabian, 1984; Svenningsson et al, 1999). The other type of interaction takes place at the membrane level and implies an intermolecular cross talk, related to the ability of A2A and D2 receptors to form receptor heteromers (Hillion et al, 2002; Canals et al, 2003; Ciruela et al, 2004). In this interaction, stimulation of A2A receptor results in decrease in the binding affinity of D2 receptor for dopamine (Ferré et al, 1991; Dasgupta et al, 1996). Despite that a large and still growing number of GPCR heteromers have been described based on biochemical, pharmacological, and/or structural data, for most of these GPCR heteromers, it remained very difficult up to now to reveal a functional significance. Moreover, this potential functional relevance was hard to distinguish from the more classical functional interactions between GPCR related to their targeting of common intracellular targets. So it is for the functional significance of the intramembrane A2A–D2 receptor interaction that depends on A2A–D2 receptor heteromerization, one of the most studied heteromer in the central nervous system. Here, we present results that indicate that in striatal neurons A2A and D2 receptors regulate NMDA-mediated neuronal excitation resulting in a depolarized plateau potential and spike firing, through a mechanism requiring scaffolding proteins of the Shank family and A2A–D2 receptor heterodimerization.
MATERIALS AND METHODS
Animals and Slice Preparation
Medium-sized spiny striatal neurons were recorded in acute corticostriatal slices obtained from 17- to 25-day-old Wistar rats (Iffa-Credo, Belgium), wild-type or adenosine A2A receptor knockout (A2A R−/−) mice generated on a CD1 background (Ledent et al, 1997) as previously described and D2-enhanced green fluorescent protein (EGFP) mice (Gong et al, 2003). Animals were anesthetized with halothane and killed by decapitation. The brain was quickly removed and placed in ice-cold (4°C) artificial cerebrospinal fluid (ACSF) saturated with 95% O2–5% CO2 and containing the following (in mM): 126 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1 MgCl2, 2 CaCl2, 18 NaHCO3, and 11 glucose (pH 7.2–4, 290mOsm/l) (Hopf et al, 2003). Coronal slices (200 and 280 μM thick for mice and rats, respectively) containing the nucleus accumbens were cut in ice-cold ACSF using a vibratome (VT 1000S; Leica). Slices were incubated in ACSF (bubbled with 95% O2–5% CO2) at 32°C for at least 1 h before recording. For experiments, slices were then transferred into a recording chamber where they were continuously superfused (2–3 ml/min) with ACSF warmed to 32°C. All procedures conformed with the standards of the Institutional Ethical Committee of the School of Medicine of the Université Libre de Bruxelles.
Patch-Clamp Recording
Whole-cell and perforated-patch-clamp recordings were performed on individual neurons from the ventral striatum, accumbens nucleus, identified by using infrared differential interference contrast microscopy (Axioskop 2FS, × 40/0.80 W; Zeiss). Fluorescent MSNs were identified with UV lamp (mercury) and an FITC filter (excitation BP 450/490, beamsplitter FT 510, emission LP 515). Recording pipettes were pulled from borosilicate glass capillaries (Hilgenberg GmbH, Malsfeld, Germany) on a P-2000 pipette puller (Sutter Instruments, Novato, CA, USA) and presented resistances of 5–8 MΩ when filled with the patch pipette solutions. These pipettes were used for both whole-cell and perforated-patch recordings. The pipette solution for whole-cell recordings consisted of the following (in mM): 119 KMeSO4, 1 MgCl2, 0.1 CaCl2, 10 HEPES, 1 EGTA, 12 phosphocreatine, 2 Na2ATP, 0.7 Na2GTP, pH 7.2–3 adjusted with KOH, 280–300 mOsm/l (Olson et al, 2005). The pipette solution for perforated-patch recordings consisted of the following (in mM): 80 K2SO4, 10 NaCl, 15 glucose, 5 HEPES, pH 7.2–3 adjusted with KOH and 100 μg/ml nystatin (Horn and Marty, 1988).
Passive cellular parameters were extracted in voltage clamp by analyzing current relaxation induced by a 10 mV hyperpolarized step from a holding potential of −80 mV as described previously (D’Angelo et al, 1995). In the perforated-patch configuration, access resistance (Ra) was monitored to ensure that voltage attenuation in current clamp mode was always less than 10%. In addition, data from cells that showed > 15% change in Ra were excluded from further analysis. All recordings were made with an Axopatch-200B amplifier (Axon Instruments) in the fast current clamp mode. Membrane potential signal was filtered at a cutoff frequency of 2 kHz and subsequently digitized at 5 kHz using the acquisition software Pulse (HEKA; Lambrecht-Pfalz, Germany) in combination with an ITC-16 AD/DA converter (Instrutech, NY, USA). Data were analyzed with Igor Pro software (WaveMetrics, Lake Oswego, OR, USA).
All drugs were dissolved in the bath solution and then were applied to the preparation by superfusion. The drug solution reached a steady-state concentration in the experimental chamber in 2 min. After reaching this steady-state period, the response to the drug was measured after a prolonged application (up to 5 min), to obtain a maximal effect of the drug. During the last minute of the drug application period, a continuous 30 s period of recording was used to compute the mean firing frequency of action potentials and the average membrane potential.
Data Analyses and Statistics
Data were statistically compared with one-way analysis of variance followed by a Bonferroni’s post hoc test or when appropriate, paired Student’s t-test, and Fisher’s exact test for comparison of the proportion of responding neurons. Significance was assessed at p< 0.05. All data are reported as means ± SEM.
Peptide Synthesis
CaV1.2- and CaV1.3-PDZ-binding peptides were synthesized by the solid-phase method using the Fmoc (9-fluorenylmethoxy carbonyl) strategy on a Symphony PTI Multiplex synthesizer (Protein Technologies, Tucson, AZ, USA). The peptides (>95%) were purified on reverse phase and ion exchange chromatographies. Peptide purity was assessed by capillary electrophoresis and the sequence conformity was verified by sequencing and electrospray mass spectrometry (Lab de chimie biologique et de la nutrition, ULB, Belgium). CaV1.2-PDZ-binding peptide (‘VSNL peptide’) sequence was SEEALPDSRSYVSNL, and CaV1.3-PDZ-binding peptide (‘ITTL peptide’) sequence was EEEDLADEMICITTL (Zhang et al, 2005). Adenosine A2A and dopamine D2 receptors interacting peptides were also synthesized. A2A receptor-mimicking peptide (‘SAQES peptide’ and ‘SAQEpS peptide’) sequences, corresponding to an epitope localized in the C terminus, were SAQESQGNT and SAQEpSQGNT and sequence of their control peptide (‘AAQEA peptide’) was AAQEAQGNT (Woods and Ferré, 2005). Peptides were applied at the concentration of 3 μM in the patch pipette solution.
Drugs and Reagents
All reagents were obtained from Sigma (St Louis, MO, USA). Appropriate drug stock solutions were made and diluted with ACSF just before application. All drugs were bath-applied. Drugs used were NMDA, R(−)-propylnorapomorphine hydrochloride (NPA), 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolol[1,5-c]pyrimidine (SCH 58261; Sigma), sulpiride, 2-[4-[(2-carboxyethyl)-phenyl]ethyl-ami-no]-5′-N-(ethylcarbamoyl)adenosine (CgS 21680; Tocris, Bristol, UK).
RESULTS
Recordings were performed on MSNs that constitute the vast majority (90–95%) of striatal neurons and are easily identified in slice preparations by their size. All these cells had resting membrane potentials of −75.00 ± 0.49 (n = 120) and input resistances of 487 ± 17 MΩ, parameters that are similar to those previously reported (Kombian and Malenka, 1994; Hernandez-Echeagaray et al, 2004). Less abundant classes of interneurons were readily identified based on their typical firing pattern (ie cholinergic and fast spiking neurons) and excluded from the present study. Recently, an in vitro model has been proposed to mimic the down- to upstate membrane potential transitions through the utilization of repetitive cortical stimulation or low micromolar concentrations of NMDA (Vergara et al, 2003; Olson et al, 2005). In the present study, we used a variant of this model as we performed recordings in the nystatin perforated-patch configuration. This configuration allows, in contrast to the whole-cell configuration, to protect the integrity of the intracellular machinery and particularly the homeostasis of calcium and second messengers. Under these conditions, application of 5 μM NMDA shifts the MSN from its hyperpolarized resting membrane potential to a depolarized plateau, inducing a continuous action potential firing (Figure 1a and b). This NMDA receptor-dependent transition of the MSN membrane potential from the resting potential to a depolarized plateau potential was reversible upon NMDA washout.
Figure 1.
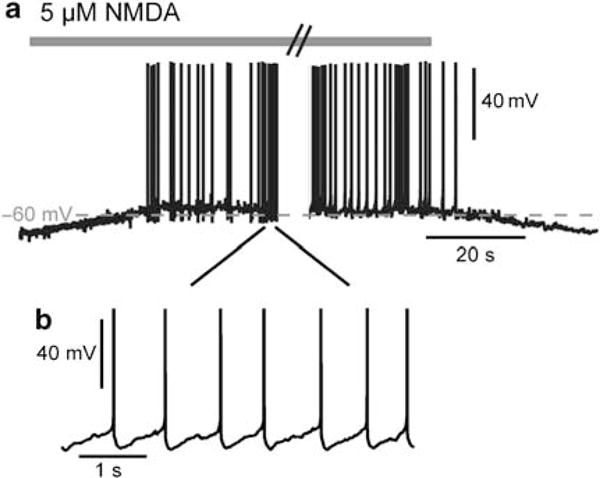
Membrane potential transition from a hyperpolarized resting potential to a depolarized plateau potential of a medium spiny neuron (MSN) in response to glutamatergic receptor stimulation. (a) Transition in a representative MSN recorded in an acute slice in perforated-patch clamp. Application of 5 μM N-methyl-D-aspartate (NMDA), which mimics cortical synaptic inputs, evoked a reversible membrane potential transition between a hyperpolarized state and a depolarized plateau potential inducing a continuous action potential firing. (b) Periodic spike firing of MSN during the 5 μM NMDA-induced upstate.
Dopamine D2 Receptors Suppress the NMDA-Induced Depolarized Plateau in Medium Spiny Neurons
To determine the role of D2 receptors in initiation and maintenance of the NMDA-induced depolarized plateau potential of MSNs, we applied the D2-like receptor agonist NPA (10 μM; Hernandez-Lopez et al, 2000). In this and following experiments, both the mean firing frequency in the NMDA-induced depolarized plateau and the mean membrane potential during NMDA application were determined (see Materials and methods). Activation of D2 receptor (Figure 2a) hyperpolarized the membrane potential from −58.15 ±1.01 to −64.32 ± 1.13 mV (n = 24; p<0.05) and significantly diminished the firing frequency from 2.4 ± 0.31 to 1.04 ± 0.43 Hz (n = 24; p<0.05; Figure 2b) in the NMDA-induced depolarized plateau. Two different populations could be distinguished in these MSNs: a D2-responsive and a D2-unresponsive population. Indeed, on 17 out of these 24 recorded neurons, the application of NPA virtually suppressed the NMDA-induced spike firing (2.05 ± 0.28 Hz for NMDA and 0.04 ± 0.02 Hz for NPA; p<0.05; Figure 2b, inset) by more than 90% and hyperpolarized the mean membrane potential from −55.66 ± 1.29 to −66.25 ± 1.04mV (p<0.05). On the other hand, in the remaining seven neurons, the frequency of the NMDA-induced spike firing, 3.24 ± 0.78 Hz, as well as the mean membrane potential, −59.32 ± 1.49 mV, was not significantly altered by D2 receptor activation (3.46 ±1.06 Hz and −59.66 ± 2.18 mV, respectively; n = 7; p>0.05; Figure 2b, inset). To confirm the specificity of these results, the selective D2 receptor antagonist sulpiride (10 μM) was subsequently applied (Figure 2a). In the presence of sulpiride, NPA-induced hyperpolarization of the membrane potential (−65.38 ± 1.67 mV for NPA and −57 ± 1.56 mV for sulpiride; n = 6; p<0.05) and inhibition in firing (0.032 ± 0.038 Hz for NPA and 1.34 ± 0.37 Hz for sulpiride; n = 6; p< 0.05; Figure 2c) were fully abolished. It is worth mentioning that D2 receptor activation did not modify the membrane potential (−72.89 ± 2.96 mV for control resting potential and −72.29 ± 2.49 mV for NPA; p>0.05) or the input resistance (495.92 ± 69.6 MΩ for control and 486.71 ± 77.8MΩ for NPA; p>0.05) of MSNs in the control (data not shown; n = 8).
Figure 2.
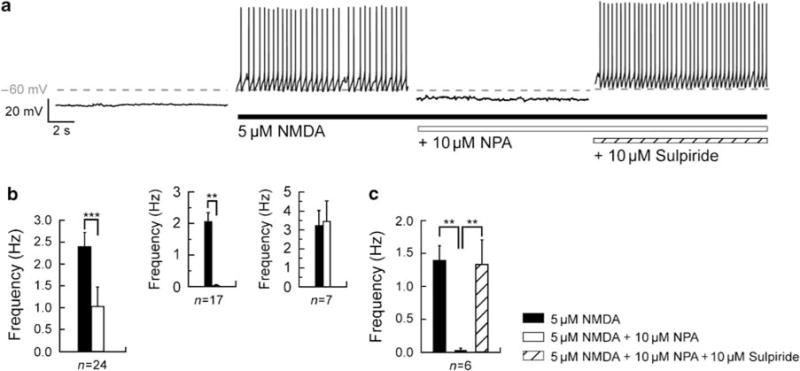
Dopamine D2 receptor suppresses the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential. (a) Consecutive traces, recorded in a single neuron, showing typical transitions where the action of NMDA (5 μM) was recorded before and in the presence of D2 receptor agonist R(−)-propylnorapomorphine hydrochloride (NPA, 10 μM) and D2 receptor antagonist sulpiride (10 μM). Notice that before NMDA application the recorded medium spiny neuron is in a hyperpolarized resting potential (−78 mV) and in response to NMDA depolarized to plateau potential (−60 mV). In the presence of NMDA, application of NPA suppresses the plateau potential and inhibits the action potential firing. Subsequent application of sulpiride blocks the D2 effect and reestablishes the depolarized plateau potential. (b) Summary histogram obtained from 24 different neurons illustrating the effect of the D2 receptor agonist on the firing frequency. Application of NPA (10 μM) significantly reduced the frequency of action potential firing. In 17 out of 24 recorded neurons, application of NPA totally reverses the depolarized firing plateau, whereas 7 out of 24 recorded neurons do not respond to the activation of D2 receptors. These data show D2-responsive and -unresponsive populations. (c) Summary histogram illustrates the significant reversed effect of D2 receptor antagonist on the firing frequency of D2-responsive neurons (n = 6) (data represent mean ± SEM; **p <0.01, ***p <0.001).
D2 Receptor Modulation of the NMDA-Induced Depolarized Plateau Is Disrupted by Blockade of Shank-Cav1.3a Interaction
One of the major consequences of dopamine D2 receptor activation in MSNs is the suppression of L-type Ca2+ channel currents, through the activation of the Ca2+/calmodulin-dependent protein phosphatase PP2B or calcineurin (Hernandez-Lopez et al, 2000). Olson et al (2005) have shown that the D2 dopamine receptor preferentially modulates the activity of Cav1.3a Ca2+ channels as compared to Cav1.2 Ca2+ channels. This selective modulation of Cav1.3a appears to be dependent on the physical interaction between synaptic scaffolding proteins of the Shank family and Cav1.3a Ca2+ channels mediated by a PDZ-binding domain containing an ITTL motif (Olson et al, 2005; Zhang et al, 2005). We therefore reasoned that such an interaction between Shank and Cav1.3a subunits should also be critical for the modulation of the transition to a depolarized plateau potential by the D2 receptor. To test this hypothesis, we used peptides containing the CaV1.3a PDZ-binding ITTL motif, which is supposed to competitively inhibit the interaction and disrupt the modulation, or the Cav1.2 VSNL PDZ-binding domain, which should not. As we used the perforated-patch configuration, which precludes the transfer of peptides into the recorded neuron, we modified our protocol as follows and illustrated in Figure 3a. In a first step, the peptide of interest was dialyzed into the neuron through the patch pipette in a whole-cell recording configuration during 5–10 min and then the pipette was gently removed. After 10 min (to allow the neuron to recover), the same neuron is subsequently recorded with the perforated-patch configuration (Figure 3a). We first demonstrated that these repeated manipulations (whole-cell followed by perforated patch) neither modify intrinsic neuronal parameters nor the NMDA-induced membrane shift when considering all our series of recorded neurons. Indeed, membrane potential and input resistance after repatching were −75.66 ± 0.62 mV and 482 ± 20 MΩ, n = 44, respectively. These values were not different than those obtained on neurons that were not submitted to this serial protocol, −74.62 ± 0.69 mV and 489 ±24 MΩ, n = 76; p>0.05). The membrane shift to NMDA was also similar to that obtained on neurons that were not submitted to this serial protocol (−58.74 ± 0.66 mV for unloaded neurons, n = 76 and −59.65 ± 0.82 for whole-cell loaded neurons after repatching, n = 44; p>0.05). To be sure that this technical approach did not perturb the D2 receptor-mediated modulation of potential transition, we first made the recording without dialyzing any peptide. In this condition, three out of five recorded neurons exhibited a D2 receptor-mediated inhibition of the NMDA-induced spike firing (2.02 ± 0.43 Hz for NMDA and 0.22 ± 0.22 Hz for NPA; p<0.05; data not shown).
Figure 3.
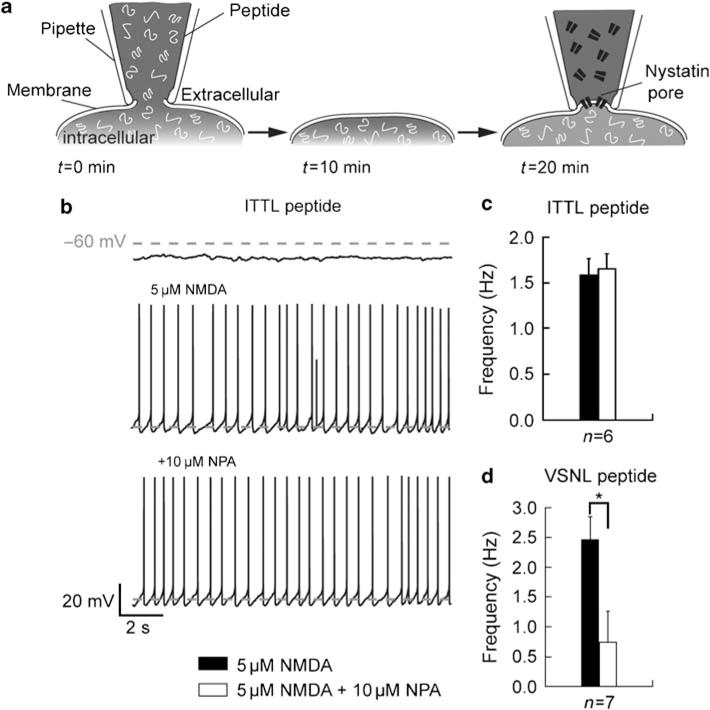
Peptide targeting the Shank-CaVl.3a interaction blocks D2 receptor modulation of the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential. (a) Method used to dialyze specific peptide in the recorded medium spiny neuron (MSN) using the whole-cell configuration of the patch-clamp technique. The selective peptide is dialyzed in the neuron through the patch pipette during 5–10 min and then the pipette is gently removed. After 10 min, to allow the neuron to recover, the same neuron is subsequently recorded using the perforated-patch configuration. (b) Consecutive traces of an MSN dialyzed with the ITTL peptide targeting the Shank PDZ-binding domain interacting with CaVl.3a L-type Ca2+ channels. In this dialyzed neuron, D2 receptor activation by R(−)-propylnorapomorphine hydrochloride (NPA, 10 μM) does not affect NMDA receptor-induced depolarized plateau potential. (c) Histogram showing the effect of D2 receptor activation on the firing frequency on neurons dialyzed with the ITTL peptide. Application of NPA (10 μM) did not affect the frequency of action potential firing (n = 6). (d) Effect of NPA on the firing frequency of neurons dialyzed with the VSNL peptide targeting the shank PDZ-binding domain interacting with Cavl.2 L-type Ca2+ channels. On seven recorded neurons, D2 receptor activation strongly decreases the action potential firing frequency (data represent mean ±SEM; *p<0.05).
Then, peptides mimicking the Cav1.3a and Cav1.2 PDZ-binding domains were introduced into neurons. We first demonstrated that these peptides do not influence the basal membrane properties with resting membrane potential of −68.65 ± 1.2 mV and input resistance of 579 ± 59.57 MΩ in basal condition (n = 24), −67.03 ±1.5 mV and 570 ±50.13 MO for ITTL peptide (n = 6), and −67.77 ± 1.36 mV and 497 ± 58.13 MΩ for VSNL peptide (n = 7) (p>0.05; data not shown). Dialysis of the ITTL peptide completely disrupted the ability of the D2-like agonist to abolish the depolarized plateau potential (none D2-responding neurons out of six, 1.58 ± 0.18 Hz for NMDA and 1.66 ± 0.16 Hz for NPA; n = 6; p>0.05; Figure 3b and c) whereas dialysis of the VSNL peptide did not affect the D2 receptor-mediated modulation (five D2-responding neurons out of seven, 2.46 ± 0.38 Hz for NMDA and 0.74 ±0.51 Hz for NPA; n = 7; p<0.05; Figure 3d). Under these two conditions, the proportion of D2-responding neurons was significantly different (p < 0.05, Fisher’s exact test), which underlines the specific effect of the ITTL peptide vs the VSNL peptide on the Shank–Cav1.3a interaction. These experiments demonstrated that as suggested by Olson et al (2005) the D2 receptor-mediated inhibition of Cav1.3a channel currents, dependent on Cav1.3a–Shank interaction, could be considered as a major molecular mechanism for the D2 receptor-induced abolition of down- to upstate transitions in MSNs.
Adenosine A2A Receptors do not Affect the NMDA- Induced Depolarized Plateau in Medium Spiny Neurons
The role of A2A receptors in the modulation of corticostriatal synaptic transmission and in the control of MSN intrinsic excitability is poorly understood and it is not known whether activation of this receptor may affect their membrane potential oscillations. We therefore investigated the role of A2A receptor activation on the NMDA receptor-induced transition to the depolarized plateau by applying the selective A2A receptor agonist CGS 21680 (1 μM) after the application of 5 μM NMDA (Figure 4a). Under this condition, the depolarized average membrane potential (−58.99 ± 2.36 mV for NMDA and −57.57 ± 2.82 mV for CGS 21680; n = 8; p>0.05) and the firing frequency (2.33 ± 0.44 Hz for NMDA and 2.63 ± 0.48 Hz for CGS 21680; n = 8; p>0.05; Figure 4b) were not significantly altered.
Figure 4.
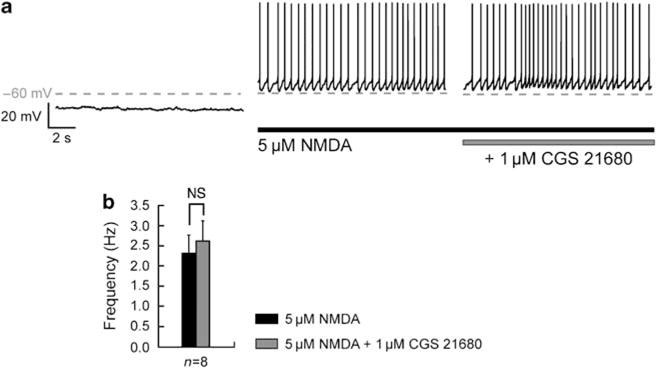
Adenosine A2A receptor does not affect the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential. (a) Typical recording on a single neuron, showing the NMDA-induced depolarized plateau potential before and after application of A2A receptor agonist, 2-[4-[(2-carboxyethyl)-phenyl]ethyl-amino]-5′-N-(ethylcarbamoyl)adenosine (CGS 21680, 1 μM). (b) Statistics illustrated in the histogram show the effect of A2A receptor activation on firing frequency. Application of CGS 21680 (1 μM) does not affect the frequency of action potential firing (n = 8) (data represent mean ± SEM; NS, not significant).
To exclude that the absence of effect of CGS 21680 is due to the fact that a maximal effect is already afforded by NMDA, we activated A2A receptor after the application of 3 μM NMDA that does not allow the neuron to reach the depolarized plateau. Under this condition, on the nine recorded neurons the average membrane potential was not significantly modified (−69.87 ± 2.49 mV for 3 μM NMDA and −69.08 ± 2.67 mV for 3 μM NMDA + 1 μM CGS 21680; n = 9; p>0.05).
These results highly suggest that adenosine acting at the A2A receptor is unable to affect the transition to the depolarized plateau per se in these neurons and show that it cannot assist NMDA in bringing about it. Even though the A2A receptor is unable to affect this transition per se, its activation could still modify the response of MSNs to D2 receptor activation by means of A2A–D2 receptor interactions (Ferré et al, 1991, 1993; Schiffmann and Vanderhaeghen, 1993; Schiffmann et al, 2007).
A2A Receptors Counteract the D2 Receptor-Mediated Suppression of NMDA-Induced Depolarized Plateau in Medium Spiny Neurons
To address the question of a functional role of A2A–D2 receptor interaction, we activated adenosine A2A receptors on D2-responsive MSNs (Figure 5a). On nine D2-responsive neurons, activation of A2A receptor by the selective A2A receptor agonist, CGS 21680 (1 μM), totally counteracted the effect of 10 μM NPA on the firing frequency (0.058 ± 0.039 Hz for NPA and 2.66 ± 0.66 Hz for CGS 21680; p<0.05; Figure 5b) and on the average membrane potential (−66.07 ± 1.39 mV for NPA and −57.17 ± 1.75 mV for CGS 21680; p< 0.05; Figure 5c, inset). This A2A receptor-mediated modulation of the NMDA-induced depolarized plateau in D2-responsive neurons was blocked by the selective A2A receptor antagonist, SCH 58261 (1 μM) (n = 2; Figure 5a). To confirm the specificity of this adenosine A2A receptor effect, we performed the same experiments on neurons from A2A receptor knockout mice and their wild-type littermates (Figure 5d). In wild-type mice, we obtained the same results than in rats with significant modulations of the firing frequency by D2 receptors in four out six recorded neurons (1.2 ± 0.11 for NMDA and 0 ± 0Hz for NPA; n = 4; p<0.05) and a counteraction by A2A receptors in these four D2-responsive neurons (1.61 ± 0.24Hz; n = 4; p<0.05 as compared to NPA; Figure 5e) as well as the average membrane potential (−60.94 ±1.78 for NMDA and −65.34 ± 2.16 mV for NPA; n = 4; p< 0.05). Conversely, in slices from A2A receptor knockout animals, four out of six recorded neurons exhibited a total suppression of the NMDA-induced depolarized plateau potential (1.34 ± 0.34 Hz for NMDA and 0±0Hz for NPA; p<0.05) and shifted from −55.92 ± 1.59 mV to a more hyperpolarized state of −65.11 ± 1.28mV after the application of NPA (p<0.05) whereas the subsequent activation of A2A receptors on the four D2-responsive neurons had no effect (0 ± 0 Hz, −63.77 ± 0.94mV; p>0.05 as compared to NPA; Figure 5f; p<0.05 for the comparison of proportions of A2A-responding neurons, Fisher’s exact test). These results support the conclusion that the A2A receptor exerts an antagonism on the effect of the D2 receptor and, in view of the absence of effect using the A2A agonist alone, suggest that A2A receptors need the stimulation of D2 receptors to generate a response effectively. Such a functional interaction between A2A and D2 receptors, in which stimulation of A2A receptors counteracts the effects of D2 receptor stimulation has been previously suggested to be dependent on the intramembrane A2A–D2 receptor interaction (Ferré et al, 1993; Salim et al, 2000; Stromberg et al, 2000), which is now known to be dependent on A2A–D2 receptor heteromerization (Dasgupta et al, 1996; Salim et al, 2000; Hillion et al, 2002; Canals et al, 2003; Ciruela et al, 2004).
Figure 5.
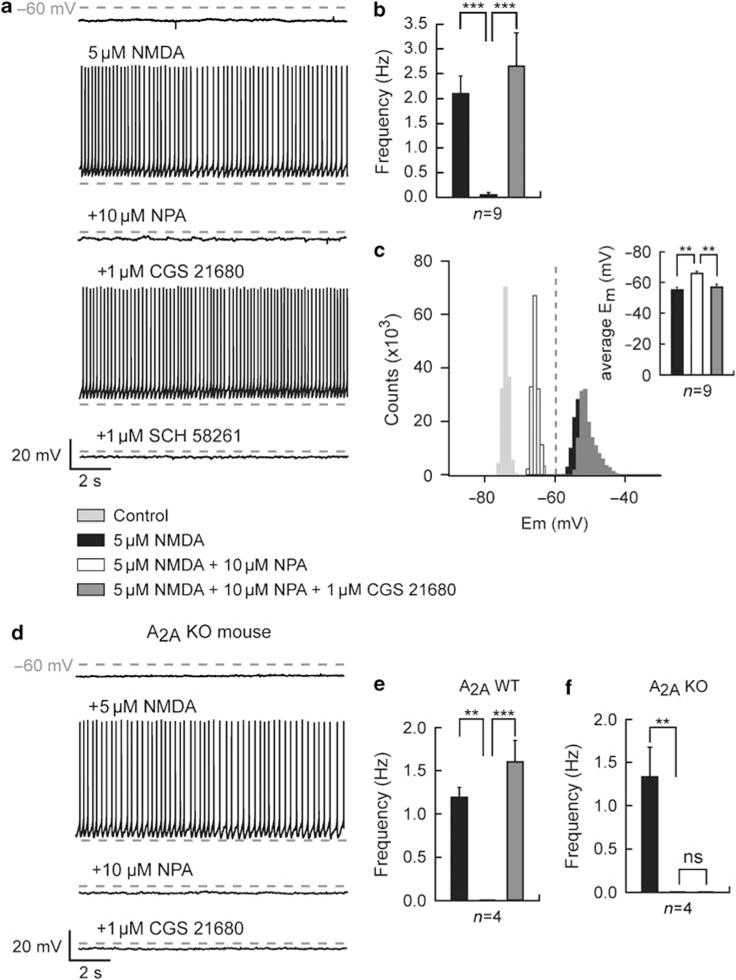
Interaction of dopamine D2 and adenosine A2A receptors modulates the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential on D2-responsive neurons. (a) Consecutive traces showing typical transitions where the action of NMDA (5 μM) was recorded before and in the presence of D2 receptor agonist R(−)-propylnorapomorphine hydrochloride (NPA, 10 μM), A2A receptor agonist 2-[4-[(2-carboxyethyl)-phenyl]ethyl-amino]-5′-N-(ethylcarbamoyl)adenosine (CGS 21680, 1 μM), and A2A receptor antagonist 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-l,2,4-triazolol[l,5-c]pyrimidine (SCH 58261, 1 μM). On a D2-responsive neuron, subsequent application of CGS 21680 totally counteracts the effect of D2 receptor activation, ie the inhibition of the depolarized plateau potential and firing frequency. The A2A receptor modulation on this D2-responsive neuron was reversed by the selective A2A receptor antagonist SCH 58261 (1 μM). (b) Summary histogram obtained from nine D2-responsive neurons illustrates the antagonistic effect of A2A receptors activation on the action potential firing frequency (n = 9). (c) Typical all-point histogram from a single neuron shows the membrane potential distributions before and after additional application of NMDA, NPA, and CGS 21680. In these conditions, activation of NMDA receptors set and A2A receptors reset the neurons in a depolarized state, whereas activation of D2 receptors holds the neuron in a hyperpolarized state. In inset, a summary histogram illustrates the significant modulation of the average membrane potential after the subsequent application of NMDA, NPA, and CGS 21680. (d) Consecutive traces of a D2-responsive medium spiny neuron (MSN) from an A2A receptor knockout mouse. As expected, subsequent application of CGS 21680 (1 μM) fails to reverse the D2 receptor-induced hyperpolarized potential. (e, f) Summary histograms of the effects of A2A receptor activation on D2-responsive cells of wild-type (e, n = 4 out of 6 recorded neurons) and A2A receptor null mice (f, n = 4 out of 6 recorded neurons). Application of CGS 21680 reverses the D2 receptor-induced hyperpolarized potential in wild-type, whereas it does not affect the firing frequency of D2-responsive MSNs from A2A receptor null mice (data represent mean ± SEM; **p<0.0l, ***p<0.00l).
D2 Receptor-Mediated Suppression of NMDA-Induced Depolarized Plateau and its Reversal by A2A Receptors is not Dependent on a Presynaptic Mechanism
The Cav1.3 subunits that form a major class of L-type Ca2 + channel in neurons open at a rather hyperpolarized membrane potentials likely to be achieved during modest synaptic stimulation (Koschak et al, 2001; Xu and Lipscombe, 2001). Therefore, these channels have been even considered as low-threshold calcium channels (see eg Olson et al, 2005) and have been involved as a major actor of the transitions between down- and upstates in MSNs (Olson et al, 2005). Nevertheless, as, in addition to their postsynaptic side of interaction, D2 and A2A could also act presynaptically (Schiffmann et al, 2007), we have performed experiments with current injection instead of NMDA application to exclude a presynaptic influence. In this condition, we demonstrated that D2 receptor activation significantly decreased the firing frequency (2.44 ± 0.31 Hz for injected current and 1.38 ± 0.29 Hz for NPA; n = 9; p<0.05; Figure 6a and b) and that this effect is counteracted by the coapplication of the A2A agonist (1.38 ± 0.29 Hz for NPA and 3.13 ± 0.22 Hz for CGS 21680; n = 9; p<0.05; Figure 6a and b). These data similar to the effects on the NMDA-induced depolarized plateau strongly suggest that presynaptic influences are not a major mechanism and reinforce the hypothesis of the involvement of postsynaptic Cav1.3a as a target of the modulation.
Figure 6.
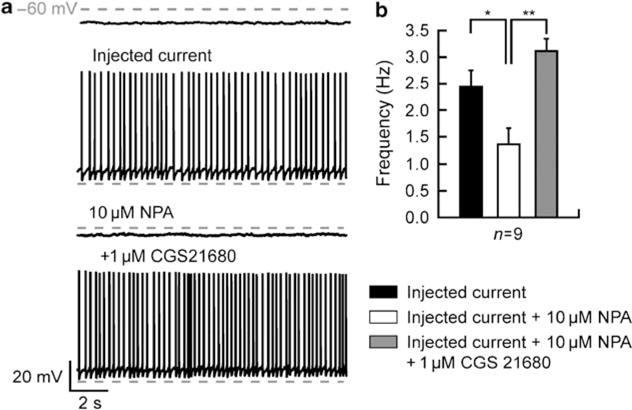
Modulatory interaction of dopamine D2 and adenosine A2A receptors on the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential is not dependent on a presynaptic mechanism. (a) Consecutive traces, recorded in a single neuron submitted to the injection of current (to mimic the action of NMDA), showing typical transitions recorded before and in the presence of D2 receptor agonist R(−)-propylnorapomorphine hydrochloride (NPA, 10μM) and A2A receptor agonist 2-[4-[(2-carboxyethyl)-phenyl]ethyl-amino]-5′-N-(ethylcarbamoyl)adenosine (CGS 21680, 1 μM). The application of NPA suppresses the depolarized the plateau potential and inhibits the action potential firing induced by the injection of current. Subsequent application of CGS 21680 blocks the D2 effect and reestablishes the depolarized plateau potential. (b) Summary histogram obtained from nine different neurons illustrating the effect of the D2 receptor agonist on the firing frequency. Application of NPA (10 μM) significantly reduces the frequency of action potential firing. Activation of A2A receptors by CGS 21680 counteracts the effects of D2 receptor activation on the action potential firing frequency (data represent mean±SEM; *p<0.05, **p<0.0l).
D2 Receptor-Mediated Suppression of NMDA-Induced Depolarized Plateau and its Reversal by A2A Receptors in Striatopallidal MSNs
To identify definitively the striatal neurons exhibiting this D2–A2A antagonistic response, we performed experiments on brain slices from mice expressing the EGFP under the control of the D2 receptor gene promoter (the ‘D2-GFP’ mice; Gong et al, 2003) (Figure 7a). Several groups have demonstrated that in this mouse strain GFP-positive neurons are specifically striatopallidal neurons. D2 receptor activation abolished the NMDA-induced firing pattern in all GFP-positive recorded neurons (2.64 ± 0.4 Hz for NMDA and 0 ± 0 Hz for NPA; n = 6; p< 0.05) and that this effect is counteracted by the coapplication of the A2A agonist (2.81 ± 0.68 Hz, p<0.05) (Figure 7b and c). These data definitively identified the neurons targeted by the D2–A2A modulation as striatopallidal neurons.
Figure 7.
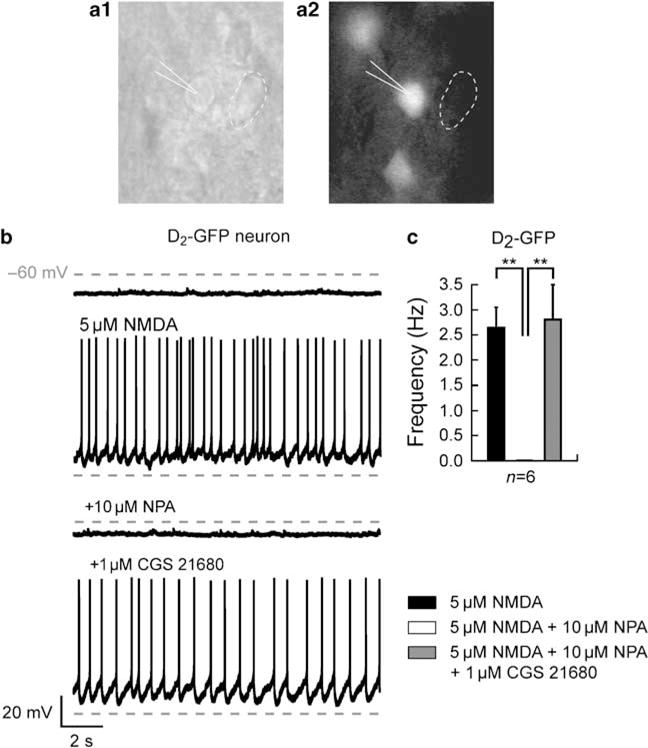
Modulatory interaction of dopamine D2 and adenosine A2A receptors on the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential occurs in striatopallidal medium spiny neurons (MSNs). Striatal acute slice from D2-enhanced green fluorescent protein (EGFP) mice in phase contrast (al) and during epifluorescence (a2). The drawing pipette identifies a D2-EGFP-positive neuron and the dashed circle a non-D2-EGFP-positive neuron. (b) Consecutive traces recorded in a single D2-EGFP-positive neuron, where the action of NMDA (5 μM) was recorded before and in the presence of D2 receptor agonist R(−)-propylnorapomorphine hydrochloride (NPA, 10 μM) and A2A receptor agonist 2-[4-[(2-carboxyethyl)-phenyl]ethyl-amino]-5′-N-(ethylcarbamoyl)adenosine (CGS 21680, 1 μM). In the presence of NMDA, application of NPA suppresses the plateau potential and inhibits the action potential firing on the D2-EGFP-positive recorded neuron. Subsequent application of CGS 21680 blocks the D2 effect and reestablishes the depolarized plateau potential. (c) Summary histogram obtained from six D2-EGFP-positive neurons illustrating the effect of the D2 receptor agonist on the firing frequency. Application of NPA (10 μM) totally abolishes the frequency of action potential firing in all recorded neurons, which is fully reversed by A2A receptors activation.
A2A Receptors Counteract the D2 Receptor-Mediated Suppression of NMDA-Induced Depolarized Plateau Through A2A–D2 Receptor Heteromerization
To analyze if the functional interaction between A2A and D2 receptors could be related to a direct receptor-receptor interaction at the membrane level through heteromerization, we used the strategy of specific competitive peptides dialysis as described above. We first demonstrated that by using this approach, recordings without dialyzing any peptide did not affect the A2A receptor modulation. Indeed, under this condition, in the three D2 receptor-responsive neurons (see above) out of five, the inhibition of the NMDA-induced spike firing (0.22 ± 0.22 Hz) was fully antagonized by a subsequent activation of A2A receptor (1.87 ± 0.25 Hz; n = 3; p<0.05; data not shown). Ciruela et al (2004) have shown that a domain centered on a phosphorylated serine in a SAQES motif in the C-tail of the adenosine A2A receptor is involved in A2A–D2 receptor heteromerization (Ciruela et al, 2004; Woods and Ferré, 2005). If the A2A antagonistic modulation of D2 receptor function requires a direct protein-protein interaction with the D2 receptor, the peptide containing the SAQES motif should block the A2A receptor modulation by disrupting A2A–D2 receptor heteromerization. We therefore recorded series of neurons loaded with either a phosphorylated (SAQEpS peptide) or a nonphosphorylated (SAQES peptide) form of the SAQES peptide. From the 12 recorded neurons that were dialyzed with the SAQEpS peptide, only 3 neurons exhibited a full D2 receptor-mediated inhibition of the NMDA-induced spike firing that was not reversed by A2A receptor activation (Figure 8b, inset). For the whole population of recorded cells, this D2 receptor effect was not significant (1.65 ± 0.27 Hz for NMDA and 1.49 ± 0.38 Hz for NPA; n = 12; p>0.05; Figure 8b) although it was significant for the three responding neurons (1.52 ± 0.28 Hz for NMDA and 0±0Hz for NPA; n = 3; p<0.05; 0±0Hz for CGS 21680, p>0.05 as compared to NPA; Figure 8b, inset). On the other hand, the ability of the D2-like agonist to modulate the spike firing frequency in the depolarized plateau was unaffected by dialysis with the SAQES peptide (1.17 ± 0.16 Hz for NMDA and 0.11 ± 0.1 Hz for NPA; n = 7; p<0.05) with six D2-responding neurons out of seven (p<0.05, Fisher’s exact test). In contrast, dialysis with this nonphosphorylated SAQES peptide also blocked the ability of A2A receptors to counteract the D2 receptor effect (0.11 ± 0.1 Hz for NPA and 0.22 ± 0.15 Hz for CGS 21680; n = 7; p>0.05; Figure 8a–c) with only one out of six D2-responding neurons exhibiting a reversal by the A2A agonist. As the blockade of the A2A effect appeared similar for both peptides, we hypothesized that the SAQES peptide was endogenously phosphorylated during the loading and recovery periods, allowing it to disrupt A2A–D2 receptor heteromerization as with the SAQEpS peptide. When we dialyzed a nonphosphorylable peptide containing an AAQEA motif in which the serine residues have been substituted by alanine, the ability of the D2-like agonist to inhibit the NMDA-induced spike firing was unaffected (1.02 ± 0.09 Hz for NMDA and 0.26 ± 0.16 Hz for NPA; n = 7; p<0.05), with five D2-responding neurons out of seven. Importantly, in this condition, the ability of A2A receptor agonist to counteract the D2 receptor-mediated firing inhibition (1.2 ±0.1 Hz; n = 7; p<0.05; Figure 8c) was fully preserved with five A2A-responding neurons out of five; this latter proportion being significantly different than the one observed after loading the SAQES or SAQEpS peptides (p<0.05, Fisher’s exact test). These peptides do not influence the basal membrane properties with resting membrane potential of −68.65 ±1.2 mV and input resistance of 579 ± 59.57 MΩ in basal condition (n = 24), −67.42 ± 1.28 mV and 468 ± 63.94 MΩ for SAQES peptide (n = 7), −70.85 ± 1.06 mV and 554 ± 41.02 MΩ for SAQEpS peptide (n = 12), −70.76 ± 1.62 mV and 486 ± 32.69 MΩ for AAQE peptide (n = 7), (p>0.05; data not shown). These data are consistent with the hypothesis that A2A–D2 receptor heteromerization is a major mechanism for the modulatory activity of A2A receptors in MSNs. Moreover, it is worth mentioning that the proportion of neurons responding to the D2 receptor activation is significantly lower when neurons have been loaded with the SAQEpS peptide as compared to neurons loaded with SAQES or AAQEA peptides (p<0.05, Fisher’s exact test), suggesting that A2A–D2 receptor heteromerization could be in some way partially required to allow D2 receptor to be active.
Figure 8.
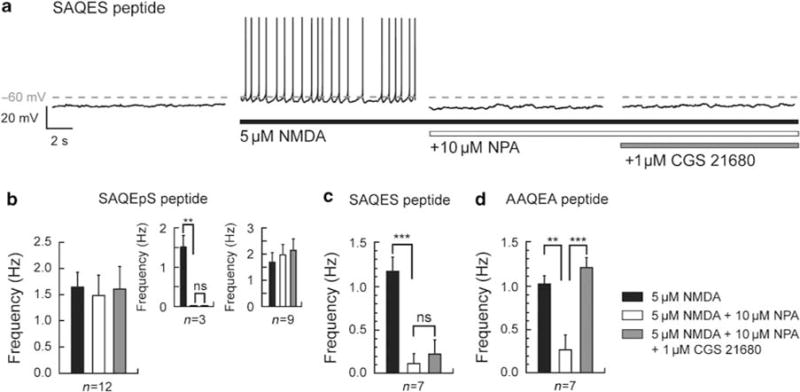
SAQES peptides disrupt functional D2–A2A receptor heteromerization. (a) Consecutive traces in a neuron dialyzed with the SAQES peptide corresponding to the C-terminal epitope of the A2A receptor that interacts with the N-terminal portion of the third intracellular loop of the D2 receptor. In this dialyzed neuron, in presence of N-methyl-D-aspartate (NMDA), application of R(−)-propylnorapomorphine hydrochloride (NPA) suppresses the transition to the depolarized plateau potential and inhibits the firing frequency. The additional application of 2-[4-[(2-carboxyethyl)-phenyl]ethyl-amino]-5′-N-(ethylcarbamoyl)adenosine (CGS 21680) does not have any effect. (b) Summary histogram of NPA effect on the firing frequency of neurons dialyzed by the phosphorylated SAQEpS peptide. NPA (10 μM) and subsequent CGS 21680 (1 μM) do not modify the frequency of action potential firing (n = 12). Histograms in insets show an NPA-induced abolition of the transition to the depolarized plateau potential in three neurons, which is not reversed by subsequent CGS 21680. (c) Data obtained from neurons dialyzed with the SAQES peptide illustrating the effect of the D2 receptor agonist and subsequent A2A receptor agonist on the firing frequency are represented in the graph bar. Application of NPA (10μM) significantly reduces the frequency of action potential firing, whereas A2A receptor activation by CGS 21680 (1 μM) does not counteract D2 receptor activation. (d) Summary histogram of D2 and D2–A2A receptor activation on neurons dialyzed with the AAQEA peptide. D2 receptor activation decreases the action potential firing frequency and this effect is totally reversed by the subsequent A2A receptor activation (data represent mean ±SEM; **p<0.0l, ***p< 0.001).
DISCUSSION
In the present study, we have presented results showing that in GABAergic striatal MSNs, the NMDA-mediated excitation, leading to a depolarized plateau potential and spike firing, is regulated by dopamine and adenosine acting at D2 and A2A receptors, respectively, through direct protein–protein interactions. In GABAergic striatal MSNs, the transitions of the membrane potential between the down- and the upstate strongly depend upon excitatory synaptic inputs and are therefore considered as one of the most important NMDA-modulated processes. However, these transitions are also influenced by intrinsic conductances that could be modulated by transmitters acting on GPCR.
To study these processes, we adapted an existing model (Vergara et al, 2003) proposed to mimic in some ways these membrane potential transitions in slice preparation by application of NMDA in the bath (Vergara et al, 2003; Olson et al, 2005). We took advantage of the preservation of the intracellular content by using the perforated-patch procedure (Gall et al, 2003), adapted to allow the use of competitive peptides. In this condition, the application of NMDA gives rise to permanent depolarized plateau potential with continuous firing instead of oscillations between down- and upstate. Preliminary experiments deserving further studies demonstrated that this difference is related to the perforated-patch configuration as compared to the whole-cell configuration used by Vergara et al (2003) and not to the striatal area (ventral vs dorsal) or the slicing orientation (coronal vs parasagittal).
D2 Receptor Modulation of the NMDA-Induced Depolarized Plateau Rely upon Shank–Cav1.3a Interaction
Transitions of the membrane potential to the depolarized plateau were promoted by augmentation of inward currents that could be carried by glutamate receptors and L-type Ca2+ channels (Vergara et al, 2003; Olson et al, 2005). This was previously shown in the dorsal striatum (Vergara et al, 2003; Olson et al, 2005) and similarly demonstrated in the present study in the accumbens nucleus. D2 receptor activation in MSNs results in the suppression of L-type Ca2+ channel currents, sustained by the Cav1.3 isoform, through a cascade involving the activation of calcineurin and dephosphorylation of these channels (Hernandez-Lopez et al, 2000; Olson et al, 2005). Moreover, this D2 receptor-mediated modulation is dependent on physical interactions between Shank proteins and Cav1.3a Ca2+ channels through a specific PDZ-binding domain in this channel (Olson et al, 2005; Zhang et al, 2005). We showed that D2 receptor activation strongly inhibits the NMDA-induced transition to a depolarized plateau potential with continuous firing, which was fully suppressed in about 60% of all recorded MSNs. This is close to the proportion of D2 receptor-expressing MSNs belonging to the striatopallidal subpopulation (Gerfen et al, 1990; Schiffmann and Vander-haeghen, 1993). This specificity of the D2 receptor effect in the striatopallidal subpopulation was firmly confirmed by using D2-GFP mice. By using peptide competition protocols, we also demonstrated that the Shank1/3–Cav1.3a protein–protein interaction is critical for this D2 receptor-mediated modulation of the depolarized plateau potential. Thus, this demonstrated that the D2 receptor-mediated inhibition of Cav1.3a channel currents is a major molecular mechanism for the D2 receptor-induced abolition of the NMDA-induced depolarized plateau in MSNs, and, hence, most probably of down- to upstate transitions in these neurons, as previously suggested (Olson et al, 2005). D2 receptor modulation of spontaneous activity and states transitions has been described in vivo (see eg Onn et al, 2000; West and Grace, 2002). In most of these studies, D2 receptor activation led to an inhibition of membrane excitability and favored the hyperpolarized membrane potential state. This is consistent with the presently reported D2 receptor-mediated inhibition of NMDA-induced excitation. It is worth to note that our results were obtained in brain slices from young animals while in vivo studies are usually conducted on adult animals. Although never described for the D2 and A2A signaling cascades in the striatum, it is not excluded that these cascades could be different at adulthood, as described for the D2 receptor in the prefrontal cortex (Tseng and O’Donnell, 2007).
Antagonistic A2A–D2 Receptors Modulation of the NMDA-Induced Depolarized Plateau Is Dependent on A2A–D2 Receptors Heterodimerization
Striatopallidal GABAergic enkephalinergic neurons coexpress predominantly D2 and A2A receptors (Schiffmann et al, 1991, 2007; Schiffmann and Vanderhaeghen, 1993; Svenningsson et al, 1999), which strongly modulate the functions of these neurons through antagonistic interactions (Schiffmann and Vanderhaeghen, 1993; Ferré et al, 1993, 1997; Stromberg et al, 2000). These tight interactions rely upon two nonexclusive putative mechanisms, intramembrane receptor–receptor interactions and the intracellular signaling cascades. There is an intramembrane A2A–D2 receptor interaction, by which stimulation of the A2A receptor decreases binding of dopamine to the D2 receptor (Ferré et al, 1991; Dasgupta et al, 1996; Salim et al, 2000). This interaction relies upon the formation of heteromers between A2A and D2 receptors (Dasgupta et al, 1996; Hillion et al, 2002; Canals et al, 2003; Ciruela et al, 2004) by means of an electrostatic epitope–epitope interaction between an arginine-rich domain of the D2 receptor (localized in its long third intracellular loop) and a phosphorylated serine localized in the C terminus of the A2A receptor (Ciruela et al, 2004; Woods and Ferré, 2005). On the other hand, there is a reciprocal interaction at the second messenger level, by which stimulation of D2 receptor inhibits A2A receptor-mediated activation of the cAMP–PKA cascade (Kull et al, 1999; Hillion et al, 2002).
We showed that activation of A2A receptor alone has no effect on the NMDA-induced depolarized plateau in MSNs even though it has been suggested to modulate NMDA current (Norenberg et al, 1998) through a signaling cascade (phospholipase C–IP3–Ca2+) that was not described by others to be activated by A2A receptor either in MSNs or elsewhere (see Schiffmann et al, 2007 for review). These results suggest that under our conditions A2A receptors need a stimulation of D2 receptors to generate a response effectively. Such a situation has been already described in other studies. For instance, stimulation of striatal A2A receptors does not have a significant effect on the release of GABA in the ipsilateral globus pallidus, but it counteracts the inhibition of pallidal GABA release induced by D2 receptor stimulation. Also, the stimulating effect of pallidal A2A receptors on GABA release in the globus pallidus is lost in the absence of D2 receptor influences (Floran et al, 2005). Similarly, in a neuroblastoma SH-SY5Y cell line coexpressing A2A and D2 receptors, A2A receptor activation had no effect on basal cytoplasmic calcium levels and on KCl-evoked responses whereas it fully counteracted the D2 effect (Salim et al, 2000).
A main possible mechanism involved in these A2A receptor-mediated modulations of D2 receptor function is the intramembrane A2A–D2 receptor interaction, which depends on A2A–D2 receptor heteromerization (see above). Another possibility could be related to the mechanism of D2 receptor modulation of the L-type Ca2+ channel (Hernandez-Lopez et al, 2000) identified as Cav1.3a (Olson et al, 2005), with an almost full phosphorylation of these channels in basal conditions.
We designed specific experiments using competitive peptides to address the A2A–D2 receptors heteromerization hypothesis by mimicking a specific domain of A2A receptor identified as an epitope involved in this interaction (see above). Both the phosphorylated and nonphosphorylated competitive peptides containing the serine residue abolished the effect of A2A receptor activation while the nonphosphorylable peptide in which the serine residues were substituted by alanine was without any blocking effect. These results strongly suggest that the nonphosphorylated peptide is endogenously phosphorylated and that disruption of the A2A–D2 receptors heteromerization precludes the activation of A2A receptor to counteract the effect of D2 receptor activation. Indeed, the SAQES peptide is a nanopeptide that sequence (SAQESQGNT) corresponds to a casein kinase I consensus site (Ciruela et al, 2004; Woods and Ferré, 2005). There is extensive evidence for the ability of different protein kinases, such as PKA, PKC, and also casein kinases, to phosphorylate short synthetic peptides (around 10 amino acids long) (Maller et al, 1978; Kuenzel and Krebs, 1985; Loog et al, 2000; Bustos et al, 2005). Hence, even though A2A receptor could be expected to reverse the D2-mediated inhibition by inducing CaV1.3 channel phosphorylation through the cAMP-PKA pathway (Surmeier et al, 1995; Qu et al, 2005), altogether, our results demonstrate that this specific protein–protein interaction is the main, if not the only one, mechanism for A2A receptor to control, through regulation of D2 receptor activity, the excitability of striatopallidal MSNs.
Regulation of NMDA Receptor-Mediated Depolarized Plateau in Striatal Neurons by a Shank/Cav1.3a Channels/A2a–D2 Receptor Heterodimers Complex: Functional Implications
The intraspine regulatory complex in which A2A–D2 receptors could be involved includes as a central player the scaffold protein of the Shank family. This protein interacts directly with the tail of CaV1.3 channels, indirectly with the guanylate kinase associated protein (GKAP) and postsynaptic density (PSD) 95 scaffold proteins, being therefore in close proximity to NMDA receptors (Kim and Sheng, 2004; Zhang et al, 2005), and indirectly, via Homer proteins, with IP3 receptors (Xiao et al, 2000). This latter interaction allows to bring CaV1.3 channels in proximity to IP3-regulated intracellular Ca2+ stores that are critical for the calcineurin-mediated D2 regulation of the L-type CaV1.3 channels (Hernandez-Lopez et al, 2000; Olson et al, 2005). There is also some evidence that GPCR as A2A and D2 receptors can also interact with synaptic scaffolding proteins (Kreienkamp, 2002; Ciruela et al, 2005) and may thus be adequately located in the spine to take part of this regulatory complex as suggested by D2 and A2A receptors ultrastructural studies (Hersch et al, 1995; Rosin et al, 2003; Ciruela et al, 2005).
Several GPCR heteromers have been recognized (Agnati et al, 2003; Milligan, 2006). However, the functional significance of these GPCR heteromers remains poorly understood in most cases. The A2A–D2 receptor heteromer is one of the most studied as compared to other receptor heteromers (reviewed in Ferré et al, 1997; Schiffmann et al, 2007). However, although it is well established that the A2A–D2 intramembrane interaction is a biochemical characteristic of this heteromer, a clear functional implication of this interaction was missing. On the other hand, the interaction of both receptors at the second messenger level (adenylyl cyclase) has been shown to be responsible for functional interactions at the gene transcription level (reviewed in Ferré et al, 1997; Schiffmann et al, 2007). The present results demonstrate that the A2A–D2 intramembrane interaction is involved in the control of neuronal excitability. Through an intermolecular cross talk in the A2A–D2 receptor heteromer, A2A receptor modulates D2 receptor-mediated suppression of L-type CaV1.3 channel currents. Thus, A2A receptor activation alone was not able to modulate the depolarized plateau, even though it could be expected to induce the CaV1.3 channel phosphorylation through the cAMP-PKA pathway (Surmeier et al, 1995; Qu et al, 2005). Furthermore, a competitive peptide mimicking the C-terminal epitope of the A2A receptor required for A2A–D2 receptors heteromerization fully abolished the modulatory effect of A2A receptor activation on the D2 receptor-mediated suppression of L-type CaV1.3 channel, which involves D2 receptor-Gβγ-phospholipase C signaling pathway (see Figure 9). Altogether, these results constitute one of the first sets of data showing a direct physiological relevance of the A2A–D2 receptor heterodimer on neuronal functions and, hence, in a broader perspective, the functional relevance of GPCR heteromers on neuronal functions.
Figure 9.
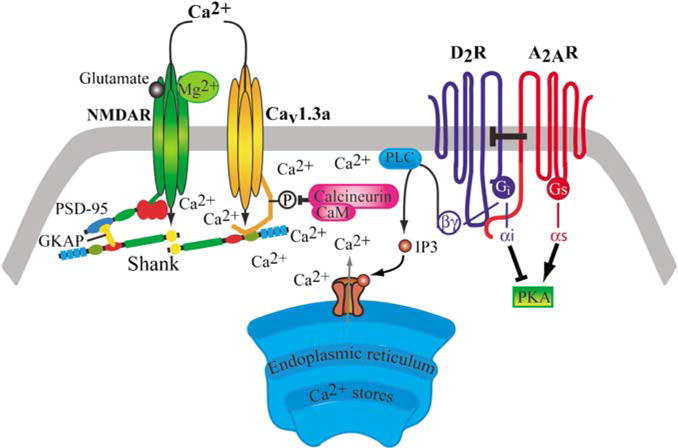
Schematic presentation of our proposed model for the involvement of protein–protein interactions, at the postsynaptic dendritic spine, in the modulation of the N-methyl-D-aspartate (NMDA)-mediated depolarized plateau potential in the striatopallidal medium spiny neuron. According to our hypothesis the D2R-mediated suppression of NMDA-induced depolarized plateau is mediated by the suppression of Cav1.3a L-type calcium channel current through the D2R-PLC signaling cascade involving the activation of calcineurin and dephosphorylation of these channels. This modulation requires the physical interaction between scaffolding Shank proteins and Cav1.3a calcium channels through a specific PDZ-binding domain. The A2AR counteracts the D2R-mediated suppression of NMDA-induced depolarized plateau via a direct A2AR–D2R interaction at the membrane level through heteromerization. NMDAR, NMDA receptor; Cav1.3a, Cav1.3a L-type calcium channel; A2AR adenosine A2A receptor; D2R, dopamine D2 receptor; Golf, G-protein activating adenylyl cyclase; Gi, G-protein inhibiting adenylyl cyclase; PLC, phospholipase C; PKA, protein kinase A; CaM, calmodulin; IP3, inositol 1,4,5-triphosphate; Shank, multiple ankyrin repeats-SH3 domain-PDZ domain-proline-rich region-sterile-α motif containing protein; PSD-95, postsynaptic density 95; GKAP, guanylate kinase-associated protein.
The ability of dopamine and adenosine acting at D2 and A2A receptors, respectively, to strongly modulate induction and maintenance of a depolarized plateau potential with continuous firing, reminiscent of the in vivo down- to upstate transitions, in striatopallidal neurons through their regulation of L-type CaV1.3 channels and hence to modulate their neuronal excitability, could also lead to long-term modifications in neuronal functions such as synaptic and nonsynaptic plasticity (Wang et al, 2006; Adermark and Lovinger, 2007). Such effects in this subpopulation of striatal neurons at the origin of the indirect pathway (Alexander and Crutcher, 1990) should therefore have major consequences on the functions of the basal ganglia system both in the motor control and in the reward processes as well as in pathologies in which they have been involved as Parkinson’s disease or drug addiction.
Acknowledgments
This work was supported by FMRE (Belgium), FNRS (Belgium), Van Buuren Foundation, FER from ULB, Action de Recherche Concertée (2002–2007) from the CFWB, EC (QLG3-20001-01056), and the intramural funds of NIDA. DG was supported by FNRS.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that except for income received from the primary employer no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;25:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutic implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Bustos VH, Marin O, Meggio F, Cesaro L, Allende CC, Allende JE, et al. Generation of protein kinase Ck1alpha mutants which discriminate between canonical and non-canonical substrates. Biochem J. 2005;391:417–424. doi: 10.1042/BJ20050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Burgueno J, Casado V, Canals M, Marcellino D, Goldberg SR, et al. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope–epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Canela L, Burgueno J, Soriguera A, Cabello N, Canela EI, et al. Heptaspanning membrane receptors and cytoskeletal/scaffolding proteins: focus on adenosine, dopamine, and metabotropic glutamate receptor function. J Mol Neurosci. 2005;26:277–292. doi: 10.1385/JMN:26:2-3:277. [DOI] [PubMed] [Google Scholar]
- D’Alcantara P, Ledent C, Swillens S, Schiffmann SN. Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience. 2001;107:455–464. doi: 10.1016/s0306-4522(01)00372-4. [DOI] [PubMed] [Google Scholar]
- D’Angelo E, De Filippi G, Rossi P, Taglietti V. Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J Physiol. 1995;484:397–413. doi: 10.1113/jphysiol.1995.sp020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Ferré S, Kull B, Hedlund PB, Finnman UB, Ahlberg S, et al. Adenosine A2A receptors modulate the binding characteristics of dopamine D2 receptors in stably cotransfected fibroblast cells. Eur J Pharmacol. 1996;316:325–331. doi: 10.1016/s0014-2999(96)00665-6. [DOI] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, O’Connor WT, Fuxe K, Ungerstedt U. The striatopallidal neuron: a main locus for adenosine–dopamine interactions in the brain. J Neurosci. 1993;13:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Floran B, Gonzalez B, Floran L, Erlij D, Aceves J. Interactions between adenosine A(2a) and dopamine D2 receptors in the control of [(3)H]GABA release in the globus pallidus of the rat. Eur J Pharmacol. 2005;520:43–50. doi: 10.1016/j.ejphar.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Gall D, Roussel C, Susa I, D’Angelo E, Rossi P, Bearzatto B, et al. Altered neuronal excitability in cerebellar granule cells of mice lacking calretinin. J Neurosci. 2003;23:9320–9327. doi: 10.1523/JNEUROSCI.23-28-09320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001;21:4498–4504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci. 2004;19:2455–2463. doi: 10.1111/j.0953-816X.2004.03344.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, et al. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, et al. Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ. Organisation of G-protein-coupled receptor signalling complexes by scaffolding proteins. Curr Opin Pharmacol. 2002;2:581–586. doi: 10.1016/s1471-4892(02)00203-5. [DOI] [PubMed] [Google Scholar]
- Kuenzel EA, Krebs EG. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci USA. 1985;82:737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull B, Ferré S, Arslan G, Svenningsson P, Fuxe K, Owman C, et al. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Loog M, Toomik R, Sak K, Muszynska G, Järv J, Ek P. Peptide phosphorylation by calcium-dependent protein kinase from maize seedlings. Eur J Biochem. 2000;267:337–343. doi: 10.1046/j.1432-1327.2000.01002.x. [DOI] [PubMed] [Google Scholar]
- Maller JL, Kemp BE, Krebs EG. In vivo phosphorylation of a synthetic peptide substrate of cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA. 1978;75:248–251. doi: 10.1073/pnas.75.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. G-protein-coupled receptor heterodimers: pharmacology, function and relevance to drug discovery. Drug Discov Today. 2006;11:541–549. doi: 10.1016/j.drudis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Assmann H, Richter M, Illes P. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids. 1998;14:33–39. doi: 10.1007/BF01345239. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, et al. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of \{alpha\}1D (Cav1.3) L-type Ca channel by protein kinase A. Am J Physiol Heart Circ Physiol. 2005;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Salim H, Ferré S, Dalal A, Peterfreund RA, Fuxe K, Vincent JD, et al. Activation of adenosine A1 and A2A receptors modulates dopamine D2 receptor-induced responses in stably transfected human neuroblastoma cells. J Neurochem. 2000;74:432–439. doi: 10.1046/j.1471-4159.2000.0740432.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha R, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Popoli P, Muller CE, Ferreé S, Fuxe K. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur J Neurosci. 2000;12:4033–4037. doi: 10.1046/j.1460-9568.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, et al. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AS, Ferré S. Amazing stability of the arginine-phosphate electrostatic interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, et al. Association of CaV1.3L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


