Abstract
Patient: Female, 18
Final Diagnosis: Inflammatory myofibroblastic sarcoma
Symptoms: Headache
Medication: —
Clinical Procedure: Craniotomy • lobectomy
Specialty: Oncology
Objective:
Rare disease
Background:
ALK gene rearrangements as oncogenic drivers have been described in many cancers, including inflammatory myofibroblastic sarcoma (IMS). The first-generation ALK inhibitor was limited in its ability to cross the blood-brain-barrier to treat brain metastasis. Drug-resistance invariably develops over time in ALK-rearranged tumors, which leads to disease progression. The newer generations of ALK inhibitors are designed to have higher potency in ALK inhibition and improved CNS penetration.
Case Report:
We report a rare case of pulmonary IMS with ALK-1 gene rearrangement and multiple brain metastases as initial presentation. After the primary lung tumor and the larger brain metastases were resected, control of residual CNS disease and subsequent progression and CNS spread was achieved with favorable clinical response by all three generations of ALK inhibitors.
Conclusions:
ALK inhibitors may be an effective therapy for this rare and unusual form of ALK-1-rearranged cancer, even in the presence of multifocal CNS metastases with leptomeningeal involvement.
MeSH Keywords: Gene Rearrangement, Neoplasm Metastasis, Sarcoma
Background
Inflammatory myofibroblastic tumor (IMT), or inflammatory pseudotumor, is an uncommon, largely indolent mesenchymal neoplasm, primarily occurring in the thoracic and abdominal cavities of children and young adults [1]. Rare examples of its malignant variant, or inflammatory myofibroblastic sarcoma (IMS), have been reported based on local recurrence and distant metastasis [1–3]. About 36–60% of IMT have been found to have anaplastic lymphoma kinase-1 (ALK-1) gene rearrangement on chromosome 2p23 [4,5]. There have been increasing reports on the treatment effects of ALK inhibitors on IMS [6–8]. Here we report a case of pulmonary IMS in an 18-year old female with multiple brain metastases as her initial presentation, whose tumors gained significant clinical response to sequential treatment with the first-, second- and third-generation ALK inhibitors (crizotinib, ceritinib, alectinib, and lorlatinib). To the best of our knowledge, this is the first case of IMS treated with all three generations of ALK inhibitors. It may provide valuable insight on the treatment of this rare and aggressive tumor.
Case Report
An otherwise healthy 18-year old Caucasian female presented to our center in January 2014 with severe headaches. Brain MRI revealed multiple brain masses with the largest (5 cm) found in anterior left frontal lobe (Figure 1). Metastatic workup showed a 6 cm right middle lobe lung mass (Figure 2). Neurosurgical excision of the left frontal brain lesion to reduce mass effects and related symptoms produced a 4.6×3×3 cm, firm, circumscribed, vascular nodular tumor. Pathologic examination showed a hypercellular spindle cell neoplasm sharply demarcated from the surrounding gliotic brain parenchyma (Figure 3). The tumor cells are arranged haphazardly, in a hemangiopericytoma-like or a storiform architectural pattern with many delicate capillaries or sinusoidal vessels, geographic areas of necrosis (Figure 4), and focal hyalinized hypocellularity (Figure 5). They contain oval vesicular nuclei, small nucleoli or chromocenters, varying amounts of pale eosinophilic cytoplasm and indistinct intercellular borders (Figure 6) with focal formation of scattered multinucleated giant cells (Figure 7) in between many infiltrating small lymphocytes and occasional plasma cells (Figure 8). Mitotic figures are readily found, averaging up to 3 mitoses per 10 high-power fields. Immunohistochemical stains show diffuse cytoplasmic immunoreactivity for ALK-1 protein (Figure 9) and no immunoreactivity for cytokeratins, glial fibrillary acidic protein (GFAP), S100 protein, CD34, CD99, or bcl-2. Fluorescence in situ hybridization (FISH) test confirmed ALK-1 gene rearrangement in tumor cells, but genomic DNA sequencing failed to reveal the fusion partner, likely due to intron interference. The patient underwent a second craniotomy one month later for resection of a rapidly progressing right parietal tumor, followed by Gamma Knife radiosurgery to several smaller brain lesions before being started on crizotinib (Pfizer, 250 mg bid) under the diagnosis of ALK-1-rearranged IMS. Two months later, after the lung lesion was noted radiologically to have reduced in size by 50% and after her CNS disease was deemed arrested, she underwent a thoracotomy and right middle lobectomy to remove the suspected primary tumor. Pathology of the lung lesion was identical to that of the two resected brain lesions. She had no significant sequelae from her medical and surgical treatments, and resumed her college studies at a local university and continued crizotinib.
Figure 1.
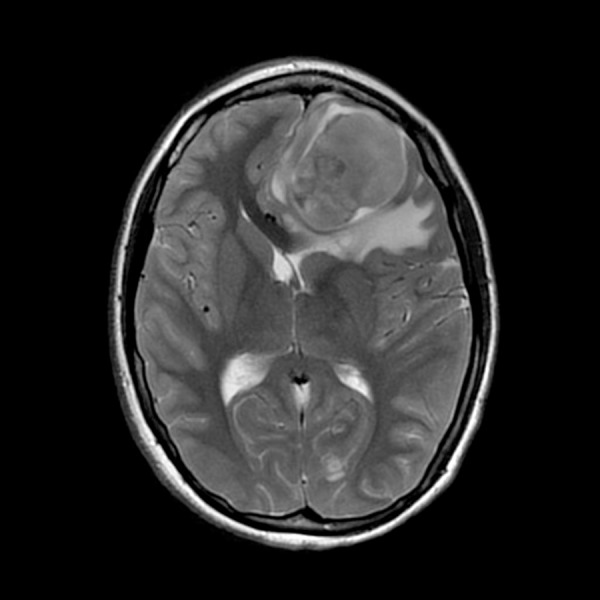
Brain MRI with contrast: Multiple brain masses were found, with the largest left frontal lesion measuring approximately 5.2×3.7 cm, in greatest perpendicular oblique transverse and AP dimensions respectively, and approximately 4.0 cm in greatest craniocaudally dimensions.
Figure 2.
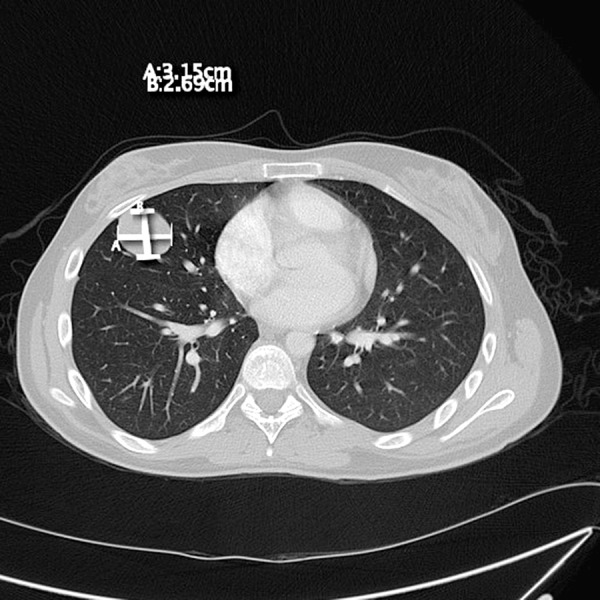
CT chest with contrast: A well circumscribed pleural-based heterogenous mass in right middle lobe measures 4×3.5 cm.
Figure 3.
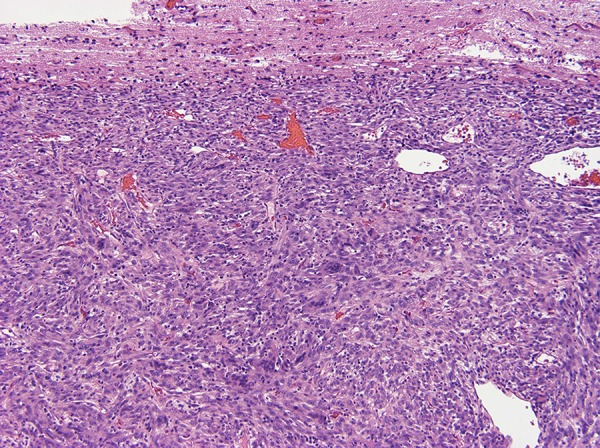
A hypercellular spindle cell neoplasm sharply demarcated from the surrounding gliotic brain parenchyma.
Figure 4.
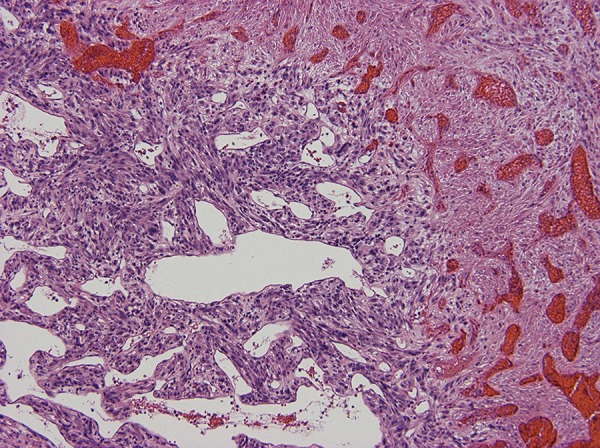
Tumor cells are arranged haphazardly, in a hemangiopericytoma-like or a storiform architectural pattern with many delicate capillaries or sinusoidal vessels, geographic areas of necrosis.
Figure 5.
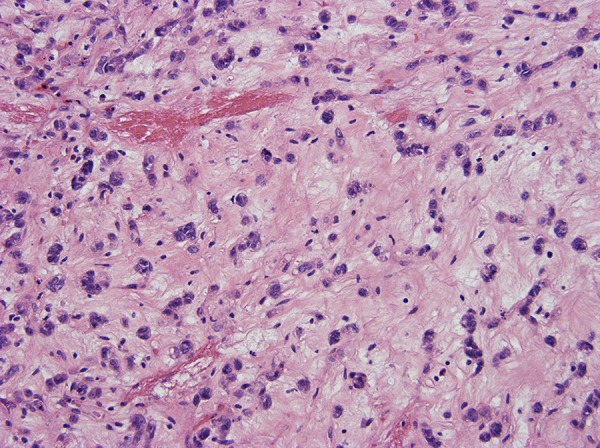
Focal hyalinized hypocellularity.
Figure 6.
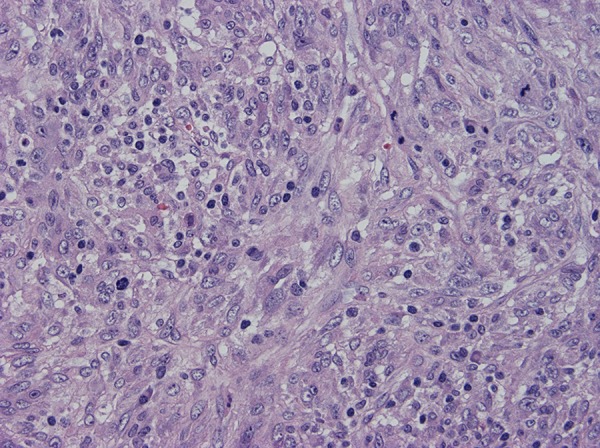
The cells contain oval vesicular nuclei, small nucleoli or chromocenters, varying amounts of pale eosinophilic cytoplasm and indistinct intercellular borders.
Figure 7.
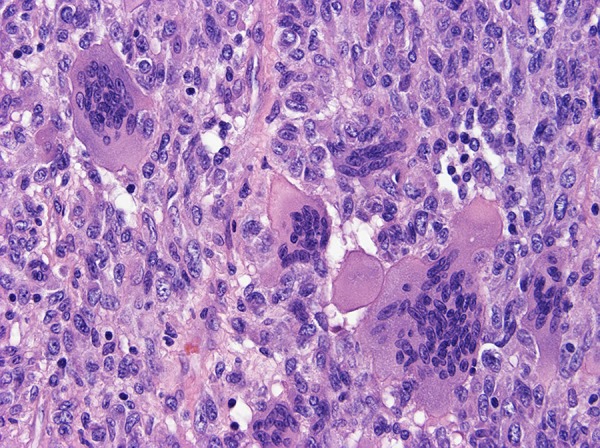
Focal formation of scattered multinucleated giant cells.
Figure 8.
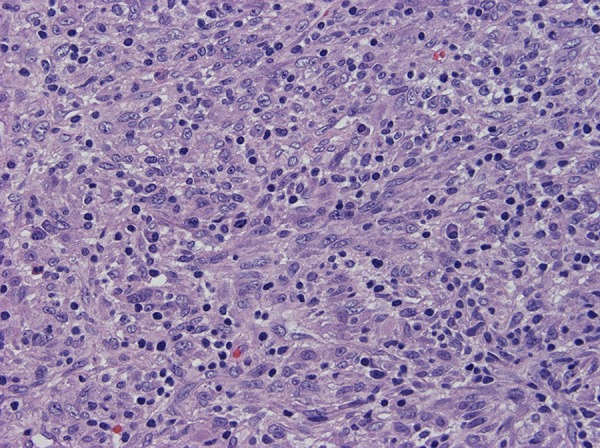
Many infiltrating small lymphocytes and occasional plasma cells. Mitotic figures are readily found, averaging up to 3 mitoses per 10 high-power fields.
Figure 9.
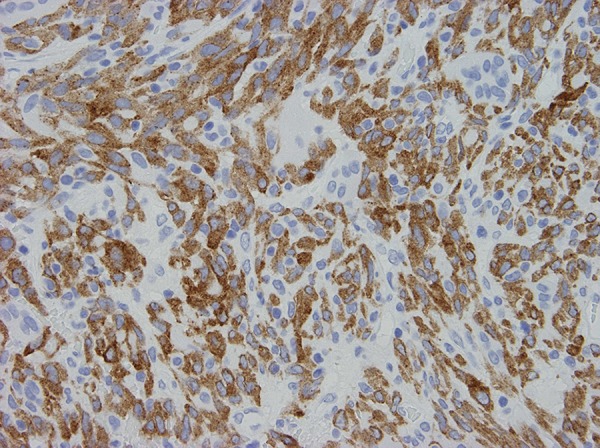
Immunohistochemical stains show diffuse cytoplasmic immunoreactivity for ALK-1 protein.
Serial laboratory and imaging studies were used to follow our patient’s disease regularly. Unfortunately, her CNS disease progressed on follow-up brain MRI after being on crizotinib (250 mg bid) for three months. Crizotinib was discontinued, and she was started on ceritinib (Novartis, 750 mg qd) with a good radiographic response. Grade 3 elevation of the patient’s transaminases required a dose reduction of this ALK inhibitor (to 450 mg qd), and after eight months her CNS disease progressed again on brain MRI. She underwent a second round of gamma knife radiosurgery treatment, followed by compassionate use of alectinib (Genentech, 600 mg bid). In January 2016, after eight months of alectinib treatment, she developed recurrent severe headaches and brain MRI identified rapid progression of several CNS lesions and the development of leptomeningeal disease in all spinal compartments (Figures 10–12). Multiple CT scans over the entire two-year time span showed no evidence of extra-CNS disease below the neck. Compassionate use of lorlatinib (Pfizer, 100 mg qd) was then administered with dramatic clinical improvement. Within a few weeks, the patient was able to discontinue IV opiate narcotics required to control her headaches. Follow-up brain MRI three months later revealed complete clearance of leptomeningeal disease in all spinal compartments and stable brain lesions without worsening signs (Figures 13, 14). The patient reported minimal headaches, for which she used Fioricet for control. The patient is alive and well 2½ years since her primary diagnosis and has returned to her college studies.
Figure 10.
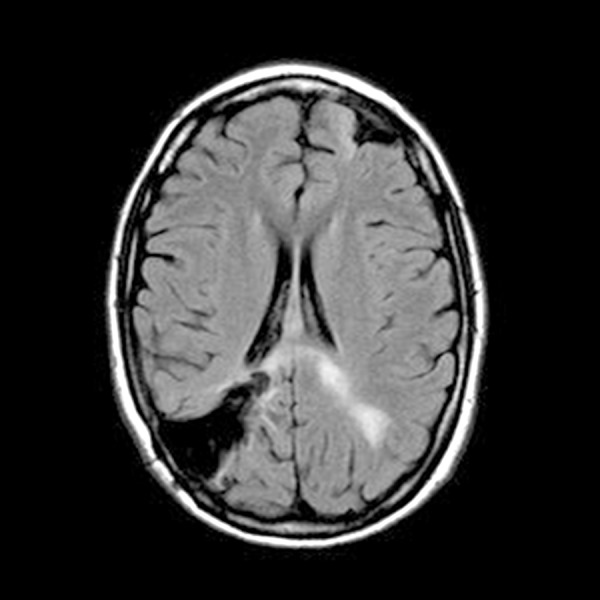
Brain MRI with contrast: Multiple mass lesions present involving the supratentorial brain, consistent with intracranial metastatic disease. Involving the left parietal lobe, there is an approximately 13×15×13 mm enhancing mass, with surrounding ischemic edema and mild associated local mass effect. Encephalomalacia present involving the right parietal lobe, consistent with resection of prior metastasis in this location.
Figure 11.
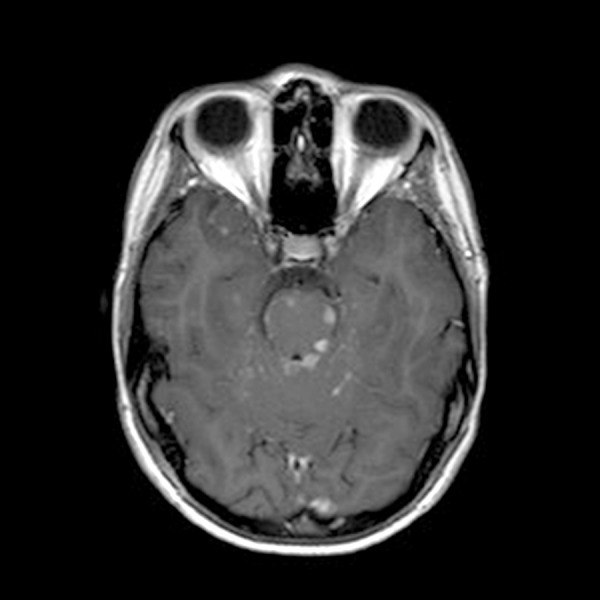
Brain MRI with contrast: Multifocal small foci of enhancement with areas of edema involving the brainstem, likely reflect leptomeningeal disease vs parenchymal metastases.
Figure 12.
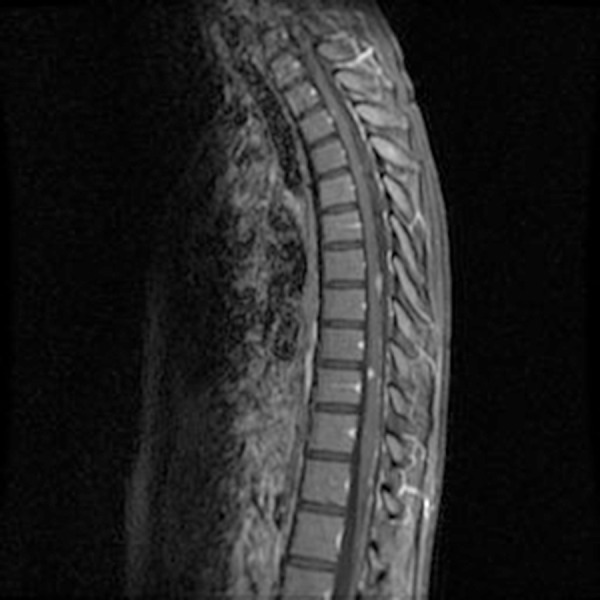
Thoracic spine MRI with contrast: Abnormal extensive leptomeningeal enhancement as noted, with dominant enhancing nodules at the level of T7–8 dorsally and T10–T11 ventrally, concerning for metastatic process.
Figure 13.
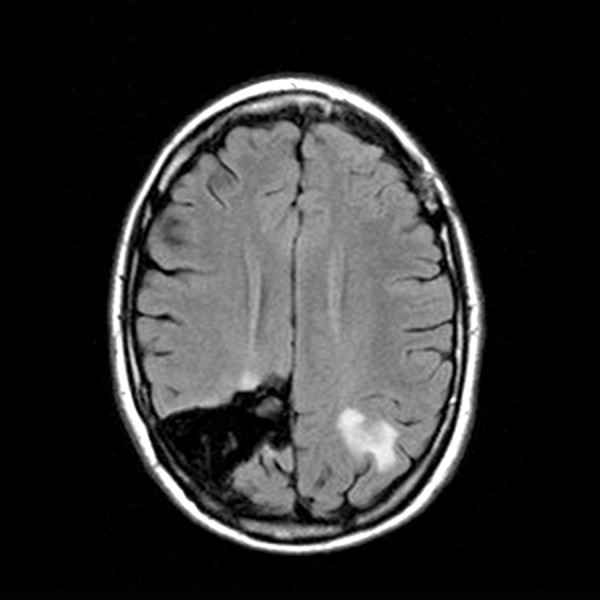
Brain MRI with contrast: A mass with contrast enhancement on the posterior left parietal lobe is slightly decreased in size.
Figure 14.
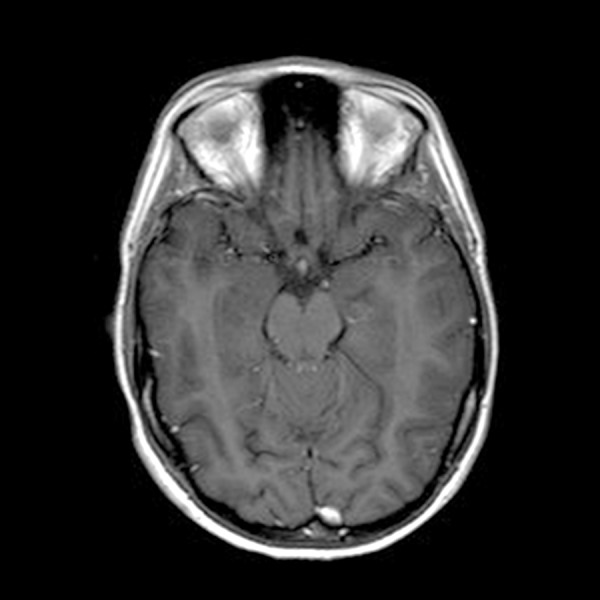
Brain MRI with contrast: The multifocal small foci of enhancement within the brainstem are no longer visualized.
Discussion
Inflammatory myofibroblastic tumor (IMT) is one of a group of mesenchymal tumors with intermediate biologic potential for malignancy [9], many of which recur after surgical intervention. A small number of these tumors behave aggressively [10]. The term inflammatory myofibroblastic sarcoma (IMS) was first suggested by Marino-Enriquez, et al. in 2011 [1], who reported a series of 11 IMS patients with an age range of 7 months to 63 years (median 39 years). Most reported cases of IMS occurred in the abdominal cavity, and the majority was found in the mesentery or omentum at presentation [1,7,11]. One reported case presented in the pleural cavity [12]. It is very unusual for IMS to present initially as multiple brain metastases as did our patient, without manifestation at the primary tumor site.
ALK-1 gene rearrangements were initially identified in a subset of anaplastic large cell lymphomas [13]. Since then, they have been reported in many other types of cancers, including non-small cell lung cancer (NSCLC), IMT/IMS, colon cancer, renal cell carcinoma, breast cancer, esophageal cancer, and neuroblastoma [14]. Over the past decade, several ALK inhibitors have been approved for the treatment of ALK-rearranged cancers, mainly for NSCLC, including first-generation crizotinib [15], and second-generation ceritinib [16] and alectinib [17,18]. The third-generation ALK inhibitor lorlatinib (PF-06463922, Pfizer) is currently under clinical trial for the treatment for ALK-rearranged NSCLC, including those refractory to first and second-generation ALK inhibitors [19].
Most of the current knowledge and experience with ALK inhibitors is derived from treatments for NSCLC. However, ALK inhibitors have been reported as effective treatments for ALK-rearranged IMT [6,8]. Liu et al. [8] reported a sustained response in a 22-year old male with abdominal IMT. Our patient was initially put on crizotinib, a first-generation ALK inhibitor after her craniotomy with tumor resection. Unfortunately, her disease progressed shortly after three months of treatment. It has been known that crizotinib has poor CNS penetration as evidenced by low concentrations detected in CNS samples during the treatment course [20], which was likely the cause of treatment failure in our patient. It was proposed that poor brain penetration of crizotinib is likely due to its high efflux by P-glycoprotein (PGP) [21].
The second-generation ALK inhibitors, which have been developed to overcome the acquired crizotinib resistance, are more potent and structurally different. Our patient was subsequently switched to the second-generation ALK inhibitors ceritinib and then alectinib with eight-month response, respectively, until the disease progressed. She was then finally put on lorlatinib, a third-generation ALK inhibitor as compassionate treatment. Lorlatinib, selectively active against both ALK and ROS1, has been designed to have better CNS penetration with improved activity against CNS metastasis [19]. After a few weeks our patient achieved significant clinical response with resolved headache and the disappearance of the leptomeningeal carcinomatosis as evidenced on MRI.
Resistance to treatment almost invariably develops after one or two years of initial of treatment with crizotinib [22]. The development of the resistance to ALK inhibitors is currently a major barrier to the successful long-term treatment for ALK-rearranged cancers [18,23]. Mechanisms responsible for resistance to crizotinib may be due to acquired ALK gene mutations, and activation of other signaling bypass pathway [24,25]. It also has been shown that multiple mechanisms of resistances may occur in the same ALK resistant tumor [26,27].
This case report illustrates the potential efficacy of serial usage of different ALK inhibitors in treating IMS that progresses with time, even in the absence of identifiable resistance mutations. Responses can be re-elicited likely due to the unique molecular characteristics of second- and third-generation ALK inhibitors. To the best of our knowledge, this is the first case of IMS that has been effectively controlled by the use of all three generations ALK inhibitors.
Conclusions
In summary, we report a rare case of pulmonary inflammatory myofibroblastic sarcoma with ALK-1 gene rearrangement and multiple brain metastases as initial presentation. After the primary lung tumor and the larger brain metastases were resected, control of residual CNS disease and subsequent progression and CNS spread was achieved with favorable clinical response by the use of all three generations of ALK inhibitors. Our experience offers support for serial ALK inhibitors as an effective therapy for this rare and unusual form of ALK-1-rearranged cancer, even in the presence of multifocal CNS metastases.
Footnotes
Conflicts of Interest
Authors declared no conflicts of interest.
References:
- 1.Marino-Enriquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–44. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM, World Health Organization. International Agency for Research on C . WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. [Google Scholar]
- 3.Zhang HH, Qi F, Zu XB, et al. Recurrence of inflammatory myofibroblastic tumor in bladder secondary to prostate treated with laparoscopic radical cystectomy. Med Sci Monit. 2012;18(8):CS63–66. doi: 10.12659/MSM.883255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–76. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 5.Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–80. [PubMed] [Google Scholar]
- 6.Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurihara-Hosokawa K, Kawasaki I, Tamai A, et al. Epithelioid inflammatory myofibroblastic sarcoma responsive to surgery and an ALK inhibitor in a patient with panhypopituitarism. Intern Med. 2014;53:2211–14. doi: 10.2169/internalmedicine.53.2546. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Kan Y, Zhao Y, et al. Epithelioid inflammatory myofibroblastic sarcoma treated with ALK inhibitor: A case report and review of literature. Int J Clin Exp Pathol. 2015;8:15328–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 10.Donner LR, Trompler RA, White RRt. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol. 1996;27:1095–98. doi: 10.1016/s0046-8177(96)90291-9. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Meng YH, Lu P, et al. Epithelioid inflammatory myofibroblastic sarcoma in abdominal cavity: A case report and review of literature. Int J Clin Exp Pathol. 2015;8:4213–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Kozu Y, Isaka M, Ohde Y, et al. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014;62:191–94. doi: 10.1007/s11748-013-0204-x. [DOI] [PubMed] [Google Scholar]
- 13.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–84. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 14.Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- 15.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A Phase II Global Study. J Clin Oncol. 2016;34:661–68. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 19.Iragavarapu C, Mustafa M, Akinleye A, et al. Novel ALK inhibitors in clinical use and development. J Hematol Oncol. 2015;8:17. doi: 10.1186/s13045-015-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–45. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 21.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–44. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 22.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 23.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–39. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 24.Lovly CM, Shaw AT. Molecular pathways: Resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20:2249–56. doi: 10.1158/1078-0432.CCR-13-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–81. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 26.Toyokawa G, Seto T. Updated evidence on the mechanisms of resistance to ALK inhibitors and strategies to overcome such resistance: Clinical and preclinical data. Oncol Res Treat. 2015;38:291–98. doi: 10.1159/000430852. [DOI] [PubMed] [Google Scholar]
- 27.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120) doi: 10.1126/scitranslmed.3003316. 120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]


