Abstract
Anemia, which is highly prevalent in oncology patients, is one of the most established negative prognostic factors for several gynecologic malignancies. Multiple factors can cause or contribute to the development of anemia in patients with gynecologic cancers; these factors include blood loss (during surgery or directly from the tumor), renal impairment (caused by platinum-based chemotherapy), and marrow dysfunction (from metastases, chemotherapy, and/or radiation therapy). Several peri- and intra-operative strategies can be used to optimize patient management and minimize blood loss related to surgery. Blood transfusions are routinely employed as corrective measures against anemia; however, blood transfusions are one of the most overused healthcare interventions. There are safe and effective evidence-based blood transfusion strategies used in other patient populations that warrant further investigation in the surgical oncology setting. Blood is a valuable healthcare resource, and clinicians can learn to use it more judiciously through knowledge of the potential risks and complications of blood interventions, as well as the ability to properly identify the patients most likely to benefit from such interventions.
Keywords: gynecologic malignancy, blood transfusion, anemia, cancer
Anemia in the cancer patient
Prevalence and pathogenesis
Secondary to disease progression or as a consequence of treatment, many cancer patients will require blood products at some point during their continuum of care. This need for blood often coincides with the development of symptomatic anemia. According to the World Health Organization, a hemoglobin (Hb) level ≥ 12 g/dL (120 g/L) is considered normal in non-pregnant women. Mild, moderate, and severe anemia are identified at Hb levels of 11.0–11.9, 8.0–10.9, and <8.0 g/dL, respectively (1). While numerical cutoffs do not reflect patient comorbidities, which contribute significantly to the variation in symptomatology, they are the main parameters used to guide transfusion practice. More than 6 million units of red blood cells (RBCs) are transfused in the United States annually, at an estimated cost of $1600–$2400 per transfusion event (2–4). Oncology patients account for 34% of this blood supply use and cost (5).
Clinicians use low Hb concentration or low hematocrit as the complete blood count parameters to define anemia. Symptoms are dependent upon the degree of anemia and the rate at which it develops. The reduction of Hb concentration to 5 g/dL maintains adequate tissue oxygen delivery in healthy resting adults, with symptoms occurring only when the Hb concentration drops below this level. In the postoperative setting, Hb levels of 7.1–8.0 g/dL have a low risk of death, with the mortality rate rising to 34.4% with Hb levels of 4.1–5.0 g/dL (6). There are several compensatory mechanisms in response to anemia, such as increased heart rate, increased respiratory rate, and a right shift of the oxygen-dissociation curve, all with the goal to maintain adequate oxygen delivery to the tissue. The main symptoms of anemia, such as dyspnea, palpitations and fatigue, are manifestations of these compensatory mechanisms. Functional impairment and a decline in subjective well-being are also highly distressing symptoms that are detrimental to a patient’s quality of life and affect their ability to tolerate cancer treatments.
Multiple factors can cause or contribute to anemia in the oncology setting. Patients should undergo a basic work-up to identify possible causes. The work-up should include reticulocyte, creatinine, iron (serum iron, total iron-binding capacity, transferrin saturation, and serum ferritin), B12, and folate measurements (7). Cancer-related anemia (CRA), which can occur in patients with malignancies, is considered a cytokine-mediated process between tumor cells and the immune system, with an overexpression of certain pro-inflammatory cytokines, specifically interleukin-1 (IL-1) and tumor necrosis factor (TNF). These cytokines have been shown to impair iron utilization, suppress erythroid maturation, and reduce erythropoietin (EPO) production (8). In CRA, hepcidin, an iron regulatory peptide, is upregulated and inhibits the transport of iron via macrophages in the duodenum, decreasing gastrointestinal absorption and the accessibility of stored iron. CRA is typically a normochromic, normocytic anemia associated with a low reticulocyte count.
The European Cancer Anemia Survey (ECAS), which was conducted across 24 European countries and 748 cancer centers, assessed more than 13,600 patients for the incidence and prevalence of anemia across various cancer types and stages of treatment. In the study, anemia was defined as an Hb level <12.0 g/dL. Gynecologic malignancies accounted for 11.6% of all cases. The ECAS found a prevalence of anemia of 39.3% and 67.0% at enrollment and during the survey, respectively. When looking specifically at patients with gynecologic cancers, 48.1% were anemic at enrollment, of which only 42.7% ever received treatment for their anemia (9). In gynecologic malignancies, the most common factors associated with the development of anemia are blood loss (during surgery or directly from the tumor), renal dysfunction (secondary to platinum-based chemotherapy), and marrow dysfunction (from metastases, chemotherapy, and/or radiation) (Figure 1).
Figure 1. The multi-factorial pathogenesis of anemia in the cancer patient.
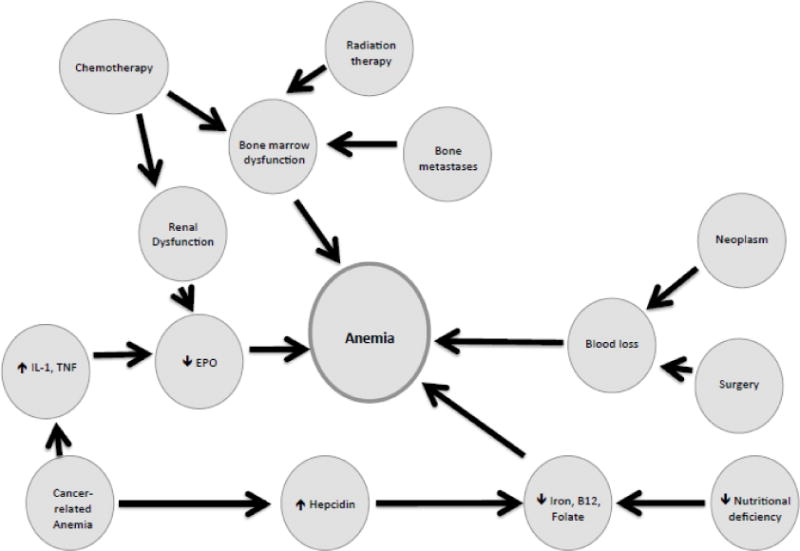
IL-1 – Interleukin 1; TNF – tumor necrosis factor; EPO – erythropoietin
Prognosis
In a systematic review assessing anemia as an independent prognostic factor for survival in patients with various cancers, Caro et al. noted reduced median survival times of 20% to 43% across cancer types in anemic patients compared to those without anemia, with an overall adjusted HRR of 1.65 (aHRR, 1.19–1.75) (10). Findings from a systematic review by Knight et al. showed that nearly all studies reported an association between anemia and decreased survival or increased mortality for multiple cancer types. The authors cautioned that the relationships between anemia and disease progression, treatment response, and overall survival may not be causal, as these findings were based on observational studies (11).
There is abundant evidence establishing anemia as one of the most prevailing prognostic factors in patients with cervical cancer (Table 1) (12–28). Most published studies exploring this relationship are retrospective and include patients who had undergone some form of radiation therapy. This poses a confounder on prognosis, since anemia may predict resistance to radiation (Refer to section: Implications for Radiation Therapy), indirectly impacting survival. Alternatively, anemia may independently characterize more aggressive tumors, representing a surrogate for poor prognosis. It is unclear whether Hb is an independent prognostic factor for outcome or a surrogate for advanced disease. What is clear is that patients with cervical cancer and anemia have shortened survivals.
Table 1.
Outcomes related to disease progression in patients with cervical cancer and anemia
| Authors | Study Design | Number of participants (n) | Stages of Disease | Population | Significant findings |
|---|---|---|---|---|---|
| Kapp et al., 1983 | Retrospective | 910 | IB–IVB | Most EBRT + IVRT | Low hematocrit (<37%) associated with lower OS and DFS, p=0.027 and p=0.01, respectively |
| Girinksi et al., 1989 | Retrospective | 386 | IIB–III | EBRT +/− IVRT | Pretreatment Hb levels (<10 g/mL versus ≥ 10 g/mL) prognostic for local and distant recurrence, RR = 1.8 (p<0.01) |
| Pederson et al., 1995 | Retrospective | 424 | IIB–IVA | Pretreatment Hb levels (value unspecified) prognostic of OS, RR = 0.88 (95% CI 0.80–0.96; p=0.008); local recurrence, RR= 0.85 (95% CI 0.75–0.96, p=0.01); and distant metastases, RR = 0.79 (95% CI 0.69–92, p=0.003) | |
| Wernerwasik et al., 1995 | Retrospective | 125 | I–II | EBRT + IVRT | Risk of recurrence increased linearly with decreasing Hb level (OR 2.02; p= 0.01) |
| Grogan et al., 1999 | Retrospective | 605 | IB–IVA | RT +/− chemotherapy | Pretreatment Hb levels (<120 g/L) prognostic of survival only on univariate analysis; average weekly nadir Hb predictive of OS on multivariate analysis (p<0.0001) |
| Haensgen et al., 2001 | Prospective | 70 | IIB, IIIB, IVA | Most EBRT + IVRT | Pretreatment Hb level <11 versus ≥11; 3-yr OS, 27% vs. 62% (p=0.006) |
| Thomas, 2001 | Retrospective | 605 | IB–IVA | Definitive RT | Significant stepwise increase in OS average weekly nadir Hb levels increased (p <0.0001) |
| Obermair et al., 2001 | Retrospective | 57 | IB–IVA | Chemo-RT | Hb nadir was the only statistically significant prognostic factor for pelvic failure (p = 0.029); severity of anemia during chemo-RT correlated with likelihood of treatment failure |
| Dunst et al., 2003 | Prospective | 87 | IIB–IVA | EBRT + IVRT | Pretreatment Hb level <11g/dL associated with significantly poorer OS (p=0.003) |
| Yalman et al., 2003 | Retrospective | 257 | II–IVA | Definitive RT | Pretreatment Hb levels (≤12.5 g/dL) prognostic of local PFS but not OS on multivariate analysis (p=0.04 and p=0.151) |
| Winter et al., 2004 | Retrospective | 494 | IIB–IVA | Chemo-RT | Average weekly nadir Hb levels ≥ 12 g/dl associated with PFS of 73% versus 40% for those with Hb <10 g/dl (p<0.0001); Hb during last third of treatment most predictive of PFS |
| Fuso et al., 2005 | Retrospective | 73 | IB2–IIB | NACT + surgery | Optimal response to chemotherapy significantly determined by pretreatment Hb levels ≥12 mg/dL, HR = 6.83 (95% CI, 2.16–21.60; p=0.001) |
| Grigeine et al., 2007 | Retrospective | 162 | II–III | EBRT+IVRT | Pretreatment Hb level (<120 g/L) associated with decreased OS, HR = 0.35 (95% CI 0.18–0.67; p=0.001) and DFS, HR = 0.41 (95% CI 0.18–0.91; p=0.04) |
| Mayr et al., 2009 | Prospective | 88 | IB2–IVA | Chemo-RT | Pretreatment Hb and nadir Hb correlated only with local control (p = 0.039 and p =0.026, respectively) but not with DSS. Median Hb during treatment (≥11.2 g/dL versus <11.2 g/dL), was prognostic of the 5-year local control, 91% versus 70% (p = 0.013) |
| Endo et al., 2014 | Retrospective | 85 | I–IVA | Concurrent chemo-RT (EBRT+IVRT) | Pretreatment Hb level (<120 g/L) prognostic of OS only on univariate analysis; HR = 1.972 (95% CI, 0.97–4.00) |
| Shin et al., 2014 | Retrospective | 805 | IB–IIA | Higher pretreatment Hb prognostic of improved DFS, HR = 0.88 (95% CI, 0.078–0.99) but not of overall survival, HR = 0.94 (95% CI, 0.80–1.10) | |
| Bishop et al., 2015 | Retrospective | 2454 | IA–III | Definitive RT | Pretreatment Hb measures were not correlated with any outcome endpoint. Minimum Hb level <10 g/dL during treatment was significantly associated with lower DSS (HR = 1.28; 95% CI 1.04–1.58) |
EBRT, external beam radiation therapy; IVRT, intravaginal radiation therapy; NACT, neoadjuvant chemotherapy; OS, overall survival; DFS, disease-free survival; DSS, disease-specific survival; H, hazard ratio; R, risk ratio; OR, odds ratio; CI, confidence interval
A retrospective analysis of 61 patients with uterine cancer who had undergone surgical treatment, Metindir et al. found that 42.6% of these patients had a pretreatment Hb level <12 g/dL. These patients were more likely to have higher rates of positive cytology and advanced International Federation of Gynecology and Obstetrics (FIGO) stage disease (29). Supporting data were shown by Njolstad et al. and Wilairat et al., who found that 5-year disease-free and overall survivals were significantly lower in patients with an Hb level <12 g/dL prior to treatment (30, 31).
The association between anemia and survival in patients with ovarian cancer was demonstrated by Munsted et al., who showed that Hb levels before and during chemotherapy significantly correlated with other important prognostic parameters, such as age at diagnosis, tumor stage, and tumor grade. Hb levels >12 g/dL prior to and during chemotherapy were significantly associated with longer survival (32). Maccio et al. found that in 91 patients with epithelial ovarian cancer, pretreatment Hb concentrations were inversely related to stage of disease and Eastern Cooperative Oncology Group (ECOG) performance status. In their analysis, the lowest Hb levels were associated with the highest concentrations of pro-inflammatory markers (IL-6, IL-1β, and TNF) (33). In a prospective review assessing the relationship of pretreatment serum Hb levels to survival in patients with epithelial ovarian carcinoma, Obermair and colleagues reported overall survival rates of 38.5% and 52.3% in patients with pretreatment Hb levels <12 g/dL and >12 g/dL, respectively (p=0.008) (34). Similar results of recurrence and survival were corroborated by subsequent analyses (35–37).
In a study of 62 patients with vulvar cancer of all stages, of whom 30.6% had anemia, a pretreatment Hb level <12 g/dL was associated with poor prognosis, but failed to show significance on multivariate analysis. The authors suggested that anemia may be a marker for a more aggressive tumor, leading to the early development of metastases (38). In addition to other factors, such as histology and stage, these studies demonstrated the importance of anemia with regard to treatment outcomes.
Implications for chemotherapy
Anemia rates related to the use of chemotherapeutic agents vary widely based on baseline Hb values, type of malignancy, and the type/duration/cycle of chemotherapy (Table 2) (39–41). The incidence and severity of anemia are highest in patients receiving dose-dense paclitaxel-carboplatin therapy. Data from the Japanese Gynecologic Oncology Group (JGOG) study 3016 indicated grade 3–4 anemia in >50% of patients; in the dose-dense arm, 90% of patients had at least one treatment delayed due to anemia (41). Cisplatin treatment is one of the most common causes of chemotherapy-induced anemia, and >40% of patients will develop anemia during treatment with this agent. Hensley et al. identified a pretreatment Hb level <10 g/dL as a significant risk factor for transfusion need in patients undergoing therapy with carboplatin-paclitaxel (42). Furthermore, patients with ovarian cancer typically undergo large debulking surgeries, making them even more susceptible to the development of anemia.
Table 2.
Reported incidence of anemia associated with chemotherapeutic agents
| Agent(s) | Grade 1, 2 | Grade 3, 4 |
|---|---|---|
| Cisplatin | 8% | 11% |
| Carboplatin | 66% | 7–26% |
| Docetaxel | 73–85% | 2–10% |
| Paclitaxel | 93% | 0–12% |
| Topotecan | 67% | 30–40% |
| Gemcitabine | 8–63% | 2–5% |
| Cisplatin + etoposide | 59% | 16–55% |
| Cisplatin + paclitaxel | 45–60% | 5–25% |
| Carboplatin + paclitaxel | 10–59% | 3–34% |
| Dose dense paclitaxel + carboplatin | 5–90% | 10–57% |
The prospective ECAS survey found that patients actively receiving chemotherapy compared to those who were not had the highest incidence of anemia; the rate of anemia increased from 19.5% during the first cycle of chemotherapy to 46.7% after the fifth. Women with gynecologic malignancies receiving chemotherapy had the highest rate of anemia overall (88.3%) in comparison to patients who had breast, lung, colorectal or urogenital cancer; leukemias; or lymphomas (9). The mean Hb to initiate a transfusion in this study was 9.7 g/dL. In a large-scale UK audit of patients with a variety of solid tumors receiving chemotherapy, Barrett-Lee et al. noted a mean Hb level drop from 10.7 g/dL with the first cycle of chemotherapy to 9.9 g/dL by the sixth (43). The absolute indication for transfusion in patients actively receiving chemotherapy is unclear, and often will depend upon chemotherapy type, duration of treatment, and the severity of patient symptoms. Patients rarely require transfusion at Hb levels >9 g/dL, and at our institution, patients are typically transfused once their Hb level falls below 7 g/dL.
Implications for radiation therapy
There is experimental and clinical evidence demonstrating the association between anemia and radiation therapy failure. Whether this is because anemia represents more aggressive disease or whether this is an independent prognostic factor is unclear. In experimental models, anemia was found to be linked to intratumoral hypoxia, and radiation was less effective in these tissues (44). One theory to explain the link between anemia and radiation therapy resistance is that the efficacy of radiation therapy depends on tissue oxygenation and that a decrease in Hb has a more profound effect on tumor tissue compared with normal tissue. Tumor hypoxia, therefore, may confer radioresistance, which subsequently decreases locoregional control and promotes tumor progression (45). Another theory linking anemia with radiation therapy resistance is that tumor hypoxia possibly causes genomic and proteomic changes (i.e., hypoxia-inducible factors 1α and 2α) that result in a more ‘aggressive’ tumor phenotype. Implicit to both theories is that the correction of anemia (and tumor hypoxia) may reverse these negative consequences; however, as previously mentioned, blood transfusions have been reported to decrease survival independently of anemia, making these data difficult to interpret.
There are numerous publications in the cervical cancer literature that have shown the link between anemia and radiation therapy failure. There are also a few retrospective studies that suggest transfusions may improve survival in this patient population. Grogan et al. collected data on 605 patients with cervical cancer treated with radiation therapy and found that the average weekly nadir Hb during radiation therapy was significantly correlated with local control, disease-free survival and overall survival, but blood transfusions appeared to overcome the negative prognostic effects of low Hb prior to and during radiation therapy (15). Based on their analysis, the authors suggested maintaining Hb levels to at least >12 g/dL during radiation therapy, which improves both pelvic control and distant metastases. In an analysis of 204 patients with cervical cancer undergoing radiation therapy and treated with regular transfusions to maintain an Hb level >11g/dL, Kapp et al. found that transfused patients had an identical prognosis compared to non-anemic patients without transfusion, suggesting the negative effects of anemia were eliminated through the correction of Hb levels (46). Other groups have reported contradictory findings, showing the use of blood transfusion is not correlated with clinical benefit (12, 20). Although the only prospective study assessing the benefit of correcting Hb levels via blood transfusion in patients with cervical cancer was underpowered for this purpose, the study findings suggested that raising a patient’s Hb level to >12 g/dL could decrease the risk of local relapse (47). The National Comprehensive Cancer Network® (NCCN®) currently recommends transfusions to achieve an Hb level >7 g/dL in hemodynamically stable, asymptomatic patients, which is in keeping with our institutional practice (48).
Indications for transfusion
Guidelines from the AABB (formerly the American Association of Blood Banks) provide a framework for guiding transfusion decisions. While few of the studies used to generate the AABB guidelines include gynecologic oncology patients, the main AABB recommendation, which recommends adhering to a restrictive transfusion strategy (≤7 g/dL) in stable hospitalized patients, is in line with the NCCN® guidelines (49). According to the practice guidelines of the American Society of Anesthesiologists task force, red blood cell transfusion is rarely indicated when Hb concentration is >10 g/dL, and it is almost always indicated when Hb concentration is <6 g/dL (50). In surgical patients exhibiting symptoms of anemia, transfusion should be considered at an Hb concentration of ≤8 g/dL.
Unlike in the critical care and cardiac surgery literature, there is no clear transfusion trigger reported in the surgical oncology and oncology literature. The Transfusion Requirements in Critical Care (TRICC) trial enrolled 838 critically ill patients who were randomly assigned to a restrictive transfusion strategy (Hb <7 g/dL and maintained at 7 to 9 g/dL) or a liberal transfusion strategy (Hb <10 g/dL and maintained at 10 to 12 g/dL). The mortality rate during hospitalization was significantly lower in the restrictive strategy group, suggesting that a restrictive strategy of RBC transfusion was non-inferior, and perhaps superior, to a liberal strategy (51).
The recent Transfusion Requirements after Cardiac Surgery (TRACS) trial showed that the use of a restrictive perioperative transfusion strategy (Hb >9.1 g/dL versus 10.5 g/dL) resulted in non-inferior rates of 30-day all-cause mortality and severe morbidity. The authors also found that the number of transfused RBC units, irrespective of the liberal versus restrictive approach, was an independent risk factor for clinical complications or death at 30 days (52). Findings from the CRIT study showed similar results, i.e., that the number of RBC units transfused in the critically ill is an independent predictor of worse clinical outcome. The authors also noted that despite data regarding RBC transfusion thresholds and risks, practice patterns in the United States have not significantly changed (53).
In patients at high risk for anemia following hip surgery, researchers found that a liberal transfusion strategy (<10 g/dL) compared to a restrictive strategy (<8 g/dL) did not result in a decreased rate of death and in-hospital complications. The authors recommended withholding transfusions in the absence of symptomatic anemia, even in elderly patients with concomitant cardiovascular disease (54). The Transfusion Requirements in Septic Shock (TRISS) trial similarly found that in patients with septic shock, those who underwent a transfusion at an Hb threshold of 7 g/dL had similar 90-day mortality, use of life support, and number of days alive compared to those who underwent transfusion at 9 g/dL (55).
A recent Cochrane review, which included more than 6200 patients, found that a restrictive transfusion strategy did not impact the rate of adverse events, was associated with a significant reduction in hospital mortality, and did not impede functional recovery (56). In these critically ill patients, a restrictive transfusion approach is non-inferior to a liberal one.
The only prospective trial to assess transfusion requirements in surgical oncology patients randomized oncology patients who had undergone abdominal surgery to either a restrictive (<7 g/dL) or liberal (<9 g/dL) transfusion strategy. Unlike the TRICC, TRACS and CRIT studies, de Almeida and colleagues randomized 198 critically ill patients admitted to the surgical intensive care unit (ICU) after surgery and found that a liberal transfusion strategy was superior in terms of the primary outcome, 30-day mortality or severe clinical complications (major cardiovascular events, intra-abdominal infections). There were no differences in the rates of septic shock, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), ICU admissions, or length of hospital stay. The study lacks long-term follow-up data, which may have important implications in terms of recurrence rates (57).
Boone and colleagues recently examined outcomes in patients with gynecologic malignancies after their center implemented a restrictive policy (transfusion of one RBC unit at a time with an Hb level <7 g/dL). Although this was a retrospective analysis, they found that morbidity and mortality were not increased with the introduction of a restrictive transfusion policy (58). Despite the abundant literature to support lower transfusion thresholds, a survey of Society of Gynecologic Oncology members reported by Price et al. showed that 44% of respondents favored a postoperative transfusion trigger of Hb ≤8 g/dL, but 32% chose a trigger of Hb ≤9 g/dL. Furthermore, most physicians indicated they would still transfuse two units at their respective thresholds, with the level of anemia not influencing the decision on how much blood to transfuse. The reason why these clinicians chose to administer 2-unit transfusions was unclear, which is indicative of how these decisions are often reflexive rather than individualized to the patient (59). This discord may be due to a lack of prospective trials evaluating transfusion parameters within the oncology setting, as well as the lack of published guidelines specific to this patient population.
Risks associated with blood transfusion
While one of the most efficient ways to correct anemia is through a blood transfusion, 20% of all transfusions lead to an adverse event, with serious complications occurring in ~0.5% of transfusions. The more common complications associated with blood transfusions are discussed in detail below, and a comprehensive review of these complications is provided in a review by Delaney et al. (60).
Transfusion reactions
Transfusion reactions are generally categorized as acute (within 24 hours of the transfusion) or delayed (>24 hours after the transfusion), and then further classified depending on whether or not fever is present. Acute reactions presenting with fever are acute hemolytic, febrile non-hemolytic, or a transfusion-related acute lung injury (TRALI). Acute hemolytic reactions are characterized by symptoms and laboratory findings of acute intravascular hemolysis (decreased haptoglobin, increased serum lactate dehydrogenase [LDH], increased indirect bilirubin, hemoglobinuria, and hemoglobinemia). Symptoms usually appear within minutes of starting the transfusion. The most common cause of an acute hemolytic reaction is ABO incompatibility, which almost always stems from medical errors occurring outside of the laboratory.
Acute febrile non-hemolytic transfusion reactions (FNHTRs) are usually attributable to the recipient’s response to residual donor leukocytes or cytokines in the plasma of the transfused product and are most often seen with the use of cellular blood products (RBCs, platelets). The reaction is defined as a rise in the patient’s temperature of ≥1°C during or shortly after completing the transfusion that cannot be attributed to the patient’s underlying condition or any other cause. Pre-storage leukocyte reduction within 72 hours of collecting the blood product removes >99% of the donor leukocytes, which significantly reduces the incidence of FNHTR.
Allergic transfusion reactions are quite common and most often manifest as mild, localized urticaria and pruritus without fever. Systematic symptoms, such as bronchospasm, nausea, vomiting and diarrhea, are also occasionally reported. The reactions occur when a transfusion recipient has preformed immunoglobulin E (IgE) antibody against an antigen in the donor plasma. Most allergic reactions respond to antihistamines, while more severe reactions/anaphylaxis may require steroids or epinephrine. Premedication with antihistamines and corticosteroids should be considered prior to subsequent transfusions only when there is a history of repeat reactions.
Lung injury
Transfusion-related acute lung injury (TRALI), which is an acute respiratory distress syndrome that occurs within 4–6 hours of a transfusion, occurs at a rate of 8.1 per 100,000 transfused components. TRALI is considered a reaction between donor leukocyte antibodies and recipient antigens, causing a downstream, cytokine-mediated interaction between neutrophils and the lung endothelium. This ultimately leads to increased microvascular permeability and pulmonary edema. Clinically, it is characterized by non-cardiogenic pulmonary edema, leading to dyspnea and hypoxemia. TRALI is the most common cause of transfusion-related fatalities, but it is often under-recognized and under-reported. As such, a consensus committee established criteria for the clinical diagnosis of TRALI (Table 3) (61).
Table 3.
Canadian Consensus Conference TRALI diagnosis criteria
|
TRALI, transfusion-related acute lung injury; ALI, acute lung injury
Transfusion-associated cardiac overload (TACO) is a common complication of blood transfusions, occurring at a rate of 1–11 per 100 RBC units transfused. Recent surgery and female gender are risk factors for the development of TACO (62). The clinical presentation of TACO includes signs of fluid overload (elevated systolic blood pressure, elevated jugular venous pressure, and pulmonary edema), dyspnea, tachypnea, and an elevated B-natriuretic peptide level. Diuresis usually leads to rapid improvement in symptoms, and fatality rates are low. A TACO diagnosis can be confirmed by the finding of new, acute pulmonary edema or vascular congestion on a chest x-ray. In high-risk patients, the development of TACO can be prevented by implementing transfusions of one unit of RBCs at a time, at a rate of 4 mL/minute (2 hours for RBCs, 3–4 hours for whole blood) (63). A diuretic before or during the transfusion may be considered in patients at greatest risk for volume overload to prevent this complication.
Immunomodulation and tumor recurrence
Allogeneic blood transfusion-related immune modulation (TRIM) is a phenomenon initially described in the renal transplant literature. Opelz et al. showed that recipients of allogeneic blood transfusions had improved renal allograft survival. Given these findings, allogeneic transfusions were administered routinely as an immunosuppressant in an attempt to prevent or delay renal allograft rejection in renal transplant patients (64). Gantt later reported that the immunosuppressive effect that is beneficial to transplant patients may be deleterious in cancer patients (65). Since then, several hundred studies on the increased risks of postoperative infections and cancer recurrence in patients receiving blood transfusions have been published (66). Although the exact mechanisms underlying TRIM continue to be debated, some researchers postulate that mechanisms suppressing host natural killer cells and activating suppressor T-lymphocytes are involved (67).
An analysis of more than 20,000 patients with colorectal cancer found that allogeneic blood transfusion was associated with an increased odds ratio (OR) of 1.66 for recurrence (95% CI, 1.–1.97) and 1.45 (95% CI, 1.19–1.46) for cancer-related mortality (68). In gynecologic malignancies, De Oliveira et al. found an association between recurrence risk and allogeneic blood transfusion in patients with epithelial ovarian cancer undergoing optimal cytoreductive surgery. Time to recurrence was 17 months in patients who had undergone a transfusion compared with 11 months for those who had not (p=0.03) (69). In cervical cancer, Azuma et al. found that after correcting for other factors, patients with stage IB cervical cancer who had not undergone a perioperative transfusion had a much higher survival rate than those who had undergone a transfusion (70). A series by Monk et al., showed that perioperative blood transfusions did not impact overall survival or time to recurrence in patients with stage IIA cervical cancer (71). Taken together, these data suggest that exposure to allogeneic transfusions should be minimized in these patients.
The immunomodulatory mechanisms of transfusions may also affect the rate of postoperative bacterial infections. There have been several randomized controlled trials investigating the association between perioperative blood transfusions and postoperative bacterial infections. A recent review of 18 randomized trials found that among hospitalized patients, a restrictive RBC transfusion strategy was associated with a reduced risk of lung and wound infections, pneumonia, and sepsis (72). In a systematic review and meta-analysis of patients who had undergone surgery for colorectal cancer, the OR for developing postoperative infection following a transfusion was 3.27 (2.05–5.20) (68). Bernard et al. found that one unit of RBCs significantly increased the risk of pneumonia (OR, 1.24) and sepsis (OR, 1.29), and that transfusions with two units increased this risk by an additional 1.25 and 1.53, respectively (73).
Peri- and postoperative blood transfusions also have been associated with an increased risk of venous thromboembolism (VTE), higher composite morbidity, mortality, and length of hospital stay (3, 74).
Infectious risk
Infectious pathogen transmission is a less-common risk in patients undergoing blood transfusion, but it can pose a serious health risk nonetheless (Table 4). Over the last 30 years, there have been huge improvements to ensure the safety of modern blood products. The introduction of nucleic acid testing (used to detect human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and West Nile virus) and the bacterial screening of platelets has hugely reduced the transmission of these pathogens. The approach to the safety of the blood supply, however, has remained largely reactive. When an emerging infectious agent (e.g., Zika, Dengue, Chikungunya) is identified as a risk to the blood supply, a new test to detect the agent must be developed, tested, and Food and Drug Administration (FDA) approved for widespread use. During this time, transfusion recipients may be at serious risk for infection. The need for a proactive strategy towards blood safety led to the development of pathogen inactivation (PI) technology.
Table 4.
Transfusion-associated risk of viral transmission
| Virus | Risk |
|---|---|
| Human Immunodeficiency Virus | 1 in 1,466,671 |
| Hepatitis C Virus | 1 in 1,148,628 |
| Hepatitis B Virus | 1 in 292,561 |
| Human T-cell Lymphotropic Virus | 1 in 2,678,836 |
While PI of blood products has been used extensively in Europe for many years, it has only recently been FDA approved in the United States. The basic concept behind PI technology is to inactivate, rather than remove or reduce, any pathogens that are present in the blood product, and prevent these pathogens from growing or reproducing, rendering them non-infectious. There are several such technologies available, but currently, only the INTERCEPT™ blood system (Cerus Corporation, Concord, California) is FDA cleared for use in plasma and platelets (75).
The INTERCEPT™ system uses a psoralen (amotosalen), which intercalates between the nucleotide base pairs of any DNA or RNA present in the blood product. The product is then photo-activated with UV-A light, which leads to the irreversible cross-linking of nucleic acids. Any remaining psoralens and photoproducts are removed prior to infusion by a charcoal filter (76). Within the platelet products, the DNA and RNA of residual white blood cells are also damaged. PI products do not need to be irradiated for graft-versus-host disease prevention. The advantage of the nonspecific targeting of all nucleotide base pairs is that unknown infectious organisms can be targeted before they are even identified. For example, studies have shown that INTERCEPT™ technology was effective against the Dengue, Chikungunya, and West Nile viruses even before they were identified in the US blood supply (77). The system’s extensive use in Europe has given credence to its safety, as there has not been an increase in reported adverse events associated with this product in comparison with conventional plasma-containing products (78).
The side effects associated with PI-treated platelets include a small amount of platelet loss, which leads to lower platelet recoveries and post-transfusion count increases, as well as minor decreases in in vivo platelet survival (76). Data from clinical studies, however, have not shown increased rates of significant bleeding in patients who receive PI products compared with conventional products (79). Plasma-treated PI has shown some decrease in clotting factors, but its functional activity remains well within the accepted range, making it acceptable for therapeutic use (76). The effects of PI on RBCs are not as well characterized but will most likely include increased RBC loss and decreased storage time (80). Despite the adverse effects of PI on blood products, the safety and risk mitigation it provides the blood supply far outweigh the minor drawbacks.
Peri-operative management of anemia
The diagnosis of anemia should be made via a screening complete blood count, ideally 3–4 weeks prior to a planned surgical procedure. This allows for adequate time to work up the underlying cause of anemia and complete a course of treatment. Potential adjunct treatments of anemia are discussed below.
Iron replacement
Iron deficiency is a common cause of anemia in oncology patients. Iron replacement is an inexpensive and readily available therapeutic option. Two prospective studies in gynecologic oncology found that intravenous (IV) iron is a well-tolerated primary prevention strategy for blood transfusions in patients with ovarian cancer receiving platinum/taxane chemotherapy. Patients randomized to receive IV iron with each cycle of chemotherapy had a higher nadir Hb level, which occurred later in the treatment course, and required fewer total RBC transfusions during the study period (81, 82). Similar reductions in transfusions were observed in patients treated with IV iron while undergoing chemoradiation therapy for cervical cancer (83).
Erythropoiesis-stimulating agents
Erythropoiesis-stimulating agents (ESAs) are approved for the treatment of anemia associated with chemotherapy. In numerous studies, ESAs have been shown to reduce the need for blood transfusions and improve quality of life in patients with cancer (84–86). The American Society of Clinical Oncology (ASCO) and the American Society of Hematology recommend epoetin or darbepoetin for patients with chemotherapy-associated anemia and an Hb level <10 g/dL, with the goal to raise Hb concentrations to (or near) 12 g/dL (87). An increase in Hb levels is often seen 4–6 weeks after therapy, and the magnitude of the Hb increase is often greater if IV iron is used in combination with an erythropoietin (EPO) (88). Dousias et al. evaluated the effects of EPO when administered prior to radical surgery for cervical, ovarian, or endometrial cancer; they concluded that EPO significantly increased Hb levels, leading to the need for fewer blood transfusions (89). In several large multi-center trials, patients with ovarian cancer given EPO while undergoing platinum-based chemotherapy required fewer blood transfusions (90, 91).
Despite the efficacy of ESAs in reducing the need for blood transfusions, there are several reported risks. Two large meta-analyses reported an increased risk of mortality and thromboembolic events, and perhaps shorter overall survival, in patients who received these agents (92). Whether the thrombotic risk is directly linked to the biological action of the ESAs or is mediated by an increase of the red blood cell mass is unknown. Available data suggest that standard doses of EPO substantially affect primary and secondary hemostasis, triggering blood coagulation and inducing a moderate elevation of platelet counts and hyperactivity (93). Numerous authors have cautioned that treatment with ESAs in patients with cancer increases mortality risk and worsens overall survival, and multiple studies have linked EPO with tumor progression (94). The increased risk of death associated with these drugs should be balanced against their benefits (95).
The Southwest Oncology Group (SWOG) conducted a phase II trial to assess the safety of EPO treatment to correct anemia in patients undergoing chemoradiotherapy for stage IIB–IVA cervical cancer. The SWOG reported a mean 1.4 g/dL increase in Hb from the start of treatment to the completion of chemoradiotherapy, but also a higher rate of deep vein thrombosis (DVT) and lower progression-free and overall survivals compared to those of historic controls. The authors were unable to determine whether this was the result of EPO treatment or a lower baseline Hb (96). Gynecologic Oncology Group (GOG) study 191, a phase III trial to assess EPO use in anemic patients undergoing concurrent radiation and cisplatin therapy for cervical cancer, was closed prematurely due to concerns of high thromboembolic risk associated with EPO (97).
The AGO/NOGGO trial, which had a primary endpoint of 5-year disease-free survival, looked at ESA use in patients undergoing chemotherapy and radiation. The findings of the trial showed that in patients undergoing chemoradiotherapy for cervical cancer, ESA use did not lead to an increase in the rate of thrombosis, and there was no statistically significant difference in survival between patients who did and did not receive EPO (98).
For 10 years, the FDA required that prescribing professionals partake in the ESA Risk Evaluation and Mitigation Strategy (REMS), attesting to their understanding that ESAs shorten overall survival and/or increase the risk of tumor progression or recurrence in patients with certain cancers. In April 2017, the FDA determined that the ESA REMS was no longer necessary. Nevertheless, ASCO cautions that, while the REMS is no longer in place, the serious risks of shortened overall survival and/or increased risk of tumor progression/recurrence associated with these drugs remain. The prescribing information of these agents remains unchanged and notes an increased risk of death, venous thromboembolism, and stroke.
The NCCN® recently published Cancer- and Chemotherapy-Induced Anemia guidelines, which contain algorithms to weigh the risks of ESA use (namely increased thrombotic events, possible decreased survival, and shortened time to tumor progression) against those of RBC transfusions. The NCCN® suggests considering ESA use in patients with chronic kidney disease, those undergoing palliative treatment, those undergoing myelosuppressive chemotherapy without other causes of anemia, and in those who refuse blood transfusions (48).
Intraoperative management and prevention of blood loss and anemia
Acute normovolemic hemodilution
Patients with normal Hb levels entering surgery or who have been optimized prior to surgery may be candidates for acute normovolemic hemodilution (ANH). ANH is used in patients at high risk for blood loss and is performed immediately before surgery. The procedure consists of removing whole blood from the patient and replacing it with crystalloid/colloid in order to restore euvolemia and render the patient hemodilute during the surgery. During the procedure, the patient’s “blood loss” is a diluted version of their original starting whole blood. Once hemostasis is obtained, and toward the end of the procedure, the whole blood collected at the start of the procedure is re-infused into the patient. While ANH has been explored in non-gynecologic oncology patients, data specific to gynecologic surgery are lacking (99). A trial to explore ANH in the gynecologic oncology setting is currently under way.
Cell salvage
The purpose of cell salvage is to reduce or eliminate the need for allogeneic blood transfusions by recovering blood from the surgical field, and then cleaning, filtering, and re-infusing it into the patient. Circulating tumor cells in cell saver reinfusions have been reported in several cancer types, however, and theoretically, these cells can potentially lead to metastases. Studies of cell salvage during urologic and hepatic oncology surgeries have shown no increase in metastases or mortality (100). In a retrospective analysis of 156 patients who had undergone radical hysterectomy for cervical cancer, Mirhashemi et al. found no difference in the rates and patterns of recurrence between patients who had undergone cell salvage and those who had not (101). Connor et al. found that the use of cell salvage significantly reduced the need for intraoperative and postoperative blood transfusions in patients with uterine cancer, with no evidence of early disease recurrence at 2-years’ follow-up (102). The findings of a prospective study assessing cell salvage in patients undergoing radical hysterectomy for cervical cancer showed no evidence of tumor cell re-infusion, increased recurrence risk, or compromised survival after 16 years of follow-up (103). There are no data to contradict the use of blood salvage in malignant surgery, whereas there are substantial data suggesting worsened outcome when allogeneic transfusions are used (104).
Tranexamic acid
Tranexamic Acid (TxA) works by displacing plasminogen from fibrin, resulting in the inhibition of fibrinolysis. It is typically used to treat menorrhagia but has many off-label uses as well. It also has been used more often in recent years. In surgical oncology patients, two randomized controlled trials have evaluated the efficacy of TxA in preventing blood loss. Results from the first study showed that intraoperative treatment with low-dose TxA reduced intraoperative blood loss in patients undergoing prostatectomy for prostate cancer. There was no increase in the rate of thromboembolic events (at 6 months’ follow-up) (105). Findings from the second study showed that a single dose of TxA given before surgery significantly reduced blood loss and transfusion rates in women undergoing radical debulking for presumed ovarian cancer. The study, however, was insufficiently powered to detect differences in complications (106).
Reduced laboratory-associated blood loss
A key means of preventing blood loss, at any time during patient care, is to limit the amount of iatrogenic loss associated with laboratory testing. There are a number of methods by which to accomplish this, including using pediatric collection tubes, avoiding “discard” blood volume when drawing off lines with in-line flush devices, and most importantly, minimizing unnecessary tests and blood cultures. Many institutions have developed interdisciplinary teams that review test utilization on a routine basis. As part of quality improvement initiatives, these teams work with laboratory medicine and information technology services to optimize order sets and order entry alerts to decrease test overutilization.
Management of coagulopathy
Patients with known exposures to anticoagulants or antiplatelet drugs should be evaluated by a hematologist before surgery. An algorithm should be established for the management and monitoring of these patients during and after surgery. Basic conditions of coagulopathy management (e.g., body temperature, pH, ionized calcium) should be optimized prior to the use of blood products. The use of prothrombin complex concentrates, TxA, and point-of-care testing (viscoelastic methods) to direct the administration of products should be considered. The use of platelet aggregation testing can provide insight into platelet function; if platelet function is suboptimal, the patient may require a platelet transfusion or treatment with vasopressin.
Patient-centered transfusion strategies
Each hospital should have a patient blood management (PBM) program, with clearly defined transfusion thresholds for each blood product that take into account a patient’s individual clinical risk factors, symptoms, and anemia tolerance. PBM programs should focus on perioperative anemia management, coagulopathy management, blood conservation strategies, patient-centered transfusions, and patient outcome metrics. As transfusions continue to be one of the most overused interventions in US healthcare, hospital staff should be routinely educated on transfusion triggers. At our institution, an interdisciplinary committee has created transfusion guidelines in an effort to promote the judicial use of blood, cut down on the number of unnecessary transfusions, reduce adverse transfusion events, improve patient care, and conserve limited resources. According to these guidelines, transfusions are recommended in hemodynamically stable patients at a pre-/perioperative Hb level <7 g/dL or postoperative Hb level <8 g/dL. A one-unit transfusion is standard practice, and requesting ≥2 units requires justification. The implementation of a single-unit policy, followed by a reassessment of the patient, has been shown to improve blood utilization (107). Table 5 provides a brief summary of transfusion guidelines by blood component (108, 109).
Table 5.
Indications for transfusion of blood product components
| Indication |
|---|
| Platelets (× 109/L) |
|
|
|
| Fresh-Frozen Plasma |
|
|
|
| Cryoprecipitate |
|
|
|
| Albumin (5% and 25%) |
|
Blood alternatives
In rare cases, patients cannot be given conventional blood products; this may be due to choice or for religious reasons. In other cases, a patient may have a rare blood type for which compatible blood cannot be found. Many artificial blood products have been studied but have been pulled from the market due to serious adverse effects (e.g., death, acute myocardial infarction). A hemoglobin-based oxygen carrier (HBOC) is currently being studied in phase I/II clinical trials and remains in production (clinicaltrials.gov: NCT02754999). This HBOC is a pegylated bovine dual-action CO-releasing/oxygen transfer agent. Its functional components (CO, bovine hemoglobin, and polyethylene glycol) inhibit vasoconstriction, decrease RBC extravasation, limit reactive oxygen species production, enhance blood rheology, and deliver oxygen to tissues. Free hemoglobin is extremely toxic and scavenges nitric oxide (NO), which likely accounts for the severe side effects seen with previous generations of HBOCs. However, this product is pegylated and conjugated to CO, which prevents the NO scavenging. In patients with no other options, this product provides life-saving oxygen delivery support while the patient’s marrow produces new red blood cells.
The first human immortalized adult erythroid line, providing a potentially sustainable supply of erythroid cells with fewer infectious complications, has been generated recently. These cells have only been studied in mice so far, but the results have been positive, and this may be a viable alternative to donor blood in the future (110).
Conclusions
Blood transfusions are often used in patients with gynecologic malignancies; however, there has been minimal research and data to guide blood transfusion practices in this patient population. For now, clinical practice in this setting is mainly guided by anecdotal experience rather than published guidelines or data from randomized controlled trials. The indications for the administration of blood products should be to improve tissue oxygen delivery and quality of life. The transfusion trigger for this population remains undefined, and as such, protocols created by a multidisciplinary team are needed. The link between transfusion, prognosis, and recurrence is provocative but confounding, and should caution the surgeon’s decision-making. Restrictive blood transfusion strategies have been proven to be safe in several critical care settings. In light of the limited evidence-based data in gynecologic oncology, clinicians should exercise prudence in deciding which patients should undergo transfusion until well-designed trials can provide effective recommendations.
Highlights.
We review the incidence and prognosis of anemia in the cancer patient
We present indications and risks for red blood cell transfusion
We describe peri- and intra-operative management of blood
Acknowledgments
Financial Support
Supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose
References
- 1.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System [Internet] 2011 2011. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 2.Basha JDR, Cable D, Jones G. Tranfusions And Their Costs: Managing Patients Needs and Hospitals Economics. The Internet Journal of Emergency and Intensive Care Medicine. 2005;9(2) [Google Scholar]
- 3.Prescott LS, Aloia TA, Brown AJ, Taylor JS, Munsell MF, Sun CC, et al. Perioperative blood transfusion in gynecologic oncology surgery: analysis of the National Surgical Quality Improvement Program Database. Gynecologic oncology. 2015;136(1):65–70. doi: 10.1016/j.ygyno.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56(9):2173–83. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- 5.Watkins T, Surowiecka MK, McCullough J. Transfusion Indications for Patients With Cancer. Cancer Control. 2015;22(1):38–46. doi: 10.1177/107327481502200106. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion. J Gen Intern Med. 2002;17:111. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 7.Henry DH. Parenteral iron therapy in cancer-associated anemia. Vol. 2010. Hematology American Society of Hematology Education Program; 2010. pp. 351–6. [DOI] [PubMed] [Google Scholar]
- 8.Birgegård G, Aapro MS, Bokemeyer C, Dicato M, Drings P, Hornedo J, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl. 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. European Journal of Cancer. 2004;40(15):2293–306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer – A systematic, quantitative review. Cancer. 2001;91(12):2214–21. [PubMed] [Google Scholar]
- 11.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med. 2004;116:11–26. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Bishop AJ, Allen PK, Klopp AH, Meyer LA, Eifel PJ. Relationship Between Low Hemoglobin Levels and Outcomes After Treatment With Radiation or Chemoradiation in Patients With Cervical Cancer: Has the Impact of Anemia Been Overstated? Int J Radiat Oncol. 2015;91(1):196–205. doi: 10.1016/j.ijrobp.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Dunst J, Kuhnt T, Strauss HG, Krause U, Pelz T, Koelbl H, et al. Anemia in cervical cancers: Impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol. 2003;56(3):778–87. doi: 10.1016/s0360-3016(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 14.Girinski T, Pejoviclenfant MH, Bourhis J, Campana F, Cosset JM, Petit C, et al. Prognostic Value of Hemoglobin Concentrations and Blood-Transfusions in Advanced-Carcinoma of the Cervix Treated by Radiation-Therapy – Results of a Retrospective Study of 386 Patients. Int J Radiat Oncol. 1989;16(1):37–42. doi: 10.1016/0360-3016(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 15.Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86(8):1528–36. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Haensgen G, Krause U, Becker A, Stadler P, Lautenschlaeger C, Wohlrab W, et al. Tumor hypoxia, p53, and prognosis in cervical cancers. Int J Radiat Oncol. 2001;50(4):865–72. doi: 10.1016/s0360-3016(01)01523-1. [DOI] [PubMed] [Google Scholar]
- 17.Kapp DS, Fischer D, Gutierrez E, Kohorn EI, Schwartz PE. Pretreatment Prognostic Factors in Carcinoma of the Uterine Cervix – a Multivariable Analysis of the Effect of Age, Stage, Histology and Blood Counts on Survival. Int J Radiat Oncol. 1983;9(4):445–55. doi: 10.1016/0360-3016(83)90060-3. [DOI] [PubMed] [Google Scholar]
- 18.Obermair A, Cheuk R, Horwood K, Janda M, Bachtiary B, Schwanzelberger B, et al. Impact of hemoglobin levels before and during concurrent chemoradiotherapy on the response of treatment in patients with cervical carcinoma – Preliminary results. Cancer. 2001;92(4):903–8. doi: 10.1002/1097-0142(20010815)92:4<903::aid-cncr1399>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen D, Sogaard H, Overgaard J, Bentzen SM. Prognostic Value of Pretreatment Factors in Patients with Locally Advanced-Carcinoma of the Uterine Cervix Treated by Radiotherapy Alone. Acta Oncol. 1995;34(6):787–95. doi: 10.3109/02841869509127188. [DOI] [PubMed] [Google Scholar]
- 20.Thomas G. The effect of hemoglobin level on radiotherapy outcomes: The Canadian experience. Semin Oncol. 2001;28(2):60–5. doi: 10.1016/s0093-7754(01)90215-5. [DOI] [PubMed] [Google Scholar]
- 21.Wernerwasik M, Schmid CH, Bornstein L, Ball HG, Smith DM, Madocjones H. Prognostic Factors for Local and Distant Recurrence in Stage-I and Stage-Ii Cervical-Carcinoma. Int J Radiat Oncol. 1995;32(5):1309–17. doi: 10.1016/0360-3016(94)00613-P. [DOI] [PubMed] [Google Scholar]
- 22.Yalman D, Aras AB, Ozkok S, Duransoy A, Celik OK, Ozsaran Z, et al. Prognostic factors in definitive radiotherapy of uterine cervical cancer. Eur J Gynaecol Oncol. 2003;24(3–4):309–14. [PubMed] [Google Scholar]
- 23.Winter WE, Maxwell GL, Tian CQ, Sobel E, Rose GS, Thomas G, et al. Association of hemoglobin level with survival in cervical carcinoma patients treated with concurrent cisplatin and radiotherapy: a Gynecologic Oncology Group Study. Gynecologic oncology. 2004;94(2):495–501. doi: 10.1016/j.ygyno.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Fuso L, Mazzola S, Marocco F, Ferrero A, Dompe D, Carus AP, et al. Pretreatment serum hemoglobin level as a predictive factor of response to neoadjuvant chemotherapy in patients with locally advanced squamous cervical carcinoma: a preliminary report. Gynecologic oncology. 2005;99(3 Suppl 1):S187–91. doi: 10.1016/j.ygyno.2005.07.079. [DOI] [PubMed] [Google Scholar]
- 25.Grigiene R, Valuckas KP, Aleknavicius E, Kurtinaitis J, Letautiene SR. The value of prognostic factors for uterine cervical cancer patients treated with irradiation alone. BMC Cancer. 2007;234 doi: 10.1186/1471-2407-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr NA, Wang JZ, Zhang DQ, Montebello JF, Grecula JC, Lo SS, et al. Synergistic effects of hemoglobin and tumor perfusion on tumor control and survival in cervical cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1513–21. doi: 10.1016/j.ijrobp.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 27.Endo D, Todo Y, Okamoto K, Minobe S, Kato H, Nishiyama N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: a retrospective analysis in a Japanese cohort. J Gynecol Oncol. 2015;26(1):12–8. doi: 10.3802/jgo.2015.26.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin NR, Lee YY, Kim SH, Choi CH, Kim TJ, Lee JW, et al. Prognostic value of pretreatment hemoglobin level in patients with early cervical cancer. Obstet Gynecol Sci. 2014;57(1):28–36. doi: 10.5468/ogs.2014.57.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metindir J, Dilek GB. Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin. 2009;135(1):125–9. doi: 10.1007/s00432-008-0430-2. [DOI] [PubMed] [Google Scholar]
- 30.Njolstad TS, Engerud H, Werner HMJ, Salvesen HB, Trovik J. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecologic oncology. 2013;131(2):410–5. doi: 10.1016/j.ygyno.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Wilairat W, Benjapibal M. Presence of Anemia and Poor Prognostic Factors in Patients with Endometrial Carcinoma. Asian Pac J Cancer P. 2012;13(7):3187–90. doi: 10.7314/apjcp.2012.13.7.3187. [DOI] [PubMed] [Google Scholar]
- 32.Munstedt K, Kovacic M, Zygmunt M, von Georgi R. Impact of hemoglobin levels before and during chemotherapy on survival of patients with ovarian cancer. Int J Oncol. 2003;23(3):837–43. [PubMed] [Google Scholar]
- 33.Maccio A, Madeddu C, Massa D, Mudu MC, Lusso MR, Gramignano G, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. Blood. 2005;106(1):362–7. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 34.Obermair A, Handisurya A, Kaider A, Sevelda P, Kölbl H, Gitsch G. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients. Cancer. 1998;83(4):726–31. [PubMed] [Google Scholar]
- 35.Warner LL, Dowdy SC, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. The impact of perioperative packed red blood cell transfusion on survival in epithelial ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2013;23(9):1612–9. doi: 10.1097/01.IGC.0000436089.03581.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m(2) Ann Oncol. 2000;11(8):1029–33. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 37.Eichbaum MHR, Weiss LME, Bruckner T, Schneeweiss A, Sinn HP, Gebauer G, et al. Prognostic impact of hemoglobin levels before and during carboplatin/taxane-based chemotherapy in patients with primary invasive epithelial ovarian cancer. Med Sci Monitor. 2009;15(4):Cr156–Cr63. [PubMed] [Google Scholar]
- 38.Hefler L, Mayerhofer K, Leibman B, Obermair A, Reinthaller A, Kainz C, et al. Tumor anemia and thrombocytosis in patients with vulvar cancer. Tumor biology. 2000;21(5):309–14. doi: 10.1159/000030136. [DOI] [PubMed] [Google Scholar]
- 39.Dicato M, Plawny L, Diederich M. Anemia in cancer. Ann Oncol. 2010;21:167–72. doi: 10.1093/annonc/mdq284. [DOI] [PubMed] [Google Scholar]
- 40.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. Journal of the National Cancer Institute. 1999;91(19):1616–34. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 41.Kumagai S, Sugiyama T, Shoji T, Michimae H, Katsumata N, Aoki D, et al. Does Severe Anemia Caused by Dose-Dense Paclitaxel-Carboplatin Combination Therapy Have an Effect on the Survival of Patients With Epithelial Ovarian Cancer? Retrospective Analysis of the Japanese Gynecologic Oncology Group 3016 Trial. International Journal of Gynecological Cancer. 2011;21(9):1585–91. doi: 10.1097/IGC.0b013e318229266a. [DOI] [PubMed] [Google Scholar]
- 42.Hensley ML, Lebeau D, Leon L, Venkatraman E, Waltzman R, Sabbatini P, et al. Identification of risk factors for requiring transfusion during front-line chemotherapy for ovarian cancer. Gynecologic oncology. 2001;81(3):485–9. doi: 10.1006/gyno.2001.6185. [DOI] [PubMed] [Google Scholar]
- 43.Barrett-Lee P, Bailey N, O’Brien M, Wager E. Large-scale UK audit of blood transfusion requirements and anaemia in patients receiving cytotoxic chemotherapy. British journal of cancer. 2000;82(1):93. doi: 10.1054/bjoc.1999.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stüben G, Thews O, Pöttgen C, Knühmann K, Vaupel P, Stuschke M. Recombinant human erythropoietin increases the radiosensitivity of xenografted human tumours in anaemic nude mice. Journal of Cancer Research and Clinical Oncology. 2001;127(6):346–50. doi: 10.1007/s004320000215. [DOI] [PubMed] [Google Scholar]
- 45.Klopp AH, Eifel PJ, editors. Seminars in radiation oncology. Elsevier; 2012. Biological predictors of cervical cancer response to radiation therapy. [DOI] [PubMed] [Google Scholar]
- 46.Kapp KS, Poschauko J, Geyer E, Berghold A, Oechs AC, Petru E, et al. Evaluation of the effect of routine packed red blood cell transfusion in anemic cervix cancer patients treated with radical radiotherapy. Int J Radiat Oncol. 2002;54(1):58–66. doi: 10.1016/s0360-3016(02)02896-1. [DOI] [PubMed] [Google Scholar]
- 47.Bush RS. The significance of anemia in clinical radiation therapy. Int J Radiat Oncol Biol Phys. 1986;12(11):2047–50. doi: 10.1016/0360-3016(86)90146-x. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers GM, 3rd, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, et al. Cancer- and chemotherapy-induced anemia. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(5):628–53. doi: 10.6004/jnccn.2012.0064. [DOI] [PubMed] [Google Scholar]
- 49.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016 doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 50.Stehling LC, Doherty DC, Faust RJ, Greenburg AG, Harrison CR, Landers DF, et al. Practice guidelines for blood component therapy – A report by the American Society of Anesthesiologists Task Force on blood component therapy. Anesthesiology. 1996;84(3):732–47. [PubMed] [Google Scholar]
- 51.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care (vol 340, pg 409, 1999) New Engl J Med. 1999;340(13):1056. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 52.Hajjar LA, Vincent JL, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion Requirements After Cardiac Surgery The TRACS Randomized Controlled Trial. Jama-J Am Med Assoc. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 53.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: Anemia and blood transfusion in the critically ill – Current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 54.Carson JL, Rhoads GG, Magaziner J. Transfusion Thresholds in High-Risk Patients after Hip Surgery REPLY. New Engl J Med. 2012;366(13):1254–5. doi: 10.1056/NEJMc1201459. [DOI] [PubMed] [Google Scholar]
- 55.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. New Engl J Med. 2014;371(15):1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 56.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Db Syst Rev. 2012;(4) doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Almeida JP, Vincent JL, Galas FRBG, de Almeida EPM, Fukushima JT, Osawa EA, et al. Transfusion Requirements in Surgical Oncology Patients A Prospective, Randomized Controlled Trial. Anesthesiology. 2015;122(1):29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 58.Boone JD, Kim KH, Marques M, Straughn JM. Compliance rates and outcomes associated with a restrictive transfusion policy in gynecologic oncology patients. Gynecologic oncology. 2014;132(1):227–30. doi: 10.1016/j.ygyno.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 59.Price FV, Kelley JL, Edwards RP, Hasley PB, Amin RM. A Survey of Blood-Transfusion Practices of Gynecologic Oncologists. Gynecologic oncology. 1995;59(1):45–50. doi: 10.1006/gyno.1995.1266. [DOI] [PubMed] [Google Scholar]
- 60.Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, et al. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825–36. doi: 10.1016/S0140-6736(15)01313-6. [DOI] [PubMed] [Google Scholar]
- 61.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland I, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44(12):1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 62.Edgren G, Kamper-Jorgensen M, Eloranta S, Rostgaard K, Custer B, Ullum H, et al. Duration of red blood cell storage and survival of transfused patients (CME) Transfusion. 2010;50(6):1185–95. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrzejewski C, Casey MA, Popovsky MA. How we view and approach transfusion-associated circulatory overload: pathogenesis, diagnosis, management, mitigation, and prevention. Transfusion. 2013;53(12):3037–47. doi: 10.1111/trf.12454. [DOI] [PubMed] [Google Scholar]
- 64.Opelz G. Immunological Reactivity of Transplant Recipients. Lancet. 1973;1(7799):373–4. doi: 10.1016/s0140-6736(73)90164-5. [DOI] [PubMed] [Google Scholar]
- 65.Gantt CL. Red-Blood-Cells for Cancer-Patients. Lancet. 1981;2(8242):363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 66.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. BJA: British Journal of Anaesthesia. 2013;110(5):690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benson D, Barnett CC., Jr Perioperative blood transfusions promote pancreas cancer progression. The Journal of surgical research. 2011;166(2):275–9. doi: 10.1016/j.jss.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 68.Acheson AG, Brookes MJ, Spahn DR. Effects of Allogeneic Red Blood Cell Transfusions on Clinical Outcomes in Patients Undergoing Colorectal Cancer Surgery A Systematic Review and Meta-Analysis. Ann Surg. 2012;256(2):235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 69.De Oliveira GS, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfusion Med. 2012;22(2):97–103. doi: 10.1111/j.1365-3148.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 70.Azuma C, Koyama M, Inagaki M, Ito S, Sawada M, Saji F, et al. The influence of perioperative blood transfusion during radical hysterectomy on the prognosis of uterine cervical cancer. Transfus Sci. 1997;18(1):55–62. doi: 10.1016/s0955-3886(96)00077-x. [DOI] [PubMed] [Google Scholar]
- 71.Monk BJ, Tewari K, Gamboavujicic G, Burger RA, Manetta A, Berman ML. Does Perioperative Blood-Transfusion Affect Survival in Patients with Cervical-Cancer Treated with Radical Hysterectomy. Obstet Gynecol. 1995;85(3):343–8. doi: 10.1016/0029-7844(94)00398-W. [DOI] [PubMed] [Google Scholar]
- 72.Rohde JM. Health Care-Associated Infection After Red Blood Cell Transfusion: A Systematic Review and Metaanalysis (vol 311, pg 1317, 2014) Jama-J Am Med Assoc. 2014;312(19):2045. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwschenberger JB. Intraoperative Transfusion of 1 U to 2 U Packed Red Blood Cells Is Associated with Increased 30-Day Mortality, Surgical-Site Infection, Pneumonia, and Sepsis in General Surgery Patients. J Am Coll Surgeons. 2009;208(5):931–7. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Rustum NR, Richard S, Wilton A, Lev G, Sonoda Y, Hensley ML, et al. Transfusion utilization during adnexal or peritoneal cancer surgery: Effects on symptomatic venous thromboembolism and survival. Gynecologic oncology. 2005;99(2):320–6. doi: 10.1016/j.ygyno.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 75.Devine DV, Schubert P. Pathogen Inactivation Technologies The Advent of Pathogen-Reduced Blood Components to Reduce Blood Safety Risk. Hematol Oncol Clin N. 2016;30(3):609–+. doi: 10.1016/j.hoc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Devine DV, Schubert P. Pathogen Inactivation Technologies: The Advent of Pathogen-Reduced Blood Components to Reduce Blood Safety Risk. Hematol Oncol Clin North Am. 2016;30(3):609–17. doi: 10.1016/j.hoc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Aubry M, Richard V, Green J, Broult J, Musso D. Inactivation of Zika virus in plasma with amotosalen and ultraviolet A illumination. Transfusion. 2016;56(1):33–40. doi: 10.1111/trf.13271. [DOI] [PubMed] [Google Scholar]
- 78.Knutson F, Osselaer J, Pierelli L, Lozano M, Cid J, Tardivel R, et al. A prospective, active haemovigilance study with combined cohort analysis of 19,175 transfusions of platelet components prepared with amotosalen-UVA photochemical treatment. Vox Sang. 2015;109(4):343–52. doi: 10.1111/vox.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osselaer JC, Messe N, Hervig T, Bueno J, Castro E, Espinosa A, et al. A prospective observational cohort safety study of 5106 platelet transfusions with components prepared with photochemical pathogen inactivation treatment. Transfusion. 2008;48(6):1061–71. doi: 10.1111/j.1537-2995.2008.01643.x. [DOI] [PubMed] [Google Scholar]
- 80.Kleinman S, Stassinopoulos A. Risks associated with red blood cell transfusions: potential benefits from application of pathogen inactivation. Transfusion. 2015;55(12):2983–3000. doi: 10.1111/trf.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Athibovonsuk P, Manchana T, Sirisabya N. Prevention of blood transfusion with intravenous iron in gynecologic cancer patients receiving platinum-based chemotherapy. Gynecologic oncology. 2013;131(3):679–82. doi: 10.1016/j.ygyno.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 82.Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecologic oncology. 2010;116(3):522–5. doi: 10.1016/j.ygyno.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Kim YT, Kim SW, Yoon BS, Cho HJ, Nahm EJ, Kim SH, et al. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecologic oncology. 2007;105(1):199–204. doi: 10.1016/j.ygyno.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 84.Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alfa administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol. 2008;26(10):1611–8. doi: 10.1200/JCO.2006.10.4620. [DOI] [PubMed] [Google Scholar]
- 85.Sabbatini P. Contibution of anemia to fatigue in the cancer patient. Oncology (Williston Park) 2000;14(11A):69–71. [PubMed] [Google Scholar]
- 86.Sabbatini P. The relationship between anemia and quality of life in cancer patients. Oncologist. 2000;5(Suppl 2):19–23. doi: 10.1634/theoncologist.5-suppl_2-19. [DOI] [PubMed] [Google Scholar]
- 87.Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update. Blood. 2008;111(1):25–41. doi: 10.1182/blood-2007-08-109488. [DOI] [PubMed] [Google Scholar]
- 88.Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22(7):1301–7. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 89.Dousias V, Stefos T, Navrozoglou I, Staikos I, Ditto A, Paraskevaidis E. Administration of recombinant human erythropoietin in patients with gynecological cancer before radical surgery. Clin Exp Obstet Gynecol. 2005;32(2):129–31. [PubMed] [Google Scholar]
- 90.Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P, Group E-I-S Epoetin alfa in platinum-treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. Br J Cancer. 2006;94(7):947–54. doi: 10.1038/sj.bjc.6603004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huinink WWT, de Swart CAM, van Toorn DW, Morack G, Breed WPM, Hillen HFP, et al. Controlled multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum-based chemotherapy. Med Oncol. 1998;15(3):174–82. doi: 10.1007/BF02821936. [DOI] [PubMed] [Google Scholar]
- 92.Leyland-Jones B, Bondarenko I, Nemsadze G, Smirnov V, Litvin I, Kokhreidze I. A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. J Clin Oncol. 2016;34(11):1197–207. doi: 10.1200/JCO.2015.63.5649. [DOI] [PubMed] [Google Scholar]
- 93.Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Seminars in thrombosis and hemostasis. 2010;36(5):537–49. doi: 10.1055/s-0030-1255448. [DOI] [PubMed] [Google Scholar]
- 94.Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(20):6373–80. doi: 10.1158/1078-0432.CCR-10-2577. [DOI] [PubMed] [Google Scholar]
- 95.Glaspy J. Update on safety of ESAs in cancer-induced anemia. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(5):659–66. doi: 10.6004/jnccn.2012.0065. [DOI] [PubMed] [Google Scholar]
- 96.Lavey RS, Liu PY, Greer BE, Robinson WR, Chang PC, Wynn RB, et al. Recombinant human erythropoietin as an adjunct to radiation therapy and cisplatin for stage IIB-IVA carcinoma of the cervix: a Southwest Oncology Group study. Gynecologic oncology. 2004;95(1):145–51. doi: 10.1016/j.ygyno.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 97.Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecologic oncology. 2008;108(2):317–25. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blohmer JU, Paepke S, Sehouli J, Boehmer D, Kolben M, Wurschmidt F, et al. Randomized phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin Alfa in patients with high-risk cervical cancer: results of the NOGGO-AGO intergroup study. J Clin Oncol. 2011;29(28):3791–7. doi: 10.1200/JCO.2010.30.4899. [DOI] [PubMed] [Google Scholar]
- 99.Barile L, Fominskiy E, Di Tomasso N, Castro LEA, Landoni G, De Luca M, et al. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. Anesth Analg. 2017;124(3):743–52. doi: 10.1213/ANE.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 100.Zulim RA, Rocco M, Goodnight JE, Smith GJ, Krag DN, Schneider PD. Intraoperative autotransfusion in hepatic resection for malignancy. Is it safe? Arch Surg. 1993;128(2):206–11. doi: 10.1001/archsurg.1993.01420140083013. [DOI] [PubMed] [Google Scholar]
- 101.Mirhashemi R, Averette HE, Deepika K, Estape R, Angioli R, Martin J. The impact of intraoperative autologous blood transfusion during type III radical hysterectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1998;181(6):1310–5. doi: 10.1016/s0002-9378(99)70369-8. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 102.Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE. Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol. 1995;86(3):373–8. doi: 10.1016/0029-7844(95)00183-R. [DOI] [PubMed] [Google Scholar]
- 103.Engle DB, Connor JP, Morris PC, Bender DP, De Geest K, Ahmed A, et al. Intraoperative autologous blood transfusion use during radical hysterectomy for cervical cancer: long-term follow-up of a prospective trial. Arch Gynecol Obstet. 2012;286(3):717–21. doi: 10.1007/s00404-012-2351-1. [DOI] [PubMed] [Google Scholar]
- 104.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Brit J Anaesth. 2013;110(5):690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. doi: 10.1136/bmj.d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lundin ES, Johansson T, Zachrisson H, Leandersson U, Backman F, Falknas L, et al. Single-dose tranexamic acid in advanced ovarian cancer surgery reduces blood loss and transfusions: double-blind placebo-controlled randomized multicenter study. Acta Obstet Gyn Scan. 2014;93(4):335–44. doi: 10.1111/aogs.12333. [DOI] [PubMed] [Google Scholar]
- 107.Meybohm P, Richards T, Isbister J, Hofmann A, Shander A, Goodnough LT, et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus Med Rev. 2016 doi: 10.1016/j.tmrv.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 109.Wilkes MM, Navickis RJ. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135(3):149–64. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- 110.Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M, et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat Commun. 2017;8:14750. doi: 10.1038/ncomms14750. [DOI] [PMC free article] [PubMed] [Google Scholar]


