Abstract
Purpose
We reviewed the literature on chronic inflammatory demyelinating polyneuropathy (CIDP) in diabetes mellitus (DM) and explored real-world data on the prevalence and treatment of CIDP within DM. Methods: A literature search of Scopus was performed for the terms chronic inflammatory demyelinating polyradiculoneuropathy, chronic inflammatory demyelinating polyneuropathy, CIDP, and prevalence, incidence, epidemiology, or diabetes; peripheral neuropathy and prevalence or diabetes. We also searched through the reference lists of the resulting publications for additional findings that may have been missed. Additional publications on guidelines for the diagnosis of CIDP and diabetic neuropathy were also included. A descriptive analysis of the 2009–2013 PharMetrics Plus™ Database was performed to estimate the prevalence and treatment of CIDP within the DM population.
Results
There is an increasing body of literature suggesting that the prevalence of CIDP tends to be higher in diabetic patients, especially in those of older age. Our real-world data seem to support published findings from the literature. For the total cohort (N = 101,321,694), the percent prevalence of CIDP (n = 8,173) was 0.008%; DM (n = 4,026,740) was 4%. The percent prevalence of CIDP without DM (n = 5,986) was 0.006%; CIDP with DM (n = 2,187) was 9-fold higher at 0.054%. For patients >50 years old, there was a significantly higher percentage of CIDP with DM than CIDP without DM. Approximately 50% of CIDP patients were treated with IVIg, 23%–24% with steroids, 1%–2% with PE, and 20%–23% received no treatment.
Conclusions
In addition to the growing evidence of higher prevalence of CIDP in DM, our findings reinforce the need for heightened awareness of the association of CIDP and DM.
Keywords: Chronic inflammatory demyelinating polyneuropathy, CIDP, Diabetes mellitus, Diabetic peripheral neuropathy, Prevalence, Diabetic peripheral neuropathy
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a heterogeneous, progressive or relapsing–remitting, immune-mediated disorder of the peripheral nervous system that has an estimated prevalence of 1–8.9 per 100,000 (Chio, Cocito, Bottacchi, et al., 2007; Hafsteinsdottir, Olafsson, & Stefansson, 2012; Laughlin, Dyck, Melton, et al., 2009; Lunn, Manji, Choudhary, et al., 1999; McLeod, Pollard, Macaskill, et al., 1999; Mygland & Monstad, 2001; Rajabally, Simpson, Beri, et al., 2009). The epidemiology of CIDP varies depending on the diagnostic criteria used (Rajabally, Simpson, et al., 2009). CIDP has typical and atypical phenotypic variants (Mathey, Park, Hughes, et al., 2015). Only half of CIDP patients have typical CIDP, which exhibits symmetrical sensory and motor symptoms. The remainder has atypical disease, which presents with predominantly focal, sensory, motor, distal or asymmetrical symptoms. Despite increased efforts to identify a biomarker, there is no definitive diagnostic marker for CIDP, and recognition of CIDP is not straightforward in some cases due to its heterogeneous nature (Jann, Bramerio, Beretta, et al., 2003; Latov, 2011; Sommer & Toyka, 2011).
A thorough literature search of Scopus—an abstract and citation database of peer-reviewed literature—was performed for all publications, including but not limited to case reports, reviews, clinical studies, meeting abstracts, book chapters, for the terms chronic inflammatory demyelinating polyradiculoneuropathy, chronic inflammatory demyelinating polyneuropathy, CIDP, and prevalence, incidence, epidemiology, or diabetes; peripheral neuropathy and prevalence or diabetes. We also searched through the reference lists of the resulting publications for additional findings that may have been missed. All publications included were in the English language, and there was no limit on year of publication. Additional publications on guidelines for the diagnosis of CIDP and diabetic neuropathy were also included.
1. Diagnosis of CIDP
The diagnosis of CIDP is based on the recognition of clinical features, neurological examination, and electrodiagnostic criteria. Electrodiagnostic studies include electromyograms and nerve conduction studies (NCSs). Nerve conduction studies and electrophysiological evidence of demyelination are required to confirm the diagnosis (AAN, 1991; EFNS/PNS, 2010; Krarup, 2003; Latov, 2014; Tesfaye, Boulton, Dyck, et al., 2010), while laboratory testing, elevated protein levels in cerebrospinal fluid (CSF), and nerve biopsy can help rule out other causes for neuropathy and support the diagnosis (EFNS/PNS, 2010; Koller, Kieseier, Jander, et al., 2005). As the disease progresses, electrophysiological evidence of axonal damage may become superimposed on the demyelinating CIDP features (Hughes, Allen, Makowska, et al., 2006). Patients with CIDP typically present with progressive weakness in both proximal and distal muscles, areflexia, sensory symptoms with proximal weakness, and preferential loss of sensation for vibration or joint position (EFNS/PNS, 2010). Clinical observations of muscle weakness and loss of sensation are manifestations of nerve demyelination that result in conduction blocks and delays in conduction speed.
The 2010 European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) guidelines are globally accepted for both clinical and research purposes (EFNS/PNS, 2010; French & Vallat, 2008; Koski, Baumgarten, Magder, et al., 2009; Rajabally, Fowle, & Van den Bergh, 2015; Rajabally, Nicolas, Pieret, et al., 2009) due to their balance between high sensitivity (73.2%) and specificity (90.8%) for CIDP (Breiner & Brannagan, 2014; Rajabally, Simpson, et al., 2009; Rajabally et al., 2015). In contrast, the American Academy of Neurology (AAN) criteria (AAN, 1991) can have a sensitivity as low as 3.6% and a specificity of 100% for CIDP. The EFNS/PNS guidelines define CIDP as “definite,” “probable,” or “possible” based on specifics surrounding motor distal latency prolongation, reduction of motor conduction velocity, prolongation or absence of F-waves, motor conduction block, and distal compound muscle action potential (CMAP) duration (EFNS/PNS, 2010). Testing of multiple limbs is more sensitive than testing of unilateral or lower limbs in optimizing electrodiagnostic testing for CIDP, particularly in atypical CIDP (Chin, Deng, Bril, et al., 2015; Rajabally, Jacob, & Hbahbih, 2005; Vo, Hanineva, Chin, et al., 2015). Additional tests that may be needed to support a diagnosis of CIDP are elevated CSF protein with a leukocyte count less than 10/mm3, magnetic resonance imaging (MRI) of the lumbosacral or cervical nerve roots or the brachial or lumbosacral plexuses, nerve biopsy, and clinical improvement after immunomodulatory treatment (Abe, Terashima, Hoshino, et al., 2015; EFNS/PNS, 2010; Midroni, de Tilly, Gray, et al., 1999). Newer techniques for detecting proximal demyelination as well as treatment response include ultrasonography (Di Pasquale, Morino, Loreti, et al., 2015; Guidon, 2015; Jang, Cho, Yang, et al., 2014; Kerasnoudis, Pitarokoili, Behrendt, et al., 2014; Kerasnoudis, Pitarokoili, Behrendt, et al., 2015; Kerasnoudis, Pitarokoili, Gold, et al., 2015), magnetic stimulation of the cauda equine (Maccabee, Eberle, Stein, et al., 2011), somatosensory evoked potentials (Devic, Petiot, & Mauguiere, 2015), MRI gadolinium enhancement of the spinal nerve roots (Midroni et al., 1999), and magnetic resonance neurography with 3-dimensional reconstruction to determine patterns of nerve hypertrophy and to differentiate the pathophysiology of CIDP subtypes (Shibuya, Sugiyama, Ito, et al., 2015).
2. Diabetes mellitus and diabetic neuropathies
According to the Centers for Disease Control and Prevention, DM affects about 9.3% of the general population in the United States in contrast to 25.9% in persons 65 years or older (CDC, 2014). Type 2 DM (T2DM) results in insulin resistance and accounts for >90% of cases (Handelsman, Mechanick, Blonde, et al., 2011; Russell & Zilliox, 2014). The prevalence of T2DM increases with age, elevated body mass index, and family history (Handelsman et al., 2011). Due to the gradual onset of T2DM, early symptoms often go unrecognized (Russell & Zilliox, 2014). Some investigators report CIDP to be more frequent in patients with T2DM (Dunnigan, Ebadi, Breiner, et al., 2013), while others report equal occurrence of CIDP in type 1 DM and T2DM (Sharma, Cross, Ayyar, et al., 2002). While it is estimated that 50% of patients with DM have some form of neuropathy, more than 80% of these cases are diabetic peripheral neuropathy (DPN)—a length-dependent, sensory more than motor, axonal neuropathy (Chin & Rubin, 2010). Early signs of DPN manifest through distal loss of sensation in the feet and/or loss of deep tendon reflexes at the ankles. The risk of developing DPN increases with the duration of DM and glycemic control, and DPN may precede the formal diagnosis of DM by years (Chin & Rubin, 2010; Handelsman et al., 2011; Russell & Zilliox, 2014).
3. Controversy: the association of CIDP and DM
In the 1975 seminal paper on CIDP (Dyck, Lais, Ohta, et al., 1975), Dr Peter J. Dyck stated, “Patients with diabetes mellitus […] who had a neuropathy whose clinical, neurophysiologic, and pathologic features were indistinguishable from the neuropathy studied here, have not been included under this designation [of CIDP] even though it may be shown eventually that pathogenetic mechanisms may be similar or alike.”
Forty years later, the association between CIDP and DM is still being debated. Diagnosing CIDP in a patient with DM is more challenging, as superimposed axonal damage can obscure electrophysiology findings of CIDP, and DPN can cause elevated CSF protein (Gorson, Ropper, Adelman, et al., 2000). One study has reported that the occurrence of CIDP is 11-fold higher in diabetic than nondiabetic patients; however, this was a smaller nonpopulation based study (Sharma, Cross, Farronay, et al., 2002). Another study estimated that CIDP occurs in 9% of patients with DM (Lozeron, Nahum, Lacroix, et al., 2002), while others have reported that there is no association between CIDP and DM (Dyck, Engelstad, Norell, et al., 2010; Laughlin et al., 2009). One publication stated that an overemphasis on electrophysiological criteria may cause confusion in the perceived association between CIDP and DM (Laughlin et al., 2009). However, that study was retrospective, had a small CIDP population (only 19 patients met the Mayo Clinic clinical and electrophysiological criteria for CIDP), was from a relatively limited patient demographic, and had only 1 patient with both CIDP and DM. Moreover, full electrophysiological characteristics of subjects were not reported. A subsequent report by the same authors investigated whether painless diabetic motor neuropathy might represent CIDP (Garces-Sanchez, Laughlin, Dyck, et al., 2011). Based on clinical presentation, electrophysiology, and a strong emphasis on sural nerve biopsy, the authors concluded that painless diabetic motor neuropathy was a painless form of diabetic lumbosacral radiculoplexus neuropathy, not CIDP.
Patients with both CIDP and DM tend to have extensive axonal loss with more severe neuropathy yet may respond to treatment (Dunnigan, Ebadi, Breiner, et al., 2014; Gorson et al., 2000). A small study comparing 14 patients with CIDP and DM to 60 patients with CIDP alone, found that CIDP patients with DM were older (67 years vs. 49 years, respectively) and had similar response rates to corticosteroids, intravenous immunoglobulin (IVIg), plasma exchange (PE), and cyclophosphamide (Gorson et al., 2000). Patients with CIDP and DM exhibited more severe axonal loss, likely the result of underlying diabetic axonal polyneuropathy (Dunnigan et al., 2013). In contrast to patients with DPN (n = 56), diabetic patients with CIDP (n = 67) had more extensive slowing of motor nerve conduction velocity (32.4 ± 6.4 m/s vs. 35.2 ± 3.4 m/s, P = 0.006). Diabetic patients with CIDP also tended to be older (65.1 ± 13.7 years vs. 55 ± 16 years, P = 0.0003), have shorter duration of diabetes (16.5 ± 13.5 years vs. 24.0 ± 15.6 years, P = 0.005), have more severe neuropathy (ie, higher Toronto Clinical Neuropathy Score, higher vibration perception threshold, and more weakness), and have better glycemic control compared with diabetic patients who did not have CIDP but had slowed conduction velocities (HbA1c 7.7 ± 2.0% vs. 9.6 ± 2.4%, P = 0.003).
EFNS criteria are less predictive of treatment response in CIDP patients with DM (Abraham, Breiner, Katzberg, et al., 2015). CIDP patients with DM were more likely to respond to treatment if they fulfilled 2 EFNS/PNS electrophysiological criteria whereas CIDP patients without diabetes were likely to respond with only 1 criterion met (Table 1). In diabetics, fulfilling more diagnostic criteria for CIDP (more evidence of demyelinating neuropathy on NCSs) was associated with higher response rates to treatment (Cocito, Chio, Tavella, et al., 2006). The recent retrospective study (Dunnigan et al., 2014) also demonstrated that patients with CIDP and DM were less likely to be treated even though they had similar response rates to treatment as CIDP patients without DM and higher rates of proximal weakness and ataxia (Table 2).
Table 1.
Response-to-therapy rates based on number of EFNS/PNS criteria met.
| EFNS/PNS criteria | Treatment responders % (n) | ||
|---|---|---|---|
|
| |||
| Whole cohort | CIDP without DM | CIDP with DM | |
| 0 | 30 (8/27) | 31 (5/16) | 27 (3/11) |
| 1 | 53 (20/38) | 58 (14/24) | 43 (6/14) |
| 2 | 72 (13/18) | 67 (8/12) | 83 (5/6) |
| 3 | 78 (7/9) | 67 (4/6) | 100 (3/3) |
| 4 | 71 (5/7) | 71 (5/7) | – |
| OR* (CI) | 1.83 (1.22, 2.74) | 1.53 (0.99, 2.36) | 3.73 (1.32, 10.60) |
| P | 0.003 | 0.05 | 0.01 |
Table adapted from Abraham A et al. Expert Rev Clin Immunol 2015;11(4):537–546 with permission.
Odds ratio calculated by logistic regression with treatment responder (yes or no) as the dependent variable. CI, confidence interval; CIDP, chronic inflammatory demyelinating polyneuropathy; DM, diabetes mellitus; EFNS/PNS, European Federation of Neurological Societies/Peripheral Nerve Society; OR, odds ratio.
Table 2.
Treatment details of CIDP patients without and with DM.
| CIDP without DM and CIDP with DM patients (N = 134) | P value | ||
|---|---|---|---|
|
| |||
| CIDP without DM (n = 67) | CIDP with DM (n = 67) | ||
| Response to treatment (n = 100) | 0.71 | ||
| Non-responders, n (%) | 29 (45) | 17 (49) | |
| Responders, n (%) | 36 (55) | 18 (51) | |
| Treatment provided, n (%) | 62 (93) | 36 (57) | <0.0001* |
| IVIg, n (%) | 58 (87) | 33 (52) | <0.0001* |
| Prednisone, n (%) | 44 (67) | 12 (19) | <0.0001* |
| PE, n (%) | 10 (15) | 3 (5) | 0.040 |
| Azathioprine, n (%) | 36 (55) | 7 (11) | <0.0001* |
| Mycophenolate mofetil, n (%) | 9 (14) | 6 (10) | 0.460 |
| Loading dose IVIg (2 g/kg) | 1.86 ± 0.4 | 1.97 ± 0.4 | 0.230 |
| IVIg treatments, n (range) | 22.4 ± 39.6 (1–200) | 7.02 ± 12.2 (0–60) | 0.020 |
| Response to IVIg treatment, n (%) | 46 (84) | 18 (56) | 0.006 |
| PE treatments, n (range) | 1.4 ± 0.9 (1–3) | 4.7 ± 0.6 (4–5) | 0.0002* |
| Response to PE treatment, n (%) | 9 (82) | 2 (67) | 0.590 |
| Clinical status (n = 100) | 0.130 | ||
| Worse, n (%) | 16 (25) | 4 (11) | |
| No change, n (%) | 13 (20) | 13 (37) | |
| Stabilized, n (%) | 21 (32) | 8 (23) | |
| Improved, n (%) | 15 (23) | 10 (29) | |
| NCS after treatment (n = 93) | 0.850 | ||
| Worse, n (%) | 8 (14) | 6 (18) | |
| Stable, n (%) | 48 (81) | 26 (76) | |
| Improved, n (%) | 3 (5) | 2 (6) | |
Table adapted from Dunnigan SK et al. PLoS One. 2014;9(2):e89344 with permission. Data are mean ± SD unless otherwise indicated. Differences in categorical variables were assessed in three-group comparisons using the χ2-test, while differences in continuous variables were assessed using the analysis of variance.
Bonferroni corrected P value for significance = 0.003. CIDP, chronic immune demyelinating polyneuropathy; DM, diabetes mellitus; IVIg, intravenous immunoglobulin; PE, plasma exchange; NCS, nerve conduction study; SD, standard deviation.
4. Real-world data analysis of health insurance administrative claims
4.1. Methods
4.1.1. Database description
Controversies on the overlap of CIDP within DM led us to investigate the prevalence of CIDP and DM in a “real-world” health insurance administrative claims database—the 2009–2013 Phar-Metrics Plus™Database (Watertown, MA, USA). Secondary objectives were to determine any impact of age on the diagnosis of CIDP patients both without and with DM and to highlight any differences in treatment patterns. This database represents a pooling of adjudicated medical and pharmacy claims for over 100 million patient lives from more than 90 different health plans across the United States. The database includes inpatient and outpatient diagnoses in International Classification of Diseases-9th Revision-Clinical Modification (ICD-9-CM) format; procedures in Current Procedural Terminology-4th Edition and the Healthcare Common Procedure Coding System; and prescription records (Blanchette, Roberts, Petersen, et al., 2011). It also includes demographic variables, product and insurance type, provider specialty, and dates inclusive of plan enrollment. This private database includes a limited number of patients enrolled in Medicaid or Medicare and, therefore, underrepresents the patient population older than 65 years. A typical limitation of any claims database analysis is that clinical data are unavailable for the diagnostic criteria by which the diagnoses of CIDP and DM were made. To ensure the robustness of this dataset, we applied stringent inclusion criteria. Patients were confirmed with DM if there were ≥2 claims based on ICD-9-CM code 250. For a CIDP confirmation, patients were required to have ≥2 CIDP claims (ICD-9-CM code 357.81) reported at least 90 days apart. Incident cases were defined as being free of a CIDP diagnosis during the 12-month baseline period before their index date.
4.1.2. Statistical analysis
Descriptive statistics were used to assess differences in the population. Chi-square tests were used to assess differences in age distributions between CIDP patients without and with DM. Statistical significance was set at an alpha level of 0.05. This study was exempt from any institutional review board review because the patient information within the database was de-identified.
4.2. Results of database analysis
In this retrospective database study (N = 101,321,694), the prevalence of patients with CIDP (n = 8,173) was 8 per 100,000 persons (0.008%), which was similar to a previously reported prevalence value of 8.9 per 100,000 persons (Laughlin et al., 2009). Reflective of the generally younger patient population in the database, the overall prevalence of patients with DM (n = 4,026,740) was 3,974 per 100,000 persons (4.0%), which was lower than the 9.3% reported in the general United States population (CDC, 2014). However, in the population older than 55 years (n = 2,461,140), the percent prevalence of patients with DM was 11,567 per 100,000 persons (11.6%). The prevalence of CIDP among nondiabetic patients (n = 5,986) was 6 per 100,000 persons (0.006%). In contrast, the prevalence of CIDP in the diabetic population (n = 2,187) was 9-fold higher at 54 per 100,000 persons (0.054%). In this claims database study, clinical data are not available, and the accuracy of the diagnoses for DM and CIDP cannot be confirmed; yet the relative risk of having both CIDP and DM at 9-fold higher than having CIDP alone is approaching the 11-fold increase previously reported by Sharma et al., using the highly specific AAN criteria (Sharma, Cross, et al., 2002).
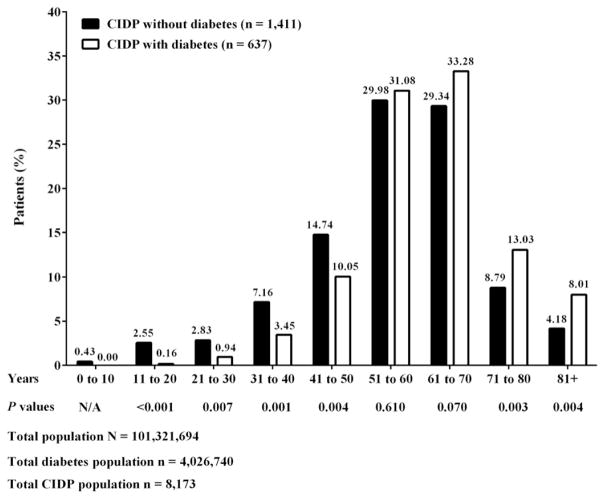
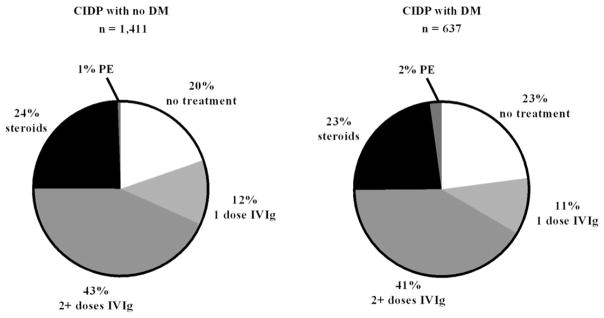
To improve the stringency around the CIDP diagnosis in this epidemiological cohort, we mandated that confirmed CIDP cases have distinct ICD-9-CM codes reported at least 90 days apart. A total of 2,048 patients had confirmed CIDP with ≥12 months pre- and post-index periods and ICD-9-CM codes of at least 90 days apart. Within this confirmed CIDP group, 1,411 (69%) patients had CIDP without DM, and 637 (31%) patients had CIDP with DM. These data were similar to the 25.7% of patients who had clinically confirmed CIDP with DM in another database study (Kalita, Misra, & Yadav, 2007). In our current study, the median ± standard deviation (SD) age was 56.4 ± 14.5 years for CIDP without DM and 61.6 ± 11.8 years for CIDP with DM. In patients aged 50 years or younger, CIDP without DM was more common than CIDP with DM (P < 0.01) (Fig. 1), suggesting that misdiagnosis of CIDP was not likely in this dataset. No difference in prevalence rates was found in patients aged 51 to 60 years in both groups (29.98% CIDP without DM vs. 31.08% CIDP with DM, P = 0.61). In those aged 61 to 70 years, there was a trend for CIDP with DM to be more common than CIDP without DM, but statistical significance was not reached (33.28% CIDP without DM vs. 29.34% CIDP with DM, P = 0.07). Despite the relatively smaller number of patients in the 2 highest age groups, the percentage of patients aged ≥71 years with CIDP and DM was statistically significantly higher than those without DM (P < 0.01). In addition, the treatment of CIDP patients without or with a concomitant diagnosis of DM was found to be similar. Approximately 50% of patients were treated with IVIg (57% CIDP without DM and 52% CIDP with DM), 23%–24% with steroids, 1%–2% with PE; and approximately 20%–23% received no treatment in both groups (Fig. 2). Although clinical data are not available, one might hypothesize that these untreated patients had only mild disability and therefore, according to EFNS/PNS guidelines, would be managed with monitoring for disease worsening (EFNS/PNS, 2010). Notably, steroid and IVIg use was similar between both groups. As only claim data were available, we cannot comment on the responsiveness of these patients to various treatments.
Fig. 1.
Percent patient distribution by age. Patients (n = 2,048) had confirmed CIDP with ≥12 months pre- and post-index period based on ICD-9-CM codes reported at least 90 days apart. CIDP, chronic inflammatory demyelinating polyneuropathy; DM, diabetes mellitus.
Fig. 2.
Treatment by percentage for CIDP patients with and without DM. Patients (n = 2,048) had confirmed CIDP with ≥12 months pre- and post-index period based on ICD-9-CM codes reported at least 90 days apart. CIDP, chronic inflammatory demyelinating polyneuropathy; DM, diabetes mellitus; IVIg, intravenous immunoglobulin; PE, plasma exchange.
In an alternative analysis evaluating this database with less stringent inclusion criteria (ie, ≥2 CIDP claims reported at no particular time interval), the reliability of this dataset became more evident. Without requiring the CIDP claims to be reported at least 90 days apart, we found similar results to those derived from the more stringent analysis previously described. A total of 3,399 patients had confirmed CIDP; 2,380 (70%) patients had CIDP without DM; and 1,019 (30%) patients had CIDP with DM. Comparable to the data described in Fig. 1, the age distribution derived from this less stringent analysis demonstrated: (A) CIDP without DM was more common than CIDP with DM (P = 0.01) in patients aged <50 years; (B) there was no difference in patients aged 51 to 60 years for both groups (29% CIDP without DM and 30% CIDP with DM, P = 0.36); however, (C) CIDP with DM was statistically significantly higher than CIDP without DM in patients aged >61 years (P = 0.01). Treatment of CIDP patients without or with a concomitant diagnosis of DM also appeared consistent with data described in Fig. 2 (approximately 40% of patients were treated with IVIg, 27% with steroids, 1%–2% with PE, and roughly 30% received no treatment).
Interestingly, the CIDP patient age distribution in this large United States database was similar to the age distribution observed in an Italian epidemiological study (N = 4,334,225), in which older patients had a higher prevalence of CIDP than younger patients (Chio et al., 2007). We, thus, conclude that diabetes may be a common comorbidity along with CIDP in patients older than 50 years. Despite the potential selection bias for fewer patients older than 65 years (due to a limited number of patients ≥65 years old enrolled in Medicare), we expect that such bias would not only underestimate the prevalence of DM observed, but also underestimate the associated prevalence of patients with CIDP and DM.
5. Treatment of CIDP with DM
At present, the decision on how to treat CIDP with DM is guided by treatment for CIDP without DM. CIDP is the most common treatable autoimmune neuropathy (Mathey et al., 2015); up to 80% of patients with CIDP respond to treatment. Clinical studies have shown that corticosteroids, PE, and IVIg all have efficacy in CIDP (Dyck, Daube, O’Brien, et al., 1986; Dyck, O’Brien, Oviatt, et al., 1982; Hahn, Bolton, Pillay, et al., 1996a; Hughes, Donofrio, Bril, et al., 2008; Mehndiratta & Hughes, 2002; Rajabally, 2015). The EFNS/PNS guidelines (EFNS/PNS, 2010) recommend IVIg or corticosteroids for sensory and motor CIDP; IVIg for pure motor CIDP; and if IVIg and corticosteroids are both ineffective, PE should be considered. Combination treatment or the addition of an immunosuppressant or immunomodulatory drug may be considered if the response to IVIg, corticosteroids, or PE is inadequate. Several clinical studies have shown that patients with CIDP and DM are also responsive to immunological treatment (Cocito, Ciaramitaro, Isoardo, et al., 2002; Dunnigan et al., 2014; Gorson et al., 2000; Jann, Bramerio, Facchetti, et al., 2009; Krendel, Costigan, & Hopkins, 1995; Sharma, Cross, et al., 2002a; Stewart, McKelvey, Durcan, et al., 1996). Treatment decisions depend on factors such as concomitant diseases, cost, therapeutic responsiveness, adverse effects particularly with long-term treatment, and a risk of relapse upon treatment withdrawal (Jann et al., 2009; Rajabally, 2015). Regardless of a DM diagnosis, our data demonstrated that steroid treatment in both groups occurred at similar rates (27%).
While IVIg may be preferred in severe disease states due to its rapid therapeutic onset, overtreatment should be avoided (Rajabally, 2015). Once maximal benefit from treatment has been achieved, it is recommended to gradually wean or even withdraw treatment (Eftimov, Vermeulen, van Doorn, et al., 2012; Hahn, Bolton, Zochodne, et al., 1996; Lünemann et al., 2015; RMC Trial Group, 2009). Improvements in the monitoring of CIDP response to treatment with consistent, standardized, and serial functional scores are needed to help physicians determine the effectiveness of a chosen therapy and avoid overtreatment. These objective measures are now required by a number of health insurance providers to continue therapy.
6. Distinguishing CIDP with concomitant diabetes from DPN
Although population-based studies have less controlled inclusion and exclusion criteria than traditional randomized, controlled trials (RCTs), this work provides meaningful real-world data that could not otherwise be described by clinical data from RCTs. As described by Booth and Tannock (Booth & Tannock, 2014), population-based studies include all patients within a given jurisdiction, including the underrepresented, the elderly, and those with comorbidity; thus, they are less prone to selection and referral biases that affect more traditional forms of observational research. The magnitude of the number of patients in this CIDP database with a concomitant diagnosis of DM indicates a need for heightened awareness of the association of CIDP and DM. This large data analysis, albeit retrospective, does highlight the need to differentiate CIDP in patients with concomitant DM, as this distinction is important to provide appropriate treatment.
While this database demonstrates that CIDP is diagnosed in DM, it remains unclear whether it is accurately diagnosed and whether adequate tools exist to aid in diagnosis. A recent publication suggested that misdiagnosis of CIDP is common (Allen & Lewis, 2015). We recognize that the number of patients in our database with a diagnosis of CIDP may be an overestimation, and if such overestimation occurred, we would expect the same level of impact on both the general CIDP population and the DM population. In the past, nerve biopsy was used to diagnose atypical CIDP. Today, nerve biopsy is seldom performed, as it is felt to be less sensitive than electrodiagnosis, and furthermore, specific abnormalities may be more difficult to detect because “inflammatory lesions in CIDP occur predominantly in the spinal roots, proximal nerve trunks, and major plexuses” (Mathey et al., 2015). Histological findings from sural nerve biopsy in CIDP are variable and nonspecific (Barohn, Kissel, Warmolts, et al., 1989; Dyck et al., 1975; Matsumuro, Izumo, Umehara, et al., 1994; Stewart et al., 1996), making it difficult “to distinguish between CIDP and DPN on histological grounds alone”(Stewart et al., 1996). There are “no distinctive pathological findings among these [CIDP with DM vs. DPN] patients” (Uncini, De Angelis, Di Muzio, et al., 1999).
Although not yet validated in large populations, a screening tool has been proposed wherein clinicians could use a combination of clinical, electrophysiological, and laboratory parameters to more accurately identify CIDP versus DPN in patients with DM (Lotan, Hellman, & Steiner, 2015). Certain parameters supportive of CIDP and not seen in DPN were given a positive (+) value (eg, progressive/relapsing motor weakness of 2–6 months and distal CMAP duration of ≥9 ms in ≥1 nerve and ≥1 other demyelinating parameter in ≥1 other nerve). Other parameters seen in DPN but not in CIDP were given a negative (−) value (eg, slowly progressive course and reduced CMAP amplitude disproportionate to motor conduction velocities). Although validated in a small number of patients (N = 57), this tool correlates well with both AAN and EFNS criteria for CIDP (AAN, 1991; Van den Bergh & Pieret, 2004). Further studies with larger populations correlating with EFNS/PNS diagnostic criteria and, importantly, response to therapy, are needed (Lotan et al., 2015).
7. Conclusion
There is an increasing body of literature suggesting that the prevalence of CIDP tends to be higher in diabetic patients, especially in those of older age. Our real-world data seem to support published findings from the literature. This retrospective health insurance administrative claims database study suggests that the prevalence of CIDP in a nondiabetic population is 6 per 100,000 persons, while the prevalence of CIDP in a patient population with DM is 9-fold higher at 54 per 100,000 persons. The association of CIDP with DM remains controversial, as both diseases have increased prevalence in patients over age 50 years. It is a challenge to identify CIDP in a diabetic population due to concomitant axonal damage. Although some patients with CIDP and DM respond to treatment, it is difficult to predict response. Because of the rising prevalence of DM throughout the world, there is a need to differentiate CIDP from DPN accurately. The overarching goal is to determine which patients with DM have a treatable neuropathy. New biomarkers for CIDP, such as corneal confocal microscopy (Stettner, Hinrichs, Guthoff, et al., 2016), are being evaluated in DPN as well as CIDP and may help differentiate these entities in the future.
Acknowledgments
This study was funded by Grifols, manufacturer of IVIg. We, the authors, thank Latoya M. Mitchell, PhD, CMPP, for medical writing assistance. We also thank Tam Nguyen-Cao, PhD, and June Davis, PhD, both of Grifols for editorial assistance. Data contained within this manuscript were presented at the American Diabetes Association Meeting in Boston, MA, on June 8, 2015, the Peripheral Nerve Society Biennial Meeting in Quebec City, Canada on June 29, 2015, and the Neurodiab 25th Annual Meeting in Elsinore, Denmark on September 13, 2015.
Footnotes
Conflict of Interest Disclosure: V.B. has received grant funding from Grifols, CSL Behring, and Alexion; has consulted for Grifols, CSL Behring, Alexion, and Bionevia; and has participated as a speaker on behalf of Grifols. D.G. and M.C.R. are employees of Grifols. C.M.B. and J.M.N. have received consulting fees from Grifols for this study. J.W.R. is supported in part by the Office of Research Development, Department of Veterans Affairs (Biomedical and Laboratory Research Service and Rehabilitation Research and Development, 101RX001030) and Baltimore GRECC. There are no other relevant conflicts of interest.
References
- AAN. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(5):617–618. [PubMed] [Google Scholar]
- Abe Y, Terashima H, Hoshino H, Sassa K, Sakai T, Ohtake A, … Yamanouchi H. Characteristic MRI Features of Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Brain and Development. 2015;37(9):894–896. doi: 10.1016/j.braindev.2015.01.006. http://dx.doi.org/10.1016/j.braindev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Abraham A, Breiner A, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Treatment responsiveness in CIDP patients with diabetes is associated with unique electrophysiological characteristics, and not with common criteria for CIDP. Expert Review of Clinical Immunology. 2015;11(4):537–546. doi: 10.1586/1744666X.2015.1018891. http://dx.doi.org/10.1586/1744666X.2015.1018891. [DOI] [PubMed] [Google Scholar]
- Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;8(6):498–504. doi: 10.1212/WNL.0000000000001833. http://dx.doi.org/10.1212/WNL.0000000000001833. [DOI] [PubMed] [Google Scholar]
- Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical Characteristics, course, and recommendations for diagnostic criteria. Archives of Neurology. 1989;46(8):878–884. doi: 10.1001/archneur.1989.00520440064022. [DOI] [PubMed] [Google Scholar]
- Blanchette CM, Roberts MH, Petersen H, Dalal AA, Mapel DW. Economic burden of chronic bronchitis in the United States: A retrospective case-control study. International Journal of Chronic Obstructive Pulmonary Disease. 2011;6:73–81. doi: 10.2147/COPD.S15882. http://dx.doi.org/10.2147/COPD.S15882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. British Journal of Cancer. 2014;110(3):551–555. doi: 10.1038/bjc.2013.725. http://dx.doi.org/10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiner A, Brannagan TH., III Comparison of sensitivity and specificity among 15 criteria for chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2014;50(1):40–46. doi: 10.1002/mus.24088. http://dx.doi.org/10.1002/mus.24088. [DOI] [PubMed] [Google Scholar]
- CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States (trans: Services U.S.D.o.H.a.H.) Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- Chin RL, Deng C, Bril V, Hartung HP, Merkies IS, Donofrio PD, … Latov N. Follow-up nerve conduction studies in CIDP after treatment with IVIG-C: Comparison of patients with and without subsequent relapse. Muscle & Nerve. 2015;52(4):498–502. doi: 10.1002/mus.24624. http://dx.doi.org/10.1002/mus.24624. [DOI] [PubMed] [Google Scholar]
- Chin RL, Rubin M. Diabetic Neuropathy. In: Poretsky L, editor. Principles of Diabetes Mellitus. 2. New York: Springer; 2010. pp. 357–370. [Google Scholar]
- Chio A, Cocito D, Bottacchi E, Buffa C, Leone M, Plano F … The PARCIDP. Idiopathic chronic inflammatory demyelinating polyneuropathy: An epidemiolog-ical study in Italy. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(12):1349–1353. doi: 10.1136/jnnp.2007.114868. http://dx.doi.org/10.1136/jnnp.2007.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito D, Chio A, Tavella A, Poglio F, Paolasso I, Ciaramitaro P, … Isoardo G. Treatment response and electrophysiological criteria in chronic inflammatory demyelinating polyneuropathy. European Journal of Neurology : the official journal of the European Federation of Neurological Societies. 2006;13:669–670. doi: 10.1111/j.1468-1331.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- Cocito D, Ciaramitaro P, Isoardo G, Barbero P, Migliaretti G, Pipieri A, … Durelli L. Intravenous immunoglobulin as first treatment in diabetics with concomitant distal symmetric axonal polyneuropathy and CIDP. Journal of Neurology. 2002;249(6):719–722. doi: 10.1007/s00415-002-0698-0. http://dx.doi.org/10.1007/s00415-002-0698-0. [DOI] [PubMed] [Google Scholar]
- Devic P, Petiot P, Mauguiere F. Diagnostic utility of somatosensory evoked potentials in chronic polyradiculopathy without electrodiagnostic signs of peripheral demyelination. Muscle & Nerve. 2015;53(1):78–83. doi: 10.1002/mus.24693. http://dx.doi.org/10.1002/mus.24693. [DOI] [PubMed] [Google Scholar]
- Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology. 2015;84(8):803–809. doi: 10.1212/WNL.0000000000001291. http://dx.doi.org/10.1212/WNL.0000000000001291. [DOI] [PubMed] [Google Scholar]
- Dunnigan SK, Ebadi H, Breiner A, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Comparison of diabetes patients with “demyelinating” diabetic sensori-motor polyneuropathy to those diagnosed with CIDP. Brain and Nehavior. 2013;3(6):656–663. doi: 10.1002/brb3.177. http://dx.doi.org/10.1002/brb3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnigan SK, Ebadi H, Breiner A, Katzberg HD, Barnett C, Perkins BA, Bril V. The characteristics of chronic inflammatory demyelinating polyneuropathy in patients with and without diabetes–an observational study. PloS One. 2014;9(2):e89344. doi: 10.1371/journal.pone.0089344. http://dx.doi.org/10.1371/journal.pone.0089344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Daube J, O’Brien P, Pineda A, Low PA, Windebank AJ, Swanson C. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. The New England Journal of Medicine. 1986;314(8):461–465. doi: 10.1056/NEJM198602203140801. http://dx.doi.org/10.1056/NEJM198602203140801. [DOI] [PubMed] [Google Scholar]
- Dyck PJB, Engelstad JK, Norell JE, Laughlin RS, Garces-Sanchez M, Massie R, Dyck PJ. inflammatory neuropathies in diabetes mellitus: The radiculoplexus neuropathies and diabetic CIDP. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2010;15:241–293. [Google Scholar]
- Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic Inflammatory Polyradiculoneuropathy. Mayo Clinic Proceedings. 1975;50(11):621–637. [PubMed] [Google Scholar]
- Dyck PJ, O’Brien PC, Oviatt KF, Dinapoli RP, Daube JR, Bartleson JD, … Windebank AJ. prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Annals of Neurology. 1982;11(2):136–141. doi: 10.1002/ana.410110205. http://dx.doi.org/10.1002/ana.410110205. [DOI] [PubMed] [Google Scholar]
- EFNS/PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2010;15(1):1–9. doi: 10.1111/j.1529-8027.2010.00245.x. http://dx.doi.org/10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van Schaik IN PREDICT Study Group. Long-Term Remission of CIDP after Pulsed Dexamethasone or Short-Term Prednisolone Treatment. Neurology. 2012;78(14):1079–1084. doi: 10.1212/WNL.0b013e31824e8f84. http://dx.doi.org/10.1212/WNL.0b013e31824e8f84. [DOI] [PubMed] [Google Scholar]
- French CIDP Study Group. Vallat JM. Recommendations on diagnostic strategies for chronic inflammatory demyelinating polyradiculoneuropathy. Postgraduate Medical Journal. 2008;84(993):378–381. doi: 10.1136/jnnp.2006.109785. http://dx.doi.org/10.1136/jnnp.2006.109785. [DOI] [PubMed] [Google Scholar]
- Garces-Sanchez M, Laughlin RS, Dyck PJ, Engelstad JK, Norell JE, Dyck PJ. Painless diabetic motor neuropathy: A variant of diabetic lumbosacral radiculoplexus neuropathy? Annals of Neurology. 2011;69(6):1043–1054. doi: 10.1002/ana.22334. http://dx.doi.org/10.1002/ana.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorson KC, Ropper AH, Adelman LS, Weinberg DH. Influence of diabetes mellitus on chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2000;23(1):37–43. doi: 10.1002/(sici)1097-4598(200001)23:1<37::aid-mus5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Guidon AC. Comment: A growing role for nerve ultrasound in diagnosis and management of CIDP? Neurology. 2015;84(8):808. doi: 10.1212/WNL.0000000000001295. http://dx.doi.org/10.1212/WNL.0000000000001295. [DOI] [PubMed] [Google Scholar]
- Hafsteinsdottir B, Olafsson E, Stefansson S. The incidence and prevalence of CIDP in Iceland. American Neurological Association. 2012;M1448:S75. [Google Scholar]
- Hahn AF, Bolton CF, Pillay N, Chalk C, Benstead T, Bril V, … Feasby TE. Plasma-Exchange Therapy in Chronic Inflammatory Demyelinating Polyneuropathy. A Double-Blind, Sham-Controlled, Cross-over Study. Brain: a Journal of Neurology. 1996;119(Pt 4):1055–1066. doi: 10.1093/brain/119.4.1055. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain: a Journal of Neurology. 1996;119(Pt 4):1067–1077. doi: 10.1093/brain/119.4.1067. [DOI] [PubMed] [Google Scholar]
- Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA … AACE Task Force for Developing a Diabetes Comprehensive Care Plan. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for Developing a Diabetes Mellitus Comprehensive Care Plan. Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2006;11(1):30–46. doi: 10.1111/j.1085-9489.2006.00061.x. http://dx.doi.org/10.1111/j.1085-9489.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K … ICE Study Group. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. The Lancet Neurology. 2008;7(2):136–144. doi: 10.1016/S1474-4422(07)70329-0. http://dx.doi.org/10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- Jang JH, Cho CS, Yang KS, Seok HY, Kim BJ. Pattern analysis of nerve enlargement using ultrasonography in chronic inflammatory demyelinating polyneuropathy. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2014;125(9):1893–1899. doi: 10.1016/j.clinph.2013.12.115. http://dx.doi.org/10.1016/j.clinph.2013.12.115. [DOI] [PubMed] [Google Scholar]
- Jann S, Bramerio MA, Beretta S, Koch S, Defanti CA, Toyka KV, Sommer C. Diagnostic value of sural nerve matrix metalloproteinase-9 in diabetic patients with CIDP. Neurology. 2003;61(11):1607–1610. doi: 10.1212/01.wnl.0000096174.86850.7f. [DOI] [PubMed] [Google Scholar]
- Jann S, Bramerio MA, Facchetti D, Sterzi R. Intravenous immunoglobulin is effective in patients with diabetes and with chronic inflammatory demyelinating polyneuropathy: Long term follow-up. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(1):70–73. doi: 10.1136/jnnp.2008.149013. http://dx.doi.org/10.1136/jnnp.2008.149013. [DOI] [PubMed] [Google Scholar]
- Kalita J, Misra UK, Yadav RK. A comparative study of chronic inflammatory demyelinating polyradiculoneuropathy with and without diabetes mellitus. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2007;14(6):638–643. doi: 10.1111/j.1468-1331.2007.01798.x. http://dx.doi.org/10.1111/j.1468-1331.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2014;125(3):635–641. doi: 10.1016/j.clinph.2013.08.014. http://dx.doi.org/10.1016/j.clinph.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Bochum Ultrasound Score Versus Clinical and Electrophysiological Parameters in Distinguishing Acute-Onset Chronic from Acute Inflammatory Demyelinating Polyneuropathy. Muscle & Nerve. 2015;51(6):846–852. doi: 10.1002/mus.24484. http://dx.doi.org/10.1002/mus.24484. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A, Pitarokoili K, Gold R, Yoon MS. Bochum ultrasound score allows distinction of chronic inflammatory from multifocal acquired demyelinating polyneuropathies. Journal of the Neurological Sciences. 2015;348(1–2):211–215. doi: 10.1016/j.jns.2014.12.010. http://dx.doi.org/10.1016/j.jns.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. The New England Journal of Medicine. 2005;352(13):1343–1356. doi: 10.1056/NEJMra041347. http://dx.doi.org/10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- Koski CL, Baumgarten M, Magder LS, Barohn RJ, Goldstein J, Graves M, … Cornblath DR. Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. Journal of the Neurological Sciences. 2009;277(1–2):1–8. doi: 10.1016/j.jns.2008.11.015. http://dx.doi.org/10.1016/j.jns.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Krarup C. An update on electrophysiological studies in neuropathy. Current Opinion in Neurology. 2003;16(5):603–612. doi: 10.1097/01.wco.0000093104.34793.94. http://dx.doi.org/10.1097/01.wco.0000093104.34793.94. [DOI] [PubMed] [Google Scholar]
- Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Archives of Neurology. 1995;52(11):1053–1061. doi: 10.1001/archneur.1995.00540350039015. [DOI] [PubMed] [Google Scholar]
- Latov N. Biomarkers of CIDP in Patients with Diabetes or CMT1. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2011;16(Suppl 1):14–17. doi: 10.1111/j.1529-8027.2011.00299.x. http://dx.doi.org/10.1111/j.1529-8027.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nature Reviews Neurology. 2014;10(8):435–446. doi: 10.1038/nrneurol.2014.117. http://dx.doi.org/10.1038/nrneurol.2014.117. [DOI] [PubMed] [Google Scholar]
- Laughlin RS, Dyck PJ, Melton LJ, 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39–45. doi: 10.1212/WNL.0b013e3181aaea47. http://dx.doi.org/10.1212/WNL.0b013e3181aaea47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I, Hellman MA, Steiner I. Diagnostic criteria of chronic inflammatory demyelinating polyneuropathy in diabetes mellitus. Acta Neurologica Scandinavica. 2015;132(4):278–283. doi: 10.1111/ane.12394. http://dx.doi.org/10.1111/ane.12394. [DOI] [PubMed] [Google Scholar]
- Lozeron P, Nahum L, Lacroix C, Ropert A, Guglielmi JM, Said G. Symptomatic diabetic and non-diabetic neuropathies in a series of 100 diabetic patients. Journal of Neurology. 2002;249(5):569–575. doi: 10.1007/s004150200066. http://dx.doi.org/10.1007/s004150200066. [DOI] [PubMed] [Google Scholar]
- Lünemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology- mode of action and clinical efficacy. Nature Reviews Neurology. 2015;11(2):80–89. doi: 10.1038/nrneurol.2014.253. http://dx.doi.org/10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- Lunn MP, Manji H, Choudhary PP, Hughes RA, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: A prevalence study in south east England. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66(5):677–680. doi: 10.1136/jnnp.66.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccabee PJ, Eberle LP, Stein IA, Willer JA, Lipitz ME, Kula RW, … Amassian VE. Upper leg conduction time distinguishes demyelinating neuropathies. Muscle & Nerve. 2011;43(4):518–530. doi: 10.1002/mus.21909. http://dx.doi.org/10.1002/mus.21909. [DOI] [PubMed] [Google Scholar]
- Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, … Lin CS. Chronic inflammatory demyelinating polyradiculoneuropathy: From pathology to phenotype. Journal of Neurology, Neurosurgery, and Psychiatry. 2015;86(9):973–985. doi: 10.1136/jnnp-2014-309697. http://dx.doi.org/10.1136/jnnp-2014-309697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumuro K, Izumo S, Umehara F, Osame M. Chronic inflammatory demyelinating polyneuropathy: Histological and immunopathological studies on biopsied sural nerves. Journal of the Neurological Sciences. 1994;127(2):170–178. doi: 10.1016/0022-510x(94)90070-1. [DOI] [PubMed] [Google Scholar]
- McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Annals of Neurology. 1999;46(6):910–913. [PubMed] [Google Scholar]
- Mehndiratta MM, Hughes RA. Corticosteroids for Chronic Inflammatory Demyelinating Polyradiculoneuropathy. The Cochrane Database of Systematic Reviews. 2002;1:CD002062. doi: 10.1002/14651858.CD002062. http://dx.doi.org/10.1002/14651858.CD002062. [DOI] [PubMed] [Google Scholar]
- Midroni G, de Tilly LN, Gray B, Vajsar J. MRI of the cauda equina in CIDP: Clinical correlations. Journal of the Neurological Sciences. 1999;170(1):36–44. doi: 10.1016/s0022-510x(99)00195-1. [DOI] [PubMed] [Google Scholar]
- Mygland Å, Monstad P. Chronic polyneuropathies in Vest-Agder, Norway. European Journal of Neurology : The Official Journal of the European Federation of Neurological Societies. 2001;8(2):157–165. doi: 10.1046/j.1468-1331.2001.00187.x. http://dx.doi.org/10.1046/j.1468-1331.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- Rajabally YA. Long-term immunoglobulin therapy for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve. 2015;51(5):657–661. doi: 10.1002/mus.24554. http://dx.doi.org/10.1002/mus.24554. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Fowle AJ, Van den Bergh PY. Which criteria for research in chronic inflammatory demyelinating polyradiculoneuropathy? An analysis of current practice. Muscle & Nerve. 2015;51(6):932–933. doi: 10.1002/mus.24496. http://dx.doi.org/10.1002/mus.24496. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Jacob S, Hbahbih M. Optimizing the use of electrophysiology in the diagnosis of chronic inflammatory demyelinating polyneuropathy: A study of 20 cases. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2005;10(3):282–292. doi: 10.1111/j.1085-9489.2005.10306.x. http://dx.doi.org/10.1111/j.1085-9489.2005.10306.x. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Nicolas G, Pieret F, Bouche P, Van den Bergh PY. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: A multicentre European study. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(12):1364–1368. doi: 10.1136/jnnp.2009.179358. http://dx.doi.org/10.1136/jnnp.2009.179358. [DOI] [PubMed] [Google Scholar]
- Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: Study of a UK population. Muscle & Nerve. 2009;39(4):432–438. doi: 10.1002/mus.21206. http://dx.doi.org/10.1002/mus.21206. [DOI] [PubMed] [Google Scholar]
- RMC Trial Group. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC Trial): A pilot, multi-centre study. The Lancet Neurology. 2009;8(2):158–164. doi: 10.1016/S1474-4422(08)70299-0. http://dx.doi.org/10.1016/s1474-4422(08)70299-0. [DOI] [PubMed] [Google Scholar]
- Russell JW, Zilliox LA. Diabetic neuropathies. Continuum: Lifelong Learning in Neurology. 2014;20:1226–1240. doi: 10.1212/01.CON.0000455884.29545.d2. http://dx.doi.org/10.1212/01.CON.0000455884.29545.d2 (5 Peripheral Nervous System Disorders) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KR, Cross J, Ayyar DR, Martinez-Arizala A, Bradley WG. diabetic demyelinating polyneuropathy responsive to intravenous immunoglobulin therapy. Archives of Neurology. 2002;59(5):751–757. doi: 10.1001/archneur.59.5.751. [DOI] [PubMed] [Google Scholar]
- Sharma KR, Cross J, Farronay O, Ayyar DR, Shebert RT, Bradley WG. Demyelinating neuropathy in diabetes mellitus. Archives of Neurology. 2002;59(5):758–765. doi: 10.1001/archneur.59.5.758. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Sugiyama A, Ito SI, Misawa S, Sekiguchi Y, Mitsuma S, … Kuwabara S. Reconstruction magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy. Annals of Neurology. 2015;77(2):333–337. doi: 10.1002/ana.24314. http://dx.doi.org/10.1002/ana.24314. [DOI] [PubMed] [Google Scholar]
- Sommer C, Toyka K. Nerve biopsy in chronic inflammatory neuropathies: In situ biomarkers. Journal of the Peripheral Nervous System: The Official Journaal of The Peripheral Nerve Society. 2011;16(Suppl 1):24–29. doi: 10.1111/j.1529-8027.2011.00301.x. http://dx.doi.org/10.1111/j.1529-8027.2011.00301.x. [DOI] [PubMed] [Google Scholar]
- Stettner M, Hinrichs L, Guthoff R, Bairov S, Petropoulos IN, Warnke C, … Kieseier BC. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Annals of Clinical and Translational Neurology. 2016;3(2):88–100. doi: 10.1002/acn3.275. http://dx.doi.org/10.1002/acn3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JD, McKelvey R, Durcan L, Carpenter S, Karpati G. Chronic inflammatory demyelinating polyneuropathy (CIDP) in diabetics. Journal of the Neurological Sciences. 1996;142(1–2):59–64. doi: 10.1016/0022-510x(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P … The Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. http://dx.doi.org/10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncini A, De Angelis MV, Di Muzio A, Callegarini C, Ciucci G, Antonini G, … Gambi D. Chronic inflammatory demyelinating polyneuropathy in diabetics: Motor conductions are important in the differential diagnosis with diabetic polyneuropathy. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 1999;110(4):705–711. doi: 10.1016/s1388-2457(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Van den Bergh PY, Pieret F. Electrodiagnostic criteria for acute and chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve. 2004;29(4):565–574. doi: 10.1002/mus.20022. http://dx.doi.org/10.1002/mus.20022. [DOI] [PubMed] [Google Scholar]
- Vo ML, Hanineva A, Chin RL, Carey BT, Latov N, Langsdorf JA. Comparison of 2-limb versus 3-limb electrodiagnostic studies in the evaluation of chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2015;51(4):549–553. doi: 10.1002/mus.24424. http://dx.doi.org/10.1002/mus.24424. [DOI] [PubMed] [Google Scholar]