Abstract
Background and Purpose
Oral anticoagulants are highly efficacious for the prevention of stroke in atrial fibrillation, and are the preferred treatment by current guidelines. The purpose of our study was to assess the utilization of antithrombotic drugs in atrial fibrillation patients at the time of ischemic stroke and the factors associated with their use.
Methods
We enrolled 759 consecutive patients admitted with ischemic stroke at Boston Medical Center, Geisinger Health System, and the University of Alabama. To be eligible, patients had to have electrocardiographically-confirmed atrial fibrillation at the time of admission or within 6 months of the index stroke. All stroke events and electrocardiograms were validated by study physicians. Patients with newly diagnosed atrial fibrillation were not eligible.
Results
The mean age was 78 years, 43% were male, 19% black, and the mean CHADS2 score 3.0. Atrial fibrillation was paroxysmal in 31%. At presentation, 181 (24%) patients were taking warfarin only, 96 (13%) both warfarin and aspirin, 294 (39%) aspirin alone, and 189 (25%) no antithrombotic therapy. The mean international normalized ratio was 1.6. Among patients with paroxysmal atrial fibrillation, one in five was taking warfarin. Although increasing stroke risk was associated with a greater likelihood of warfarin use, only 39% of highest risk CHADS2 3–6 were taking warfarin at the time of stroke.
Conclusions
Among high-risk individuals with atrial fibrillation, only 37% were taking warfarin at the time of stroke. Paroxysmal atrial fibrillation was associated with the highest risk of not receiving warfarin.
Keywords: Atrial fibrillation, oral anticoagulation, warfarin utilization, embolic stroke
1. Introduction
Stroke is a highly prevalent disease in the United States affecting more than 6.8 million Americans with an incidence of 795,000 persons/year. It is projected that by 2030 four million more people will suffer a stroke,[1, 2] and the overall medical cost associated with stroke will rise to 184.3 billion dollars. This cost represents a 157% relative increase from 2013.[3] Atrial fibrillation (AF) is an independent risk factor for stroke, increasing the risk by 5-fold. Data from the Framingham study and the Austrian stroke registry have demonstrated that strokes associated with AF have up to two times higher mortality, higher recurrence rate, and are associated with worse neurological and functional outcomes compared to non-AF strokes.[4, 5]
Oral anticoagulation (OAC) reduces the rate of ischemic stroke as well as the severity of and mortality from stroke.[6] The increased utilization of OAC in patients with AF between 1992 and 2002 resulted in a reduction in ischemic stroke rates from 46.7 to 19.5 per 1000 patient-years.[7] Use of OAC at the time of the stroke reduces the severity of ischemic stroke,[8] the short term stroke-associated mortality,[9] and results in more favorable long-term outcomes.[10] Aspirin is substantially less efficacious compared to OAC (20% vs. 60% risk reduction accordingly),[11] and currently is recommended only for the lowest risk patients.[12–14]
Despite the strong evidence supporting the use of OAC for stroke prevention in patients with AF, it remains significantly underused.[15–17] Analysis of Medicare data reveal that cost related to strokes in AF patients who are not on OAC rises to 4.8 billion USD.[18] Given the significant impact of OAC underuse, we sought to identify factors associated with OAC use at the time of ischemic stroke among individuals with previously diagnosed AF.
2. Methods
2.1. Study population
Patients admitted with ischemic stroke were identified over a 5-year period (2006–2010) from three health systems: Boston Medical Center (BMC), Geisinger Health System in Pennsylvania (GHS), and the University of Alabama at Birmingham (UAB). BMC is a teaching hospital and major safety net hospital for the city of Boston. GHS is a highly integrated health care system that serves a predominantly rural population in Pennsylvania. UAB is part of the southeastern “stroke belt” and cares for a widely diverse patient population. To be eligible, patients had to have electrocardiogram (ECG)-confirmed AF at the time of admission or within 6 months prior to the stroke, if paroxysmal. Patients with newly diagnosed AF at the time of stroke were not eligible for this study. Patients with mechanical heart valves were excluded as were patients whose stroke was secondary to a vascular procedure, infection, tumor, or vasculitis. Race was defined by self-report. Socioeconomic status was determined using zip codes. All stroke events and AF ECGs were confirmed by study physicians. Antithrombotic therapy, stroke risk factors, and the international normalized ratio (INR) were determined from stroke admission records. Antithrombotic therapy includes antiplatelet therapy with aspirin and OAC with warfarin. In this cohort, non-vitamin K oral anticoagulants were not utilized. The study was approved by the institutional review committees of the participating institutions and subjects gave informed consent.
2.2. Identification of stroke cases
Stroke events were identified using ICD-9 codes for ischemic stroke (433–434, 436). To ensure the most comprehensive search, we included ICD-9 codes identified in any position-primary or secondary. Among these ICD-9 identified stroke events, those cases associated with AF (ICD-9 code 427.31) were subject to detailed medical record review. A valid ischemic stroke was defined as a neurologic deficit of sudden onset that persisted for >24 hours, corresponded to a vascular territory, and was not explained by other etiologies, such as intracerebral hemorrhage, tumor, infection, or vasculitis.[19, 20] Stroke cases were adjudicated by site investigators based on review of the medical record. All stroke events underwent final review by site neurologists.
2.3. Statistical analysis
Analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Continuous variables are summarized as means ± standard deviations and categorical variables as counts (percentages). Bivariate analyses were performed with contingency tables, and p-values were derived with the Chi-square statistic. Multiple logistic regression analysis was performed to evaluate for independent factors associated with antithrombotic use at the time of admission. The variables included in univariate and multivariate analysis were pre-specified and based on clinically relevant factors. All p-values correspond to two-sided tests and the alpha criterion was set to 0.05.
3. Results
3.1. Patient characteristics
Across the three sites, 759 patients were identified (BMC: 176, GHS: 297, and UAB: 286; Table 1). The mean age of the study participants was 78 years and the mean CHADS2 score 3.0±1.4. Forty-three percent (328) of participants were male, 19% (143) black, and 4% (27) had a race different than white or black. Approximately one-third of patients had paroxysmal AF (PAF) and one-half were older than 80 years of age. At presentation, 24% (181) of patients were on OAC only, 13% (96) on both OAC and aspirin, 39% (293) on aspirin only, and 25% (189) were receiving neither OAC nor aspirin. The mean INR of the patients taking OAC was 1.6. 188 patients (33.6%) had INR within the therapeutic range (2.0 to 3.0) and 371 patients (66.4%) had sub-therapeutic INR. Twenty one (2.7%) patients had a prior intracranial hemorrhage and none of them was receiving OAC.
Table 1.
Baseline characteristics according to antithrombotic medication at time of ischemic stroke
| Variable | Overall n=759 | Aspirin Only n=293 | Anticoagulant Only n=181 | Aspirin + Anticoagulant n=96 | No Aspirin or Anticoagulant n=189 | |
|---|---|---|---|---|---|---|
|
|
||||||
| Age, years | Mean (SD) | 77.8 (11.0) | 78.8 (11.2) | 77.4 (9.8) | 78.2 (9.4) | 76.6 (12.4) |
| Age, %, (n) | ≥80 | 50.2% (381) | 54.9% (161) | 45.3% (82) | 44.8% (43) | 50.3% (95) |
| <80 | 49.8% (378) | 45.1% (132) | 54.7% (99) | 55.2% (53) | 49.7% (94) | |
| Sex, %, (n) | Female | 56.8% (431) | 53.6% (157) | 59.7% (108) | 54.2% (52) | 60.3% (114) |
| Male | 43.2% (328) | 46.4% (136) | 40.3% (73) | 45.8% (44) | 39.7% (75) | |
| Race, %, (n) | White | 77.6% (589) | 77.5% (227) | 78.5% (142) | 86.5% (83) | 72.5% (137) |
| Black | 18.8% (143) | 19.1%(56) | 18.2% (33) | 10.4% (10) | 23.3% (44) | |
| Other | 3.6% (27) | 3.4% (10) | 3.3% (6) | 3.1% (3) | 4.2% (8) | |
| Median Income, $ | Mean (SD) | 47,706 (17,190) | 47,597 (17,423) | 48,939 (17,845) | 46,517 (13,413) | 47,300 (17,932) |
| AF Type, %, (n | Paroxysmal | 31.4% (238) | 37.5% (110) | 17.7% (32) | 18.8% (18) | 41.3% (78) |
| Permanent | 68.6% (521) | 62.5% (183) | 82.3% (149) | 81.3% (78) | 58.7% (111) | |
| Hypertension, %, (n) | 91.2% (692) | 92.2% (270) | 90.6% (164) | 91.7% (88) | 89.9% (170) | |
| Diabetes Mellitus, %, (n) | 38.6% (293) | 40.6% (119) | 39.8% (72) | 40.6% (39) | 33.3% (63) | |
| Congestive Heart Failure; %, (n) | 39.1% (297) | 43.0% (126) | 37.0% (67) | 47.9% (46) | 30.7% (58) | |
| Coronary Artery Disease (prior MI, CABG, PCI); %, (n) | 42.3% (321) | 44.0% (129) | 41.4% (75) | 60.4% (58) | 31.2% (59) | |
| Peripheral Vascular Disease, %, (n) | 10.8% (82) | 11.6% (34) | 7.2% (13) | 14.6% (14) | 11.1% (21) | |
| Chronic Kidney Disease, %, (n) | 18.8% (143) | 20.8% (61) | 12.7% (23) | 17.7% (17) | 22.2% (42) | |
| Active Malignancy (chemo, XRT, palliative care), %, (n) | 10.7% (81) | 13.3% (39) | 8.8% (16) | 9.4% (9) | 9.0% (17) | |
| Dementia, %, (n) | 17.1% (130) | 17.4% (51) | 14.9% (27) | 19.8% (19) | 17.5% (33) | |
| Hepatitis, %, (n) | 0.7% (5) | 0.7% (2) | 0.0% (0) | 0.0% (0) | 1.6% (3) | |
| Cirrhosis, %, (n) | 1.1% (8) | 1.0% (3) | 0.6% (1) | 1.0% (1) | 1.6% (3) | |
| COPD, %, (n) | 16.2% (123) | 15.7% (46) | 20.4% (37) | 16.7% (16) | 12.7% (24) | |
| Asthma, %, (n) | 7.6% (58) | 5.8% (17) | 10.5% (19) | 7.3% (7) | 7.9% (15) | |
| Sleep Apnea, %, (n) | 7.1% (54) | 7.2% (21) | 7.2% (13) | 9.4% (9) | 5.8% (11) | |
| Prior CVA, %, (n) | 27.3% (207) | 24.9% (73) | 32.6% (59) | 34.4% (33) | 22.2% (42) | |
| Prior ICH, %, (n) | 2.8% (21) | 2.7% (8) | 0.0% (0) | 0.0% (0) | 6.9% (13) | |
| Prior TIA, %, (n) | 14.6% (111) | 11.3% (33) | 16.6% (30) | 18.8% (18) | 15.9% (30) | |
| Prior DVT or PE, %, (n) | 10.3% (78) | 7.8% (23) | 12.7% (23) | 17.7% (17) | 7.9% (15) | |
| Admitted from SNF, %, (n) | 12.0% (91) | 12.3% (36) | 6.6% (12) | 9.4% (9) | 18.0% (34) | |
AF: atrial fibrillation, CABG: coronary artery bypass grafting, COPD: chronic obstructive pulmonary disease, CVA: cerebrovascular accident, DVT: deep vein thrombosis, ICH: intracranial hemorrhage, MI: myocardial infarction, PCI: percutaneous coronary intervention, PE: pulmonary embolism, SD: standard deviation, SNF: skilled nursing facility, TIA: transient ischemic attack, XRT: radiation therapy.
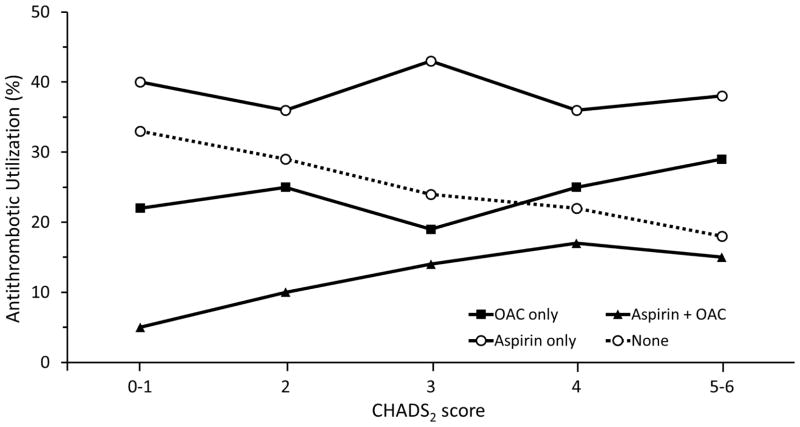
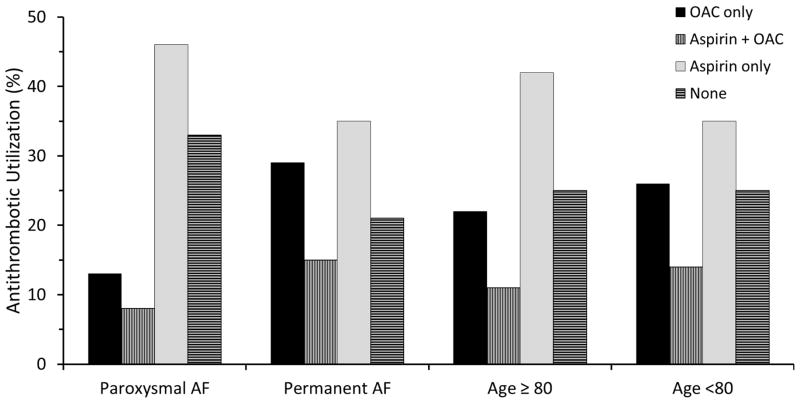
Although increasing stroke risk was associated with a greater likelihood of OAC use, only 39% of patients at highest risk CHADS2 scores of 3–6 were taking OAC at the time of stroke (Figure 1). Patients who were 80 years of age and older were less likely to be taking OAC compared to those younger than 80 years of age (45.1% vs. 54.9%, p=0.034, Figure 2). Among patients with PAF, approximately one in five was taking OAC compared to those patients with persistent or permanent AF (21% vs. 43.6%, p<0.001, Figure 2). We found no difference in CHADS2 scores between the groups: PAF, mean CHADS2 2.97 versus permanent AF, mean CHADS2 3.08. There was no difference in OAC use by sex (p=0.18), income (p=0.68), or race (p=0.18).
Figure 1. Antithrombotic utilization by CHADS2 scores.
Increasing stroke risk was associated with a greater likelihood of OAC use. However, still only 39% of patients at highest risk CHADS2 scores of 3–6 were taking OAC at the time of stroke.
Figure 2. Antithrombotic utilization across ischemic stroke patient subgroups.
Patients with paroxysmal AF were less likely to be taking OAC compared to those with persistent or permanent AF (21% vs. 43.5%, p<0.001). Patients who were 80 years of age and older were less likely to be taking OAC compared to those younger than 80 years of age (45.1% vs. 54.9%, p=0.034).
3.2. Independent factors associated with OAC use
Patients with permanent AF had a 3-fold higher likelihood of taking OAC at baseline compared to those individuals with PAF (OR: 3.25, 95% CI: 2.25–4.69, p<0.001, Table 2). Higher stroke risk was also associated with OAC use (OR: 1.22, 95% CI: 1.09–1.37, p<0.001). Patients who were 80 years of age or older were less likely to be taking OAC at time of ischemic stroke (OR: 0.51, 95% CI: 0.37–0.72, p<0.001).
Table 2.
Multivariable analysis of the influence of factors on anticoagulant use among AF patients presenting with ischemic stroke.
| Variable | OR | [95% CI] | p-value |
|---|---|---|---|
|
|
|||
| Age (≥80 vs. <80) | 0.51 | [0.37, 0.72] | <0.001 |
| CHADS2 Score (continuous) | 1.22 | [1.09, 1.37] | <0.001 |
| AF Type (Permanent vs. Paroxysmal) | 3.25 | [2.25, 4.69] | <0.001 |
| Sex (Female vs. Male) | 1.22 | [0.88, 1.68] | 0.23 |
AF: atrial fibrillation, OR: odds ratio, 95% CI: 95% confidence interval.
4. Discussion
In this study of patients with known AF, we found the prevalence of OAC use at the time of ischemic stroke to be 37%. Approximately 39% of patients were taking aspirin only, and 25% were taking neither OAC nor aspirin. Of those individuals treated with OAC, the mean INR was 1.6. Although increasing stroke risk was associated with greater likelihood of OAC use, only 39% of the highest CHADS2 risk patients were taking OAC at the time of stroke. Our study documents a gross underuse of OAC among patients with PAF; only one in five was taking OAC at time of stroke. Older patients (age ≤ 80) were also less likely to be taking OAC.
Despite the overwhelming evidence, multiple studies have documented the underuse of OAC among individuals with AF. The National Anticoagulation Benchmark Outcomes Report (NABOR) conducted in 2002 demonstrated that only 55% of high-risk patients with AF were prescribed warfarin.[17] A similar rate of OAC utilization was reported in Europe.[21, 22] The Global Anticoagulant Registry in the FIELD (GARFIELD) with 540 sites in 19 countries reported significant underuse of OAC in high-risk patients, with 38% of patients with a CHADS2 score ≤2 not being on OAC.[23] Several studies have evaluated use of antithrombotic therapy among individuals with known AF at time of incident stroke (Table 3).[6, 24–29] Of the 7 studies cited, six were from a single center outside of the United States, four were conducted over a decade ago, three included fewer than 150 patients, and validation of the AF and ischemic stroke diagnoses was variable. Across these different AF populations, OAC use ranged from 12% in the Japanese study to 42% in a small Canadian study (n=106) that excluded patients with possible contraindications to OAC therapy.
Table 3.
Studies reporting oral anticoagulation utilization at the time of incident stroke
| Study | Study period | Population | Stroke cases (N) | AF ascertainment | Stroke ascertainment | OAC use (%) | Comments |
|---|---|---|---|---|---|---|---|
| Deguchi et al.[26] | 2007–2011 | Japan Single center |
234 | ECG, 24-hour holter monitor, or chart review | CT or MRI | 14 | OAC use rate was significantly lower in PAF compared to chronic AF cases (8% vs 18.5%, p=0.02). |
| Paciaroni et al.[25] | 2000–2003 | Italy Single center |
238 | ECG on admission or prior ECG | CT | 13 | 52% of cases were not receiving any antithrombotic therapy (OAC or ASA). |
| Indredavik et al.[27] | 1996–2002 | Norway Single center |
394 | Prior ECG, chart review, patient self-report | Chart review and CT scan | 29 | Cases receiving combination therapy of ASA plus OAC were excluded. 45% of patients on OAC had INR <2. 28% of cases were not receiving any antithrombotic therapy (OAC or ASA). |
| Aronis et al. (current study) | 2006–2010 | U.S. 3 centers |
759 | ECG | Chart review CT or MRI | 37 | |
| Hylek et al.[6] | 1996–1999 | U.S. Single center |
596 | ICD-9, ECG, or both | Chart review, CT and MRI | 32 | Median INR=1.7. 62% of cases on OAC had INR<2. |
| Partington et al.[24] | 1999–2004 | Canada Single center | 106 | Canadian, institute for health information codes, chart review | ICD-10, chart review | 54 | Patients with no known diagnosis of AF a valid contraindication to warfarin use, CHADS2 <1, or a competing diagnosis for OAC use were excluded. Of the non-anticoagulated patients 46% had no documented reason for withholding OAC. |
| Pujol Lereis et al.[28] | Not reported | Argentina Single center | 112 | Not reported | Chart review, imaging | 35 | Utilization of OAC was based on whether patients had taken warfarin within 24 hours prior to admission. INR was not considered. 11% of cases had their antithrombotic treatment discontinued within 2-weeks of the index stroke event. 54% had a therapeutic INR; mean INR=2.3 (median 2.05). |
| Palm et al.[29] | 2006–2007 | Germany Registry | 106 | ECG, holter monitor | Chart review, imaging | 36 | Only cases with CHADS2 ≥ 2 were analyzed. 15% had a therapeutic INR; 35% of cases were off OAC for unexplained reasons. |
AF: atrial fibrillation, ASA: aspirin, CT: computed tomography, ECG: electrocardiogram, ICD-9: International Classification of Diseases, Ninth Revision, ICD-10: International Classification of Diseases, Tenth Revision, INR: international normalized ratio, MRI: magnetic resonance imaging, N: number, OAC: oral anticoagulation therapy PAF: paroxysmal atrial fibrillation.
Our study highlights the persisting liability associated with paroxysmal AF. Although it has been reported that patients with PAF are younger and have fewer cardiovascular risk factors, lower risk does not explain our findings.[30–32] We found no difference in CHADS2 scores between the groups: PAF, mean CHADS2 2.97 versus persistent/permanent 3.08. Results of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial initially highlighted the importance of continuing anticoagulation therapy among individuals with AF despite seeming restoration of sinus rhythm.[33] In the NABOR study, hospitalized patients with PAF were less likely to be discharged on OAC.[17] Studies of AF in Poland and Turkey[21, 34] also reported decreased use of OAC among patients with paroxysmal AF as did the Deguchi Japanese study of individuals with AF admitted with stroke.[26] Partly underlying this gap in treatment is the uncertainly regarding the duration of a single episode of AF that mandates lifelong anticoagulation. In CRYptogenic STroke And underLying AF (CRYSTAL AF) trial 8.9% of patients that suffered a cryptogenic stroke were found to have AF.[35] Subclinical AF has been detected in 10% of asymptomatic individuals and is independently associated with a 2.5-fold increase in risk of ischemic stroke or systemic embolism.[36] Asymptomatic AF is also present in 53–56% of patients after ablation for AF.[37, 38] Following ablation, AF is more likely to be shorter, regular, and more asymptomatic (up to 79% compared to 52% before ablation).[38] Ongoing randomized trials assess whether stopping OAC after successful AF ablation is safe (Table 4a). The dearth of robust evidence relating stroke risk to AF burden drives at least in part the variability in physician treatment patterns. Answers to these questions will be forthcoming given the multitude of ongoing AF detection studies utilizing various external and implantable monitoring modalities (Table 4b). In addition, the patient- and physician-specific factors that lead to discontinuation of OAC therapy and the communication strategies that underlie and influence these decisions are poorly understood.
Table 4.
PICO model for selected planned and ongoing clinical trials assessing the role of anticoagulation therapy in patients (a) after successful AF ablation and (b) patients with subclinical AF.
| Trial | Design | Patient population |
Intervention | Comparison | Definition of Silent AF |
Primary Outcome |
Clinical Trial Registration |
Study Start Date |
Estimated Completion Date |
|---|---|---|---|---|---|---|---|---|---|
| 4a. Clinical trials assessing the role of anticoagulation therapy in patients after successful AF ablation | |||||||||
| Optimal Anticoagulation for Higher Risk Patients Post- Catheter Ablation for Atrial Fibrillation Trial (OCEAN) | RCT | -CHADS2 ≥ 1 -One year after successful AF ablation -No AF recurrence (>30 sec of AF on 7- day holter) |
Rivaroxaban 20 mg daily | Aspirin 81 mg daily | N/A | Stroke, systemic embolism and silent cerebral infarction | NCT02168829 | September 2015 | December 2021 |
| Safety of Oral Anticoagulation Therapy Withdrawal After Successful Cardiac Ablation in Patients With Atrial Fibrillation and Associated High Risk Factors for Embolic Events (OAT Pilot Study) | RTC | -CHADS2 score ≥ 2 or CHA2DS2- VASc score ≥3 -Successful AF ablation -No AF recurrence (>30 sec of AF) |
Discontinuation of OAC | Continuation of OAC | N/A | Stroke or systemic embolism | NCT01959425 | August 2013 | February 2017 |
| Prevention of Silent Cerebral Thromboembolism by Oral Anticoagulation With Dabigatran After Pulmonary Vein Isolation for Atrial Fibrillation (ODIn-AF) | RTC | - CHA2DS2- VASc ≥ 2 -Successful AF ablation -No AF recurrence (assessed by 72h Holter ECG) |
Dabigatran 150 mg twice daily | No OAC | N/A | Micro- and macro- embolic lesions on cerebral MRI | NCT02067182 | October 2014 | June 2016 |
| Investigation on Appropriate Duration of Dabigatran Use After Catheter Ablation for Paroxysmal Atrial Fibrillation in Patients With Low Thromboembolic Risk | RTC | - CHA2DS2- VASc ≤ 1 - paroxysmal AF undergoing catheter ablation |
Dabigatran 150 mg twice daily | Placebo | N/A | Thromboe mbolism | NCT02313584 | December 2014 | September 2017 |
| 4b. Clinical trials assessing the role of anticoagulation therapy in patients with subclinical AF. | |||||||||
| SILENT - Subclinical AtrIal FibrilLation and StrokE PreveNtion Trial | RCT | -CHADS2 ≥ 2 - implantable device | -Intensive monitoring (device interrogation every 2 months) -AF detection triggers OAC |
-Routine monitoring -AF detection triggers OAC |
-AF for >5.5 hours per day | -Stroke or systemic embolism | NCT02004509 | February 2015 | October 2020 |
| Atrial Fibrillation Detected by Continuous ECG Monitoring Using Implantable Loop Recorder to Prevent Stroke in High-risk Individuals | RCT | ->70 years, -And at least one of: diabetes, hypertension, heart failure or previous stroke | -ILR -AF detection triggers OAC |
-Routine care -AF detection triggers OAC |
AF episode > 6 minutes | Stroke or systemic embolism | NCT02036450 | January 2014 | January 2019 |
| Apixaban for the Reduction of Thrombo- Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) | RTC | -CHA2DS2- VASc ≥ 4 -Permanent implantable cardiac device -At least one episode of device-detected AF |
Apixaban 5 mg twice daily | Aspirin 81 mg daily | AF episode > 6 minutes | Stroke or systemic embolism | NCT01938248 | April 2015 | July 2019 |
| Rhythm Evaluation for AntiCoagulaTion With COntinuous Monitoring | Pilot (Single -arm study) | -CHADS2 1–2 -Non- continuous AF |
NOAC for 30 days after an episode of AF detected ILR | N/A | AF>30 seconds | - Anticoagu lant Utilization -Stroke rate |
NCT01706146 | October 2012 | February 2015 |
AF: atrial fibrillation, ILR: implantable loop recorder, NOAC: non-vitamin K antagonist oral anticoagulant, OAC: oral anticoagulation therapy, RCT: randomized controlled trial.
Despite the increasing risk of ischemic stroke, multiple studies have demonstrated the paradox of decreased OAC use with older age and higher CHADS2 scores.[17, 21, 24, 34, 39, 40] We have previously shown that bleeding risk and risk of warfarin discontinuation increase with CHADS2 score.[39] Despite this increase in bleeding risk, the net benefit strongly supports use of OAC in these high risk groups.[41] Although the newer anticoagulants are associated with a dramatic reduction in intracranial hemorrhage, the most devastating complication of OAC therapy, the rates of the more frequent complication, gastrointestinal hemorrhage, are either similar to warfarin or increased. A better understanding of the mechanisms underlying this age-related vulnerability is needed to enhance long-term maintenance of OAC therapy.[42] Cessation of treatment following a gastrointestinal hemorrhage is associated with higher mortality and increased thrombotic risk.[43, 44] We found no difference in OAC use based on socioeconomic status or race.
Our study has several strengths. Eligible patients had ECG-confirmed AF at the time of stroke admission or within 6 months consistent with the study population definition used in recent AF trials.[45, 46] All stroke events underwent final review by site neurologists. Stroke risk factors were derived from in-depth review of the medical record as was aspirin status which is frequently missing in pharmacy administrative data due to its nonprescription, over-the-counter availability. The large number of events allowed for multivariable analyses to determine factors independently associated with OAC use at the time of the index stroke. Our multi-center cohort is the largest U.S. study to date to address this critical question.
In summary, among high-risk individuals with previously diagnosed AF, only 37% were taking OAC at the time of ischemic stroke. Paroxysmal AF and older age were associated with the highest risk of not receiving OAC, while higher CHADS2 score was associated with OAC use. Given the morbidity and mortality related to AF stroke, development of strategies to address this documented undertreatment is of paramount importance.
Acknowledgments
Grant Support: National Institutes of Health: 1RO1NS070307
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed in the acquisition, analysis and interpretation of data as well as critical revision of the manuscript for important intellectual content. Drs. Hylek, Berger, and Limdi contributed in study conception and design. Drs. Aronis, Thigpen and Hylek contributed in drafting of the manuscript. Dr. Tripodis and Ms. Quinn contributed in Statistical analysis. Study was supervised by drs. Hylek, Berger and Limdi. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Dr. Hylek has served on Advisory Boards for Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Medtronic, and Pfizer. The other authors have no conflict of interest to disclose. The National Institutes of Health has not been involved, directly or indirectly, in the collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–75. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 4.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–4. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 5.Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25:1734–40. doi: 10.1016/j.ehj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 7.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10-year perspective (1992 to 2002) Stroke. 2006;37:1969–74. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 8.Haeusler KG, Konieczny M, Endres M, Villringer A, Heuschmann PU. Impact of anticoagulation before stroke on stroke severity and long-term survival. Int J Stroke. 2012;7:544–50. doi: 10.1111/j.1747-4949.2011.00672.x. [DOI] [PubMed] [Google Scholar]
- 9.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke. 2012;43:1795–9. doi: 10.1161/STROKEAHA.111.630731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon N, Callaly E, Moore A, Ni Chroinin D, Sheehan O, Marnane M, et al. Improved late survival and disability after stroke with therapeutic anticoagulation for atrial fibrillation: a population study. Stroke. 2011;42:2503–8. doi: 10.1161/STROKEAHA.110.602235. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 14.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–36. doi: 10.1016/j.cjca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. doi: 10.1186/1471-2296-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnaiz E, Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta neurologica Scandinavica Supplementum. 2003;179:34–41. [PubMed] [Google Scholar]
- 17.Waldo AL, Becker RC, Tapson VF, Colgan KJ. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46:1729–36. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 18.Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care. 2004;10:S451–58. discussion S8–61. [PubMed] [Google Scholar]
- 19.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 20.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 21.Bednarski J, Cieszewska E, Strzelecki A, Filipiak KJ. Anticoagulant and antiplatelet therapy for stroke prevention in atrial fibrillation patients in the clinical practice of a single district hospital in Poland. Kardiol Pol. 2013;71:1260–5. doi: 10.5603/KP.a2013.0179. [DOI] [PubMed] [Google Scholar]
- 22.Arts DL, Visscher S, Opstelten W, Korevaar JC, Abu-Hanna A, van Weert HC. Frequency and risk factors for under- and over-treatment in stroke prevention for patients with non-valvular atrial fibrillation in general practice. PLoS One. 2013;8:e67806. doi: 10.1371/journal.pone.0067806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8:e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partington SL, Abid S, Teo K, Oczkowski W, O'Donnell MJ. Pre-admission warfarin use in patients with acute ischemic stroke and atrial fibrillation: The appropriate use and barriers to oral anticoagulant therapy. Thromb Res. 2007;120:663–9. doi: 10.1016/j.thromres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Paciaroni M, Agnelli G, Caso V, Venti M, Milia P, Silvestrelli G, et al. Atrial fibrillation in patients with first-ever stroke: frequency, antithrombotic treatment before the event and effect on clinical outcome. J Thromb Haemost. 2005;3:1218–23. doi: 10.1111/j.1538-7836.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 26.Deguchi I, Ogawa H, Ohe Y, Nemoto M, Tanahashi N. Rate of antithrombotic drug use and clinical outcomes according to CHADS2 scores in patients with an initial cardioembolic stroke who had nonvalvular atrial fibrillation. J Stroke Cerebrovasc Dis. 2013;22:846–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Indredavik B, Rohweder G, Lydersen S. Frequency and effect of optimal anticoagulation before onset of ischaemic stroke in patients with known atrial fibrillation. J Intern Med. 2005;258:133–44. doi: 10.1111/j.1365-2796.2005.01512.x. [DOI] [PubMed] [Google Scholar]
- 28.Pujol Lereis VA, Ameriso S, Povedano GP, Ameriso SF. Ischemic stroke in patients with atrial fibrillation receiving oral anticoagulation. J Neurol Sci. 2013;334:139–42. doi: 10.1016/j.jns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Palm F, Kleemann T, Dos Santos M, Urbanek C, Buggle F, Safer A, et al. Stroke due to atrial fibrillation in a population-based stroke registry (Ludwigshafen Stroke Study) CHADS(2) , CHA(2) DS(2) -VASc score, underuse of oral anticoagulation, and implications for preventive measures. Eur J Neurol. 2013;20:117–23. doi: 10.1111/j.1468-1331.2012.03804.x. [DOI] [PubMed] [Google Scholar]
- 30.Rizos T, Wagner A, Jenetzky E, Ringleb PA, Becker R, Hacke W, et al. Paroxysmal atrial fibrillation is more prevalent than persistent atrial fibrillation in acute stroke and transient ischemic attack patients. Cerebrovasc Dis. 2011;32:276–82. doi: 10.1159/000330348. [DOI] [PubMed] [Google Scholar]
- 31.Rabinstein AA, Fugate JE, Mandrekar J, Burns JD, Seet RC, Dupont SA, et al. Paroxysmal atrial fibrillation in cryptogenic stroke: a case-control study. J Stroke Cerebrovasc Dis. 2013;22:1405–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–61. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 33.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 34.Kaya H, Ertas F, Koroglu B, Vatan B, Cagliyan CE, Gedik S, et al. Predictors of Anticoagulant Treatment in Patients With Nonvalvular Atrial Fibrillation: Results From Atrial Fibrillation in Turkey: Epidemiologic Registry. Clin Appl Thromb Hemost. 2013 doi: 10.1177/1076029613491459. [DOI] [PubMed] [Google Scholar]
- 35.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 36.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 37.Klemm HU, Ventura R, Rostock T, Brandstrup B, Risius T, Meinertz T, et al. Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2006;17:146–50. doi: 10.1111/j.1540-8167.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 38.Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173:149–56. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 39.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, et al. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–26. doi: 10.1093/eurheartj/ehl015. [DOI] [PubMed] [Google Scholar]
- 41.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- 42.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 43.Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–91. doi: 10.1001/archinternmed.2012.4261. [DOI] [PubMed] [Google Scholar]
- 44.Raunso J, Selmer C, Olesen JB, Charlot MG, Olsen AM, Bretler DM, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J. 2012;33:1886–92. doi: 10.1093/eurheartj/ehr454. [DOI] [PubMed] [Google Scholar]
- 45.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 46.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]