Introduction
Implantable cardioverter-defibrillators (ICD) are effective in preventing sudden cardiac death in patients with left ventricular dysfunction and heart failure.1 However, inappropriate ICD shocks, most commonly caused by atrial fibrillation (AF), occur frequently, and are associated with impaired quality of life,2 increased mortality,3 and healthcare cost.4 Therefore, risk assessment of inappropriate shocks in individual patients prior to ICD implantation is critically important for the clinical decision-making.
A history of AF prior to ICD implantation is a major predictor of inappropriate shocks.3 However, patients may have asymptomatic AF5 that is clinically unrecognized prior to ICD implantation. In addition, patients may develop a new onset of AF after ICD implantation. Therefore, there is a need for a metric that predicts the risk of inappropriate shocks even in the absence of clinically recognized AF prior to ICD implantation.
Recent studies indicate that remodeling processes in the left atrial (LA) structure and function precede AF substrate maturation6,7. For example, larger LA volumes and lower LA function using tissue-tracking cardiac magnetic resonance (CMR) predict new development of AF in a substudy of a large prospective cohort.8 Therefore, we hypothesized that LA structural and functional remodeling quantified by tissue-tracking CMR predicts inappropriate shocks independent of a history of AF prior to ICD implantation. To test our hypothesis, we analyzed patients in the Prospective Observational Study of Implantable Cardioverter-Defibrillators (PROSE-ICD), a multicenter prospective cohort study to identify risk factors for arrhythmic death in primary prevention ICD candidates.9
Methods
Study Design
PROSE-ICD is a multicenter, prospective observational study of patients with cardiomyopathy eligible for a primary prevention ICD, conducted from 2003 to 2013. The details of study were described previously (online Data Supplement).9 The protocol was approved by the institutional review board, and all participants provided informed consent. Among the 1,189 participants enrolled in the PROSE-ICD study, CMR was offered to all the participants who were scheduled for ICD implantation at the Johns Hopkins Hospital, Baltimore, to assess cardiac structure and function. Fifty percent of eligible patients agreed to undergo CMR. The reasons for non-enrollment were refusal to participate in the CMR study (78% of those non-enrolled), claustrophobia (7%) and insufficient time to schedule the scan before device implantation (15%). As a result, a total of 367 participants underwent CMR prior to ICD implantation. Among the 367 participants who underwent CMR, we excluded 9 participants for poor image quality, and 196 participants in whom cine CMR of LA was performed only in the long-axis four-chamber view. Thus, we included a total of 162 participants in this study whose cine CMR of LA is available in both the long-axis four-chamber and the two-chamber views.
At enrollment, all patients underwent a baseline comprehensive history and cardiovascular physical examination, and a standard 12-lead electrocardiography. Then they underwent single-chamber or dual-chamber ICD, or cardiac resynchronization with an ICD (CRT-D) implantation based on the current guidelines.10 The systems used were manufactured by Boston Scientific (Natick, Massachusetts), Medtronic (Minneapolis, Minnesota) and St. Jude Medical (St. Paul, Minnesota). Stability and sudden onset algorithms were activated in all devices. Additional detection algorithms evaluating the atrial rate were activated in dual-chamber ICDs and CRT-Ds. The programing of tachycardia therapy cutoff rates and therapies was left to the discretion of the operators.
Patient Follow-up and Outcomes
The patients were evaluated every 6 months after implantation and after any ICD shock event reported by the patients or via remote transmission. At each visit, ICDs were interrogated to assess arrhythmic events. All stored electrograms from delivered ICD therapies were adjudicated by 2 clinical cardiac electrophysiologists blinded to patient demographic information, and each electrophysiologist independently determined the rhythm at the time of initial detection and after therapy delivery. Disagreements on the diagnosed rhythm were reviewed by a third electrophysiologist for final adjudication. In this study, the endpoint was the first occurrence of an inappropriate shock. The cause of inappropriate therapy was categorized as follows; atrial fibrillation or flutter (AF/AFL), supraventricular tachycardia including sinus tachycardia (SVT), or abnormal lead sensing. Management of inappropriate shocks was left to the discretion of the electrophysiologist who cared for the patient.
CMR Imaging and Analysis
The patients were scanned in sinus rhythm with a 1.5-T scanner, Signa CV/I (General Electric, Milwaukee, USA) or Avanto (Siemens Medical Systems, Erlangen, Germany). The details of the CMR protocol were reported previously (online Data Supplement).11 Multimodality Tissue Tracking software (MTT; version 6.0, Toshiba, Japan) was used to obtain phasic LA volumes, strain, and strain rate from four-chamber and two-chamber cine images. Details of the MTT12 and its validation13 and reproducibility13,14 have been described previously. Briefly, LA endocardial and epicardial borders were manually traced in the biplane images, excluding pulmonary veins and LA appendage (Figure 1A, 1B). Maximum LA volume (Vmax; at end systole before mitral valve opening), minimum LA volume (Vmin; at end diastole after mitral valve closure), and pre-atrial contraction LA volume (VpreA) were measured using the LA volume curve generated by the biplane Simpson’s method (Figure 1C). All volumes were subsequently indexed according to body surface area. Global longitudinal strain and strain rate curves were generated by averaging strain and strain rate in all LA segments within the biplane views, and LA maximum strain (Smax), LA pre-atrial contraction strain (SpreA), LA strain rate in left ventricular (LV) systole (SRs), LV early diastole (SRe), and LA contraction (SRa) were measured (Figure 1D,1E). The parameters of volume were calculated as follows: LA stroke volume (LASV) = LAVmax − LAVmin, LA total emptying fraction (EF) = (Vmax − Vmin)×100%/Vmax, LA passive EF = (Vmax − VpreA) ×100%/Vmax, and LA active EF = (VpreA − Vmin) ×100%/VpreA. All CMR analyses were performed blinded to clinical outcomes.
Figure 1.
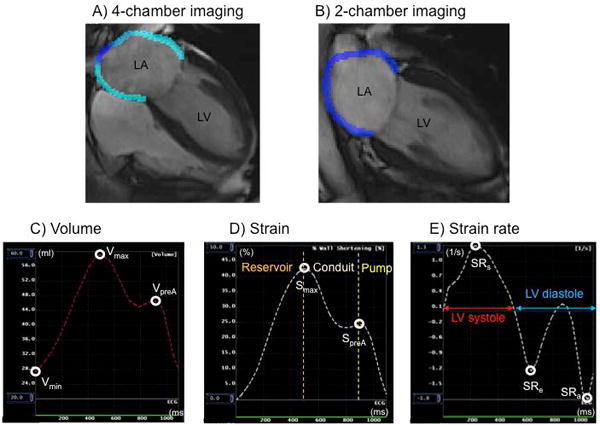
Image analysis and measurements of left atrium (LA) using tissue-tracking cardiac magnetic resonance. Tracing of LA endocardial and epicardial borders were performed at the end of left ventricular systole (A; four-chamber view, B; two-chamber view). Maximum LA volume (Vmax), minimum LA volume (Vmin), and pre-atrial contraction LA volume (VpreA) were identified from the LA volume curve (C). LA maximum strain (Smax) and pre-atrial contraction strain (SpreA) were identified from global longitudinal strain curve (D). LA strain rate in left ventricular (LV) systole (SRs), LV early diastole (SRe), and LA contraction (SRa) were identified from the LA strain curve (E).
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median [interquartile range; 25th–75th percentile], and categorical variables are expressed as frequencies and percentages. We used Pearson’s χ2 test for categorical variables and the Student t-test or Mann-Whitney U test for parametric or nonparametric continuous variables, respectively. We used Kaplan-Meyer methods and log-rank test to estimate the cumulative incidence of events. Cox proportional hazards models were used to determine predictors of inappropriate shocks. Univariable analyses of all baseline variables were performed. Multivariable analysis was performed for each LA parameter found to be significant on univariable analysis by adjusting for history of AF prior to ICD implantation, age younger than 70 years, and QRS less than 120ms. The calculated relationship was presented as a hazard ratio (HR) with a 95% confidence interval (CI). Receiver-operating characteristic (ROC) curve analysis was generated to assess the incremental value of each LA parameter individually over the base model included AF history, age and QRS duration to predict inappropriate shocks. ROC curve was also used to identify the best cut-off value of LA indices for predicting inappropriate shocks. We used JMP Pro Version 12.1.0 (SAS Institute Inc, Cary, NC, USA) to perform all statistical analyses. A difference with a p value of less than 0.05 was considered significant.
Results
Baseline Characteristics
Demographics of the study participants are presented in Table 1. Average age at baseline was 56.4±14.6 years, with 70% men. The mean ejection fraction was 27.9±10.6% with a relatively balanced distribution of cardiomyopathy etiology. Most patients received a single-chamber ICD (57%) with 21% receiving dual-chamber systems and 22% cardiac resynchronization therapy devices. The median lowest cutoff zone for tachycardia therapy was programmed to 200 (185–200) beats per minute.
TABLE 1.
Patient Demographics
| Total | No Inappropriate ICD Shock | Inappropriate ICD Shock | P Value | |
|---|---|---|---|---|
| (N = 162) | (N = 136) | (N = 26) | ||
| Age, years | 56.4 ± 14.6 | 56.7 ± 15.0 | 54.8 ± 12.3 | 0.56 |
| Male | 113 (70) | 93 (68) | 20 (77) | 0.39 |
| Race | 0.22 | |||
| Caucasian | 99 (61) | 86 (63) | 14 (54) | |
| African American | 55 (34) | 43 (32) | 12 (46) | |
| Other | 8 (5) | 7 (5) | 0 (0) | |
| NYHA class | 0.57 | |||
| I | 36 (22) | 28 (21) | 8 (31) | |
| II | 70 (43) | 59 (44) | 10 (38) | |
| III | 56 (35) | 46 (35) | 8 (31) | |
| Ischemic cardiomyopathy | 74 (46) | 63 (46) | 11 (42) | 0.71 |
| History of AF | 27 (17) | 17 (13) | 10 (38) | 0.001 |
| Hypertension | 95 (59) | 81 (60) | 14 (54) | 0.59 |
| Diabetes | 41 (25) | 37 (27) | 4 (15) | 0.20 |
| Smoker | 68 (42) | 58 (43) | 10 (38) | 0.16 |
| QRS duration, msec | 112 (96 – 140) | 113 (96 – 144) | 104 (93 – 119) | 0.08 |
| Medication | ||||
| ACE-I/ARB | 141 (87) | 121 (89) | 20 (77) | 0.09 |
| Beta-blocker | 140 (86) | 120 (88) | 20 (77) | 0.12 |
| Antiarrythmics | 13 (8) | 11 (8) | 2 (8) | 0.95 |
| CMR characteristics of LV | ||||
| LV EF, % | 27.9 ± 10.6 | 26.5 ± 11.0 | 29.2 ± 9.9 | 0.64 |
| LV EDVI, mL/m2 | 123.6 ± 40.4 | 124.7 ± 40.9 | 118.0 ± 37.9 | 0.46 |
| LV ESVI, mL/m2 | 91.4 ± 40.4 | 92.0 ± 41.0 | 88.2 ± 37.6 | 0.67 |
| LV mass index, mL/m2 | 76.9 ± 22.3 | 76.5 ± 21.4 | 79.2 ± 26.8 | 0.59 |
| LV total LGE, g | 15.5 (0 – 38.9) | 17.2 (0 – 39.7) | 5.8 (0 – 36.5) | 0.29 |
| Device type | 0.92 | |||
| Single | 92 (57) | 76 (56) | 16 (62) | |
| Dual | 34 (21) | 29 (21) | 5 (19) | |
| Dual/Biventricular | 36 (22) | 31 (23) | 5 (19) | |
| Lower rate of cutoff, bpm | 200 (185 – 200) | 200 (185 – 200) | 196 (188 – 200) | 0.47 |
| ATP used | 87 (54) | 45 (56) | 11 (42) | 0.17 |
Data are presented as mean ± standard deviation, n (%), or median (interquartile range).
ACE-I/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; AF = atrial fibrillation; ATP = antitachycardia pacing; CMR = cardiac magnetic resonance; EDVI = end-diastolic volume index; EDSVI = end-systolic volume index; ICD = implantable cardioverter defibrillators; LGE = late gadolinium enhancement; LV = left ventricle; NYHA = New York Heart Association.
Inappropriate ICD shocks
During the follow-up period of 4.0±2.9 years, 26 of 162 patients (16%) experienced one or more inappropriate shocks (Figure 2). The mean time from implantation to first inappropriate shock was 2.0±1.6 years. The most common cause of inappropriate shocks was AF/AFL, occurring in 19 patients (73%), followed by SVT in 5 patients (19%), and abnormal lead sensing in 2 patients (8%) (one T-wave oversensing and one lead fracture). Among the 26 patients, 7 patients had more than one inappropriate shock episode due to AF/AFL. Two of the 7 patients had inappropriate shocks due to both of AF and AFL.
Figure 2.
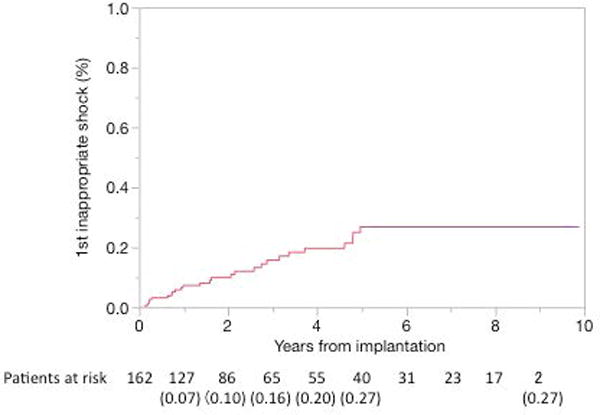
Cumulative probability of first occurrence of inappropriate ICD shock. The values of parentheses are Kaplan-Meier estimates of the cumulative probability of a first occurrence of inappropriate shock.
The patients with inappropriate shocks were more likely to have a history of AF than those without inappropriate shocks (38% vs. 13%, p=0.001) (Table 1). In 27 patients with a history of AF before device implantation, 10 patients developed inappropriate shocks due to AF/AFL. In patients without a history of AF, 16 patients developed inappropriate shocks due to AF/AFL in 9 patients, SVT in 5 patients, and abnormal lead sensing in 2 patients. There was no significant difference in other baseline characteristics between patients with and without inappropriate shocks, including the etiology of cardiomyopathy, LV ejection fraction, antiarrhythmic drugs, device type, and lower rate cutoff. However, among 27 patients with a history of AF, 1 of 9 patients (10%) on antiarrhythmic drugs including amiodarone received an inappropriate shock. In contrast, 9 of 18 patients (50%) off antiarrhythmic drugs received inappropriate shocks (hazard ratio: 0.13, p=0.037). No interventional therapy including catheter ablation was performed during the follow-up.
The LA measurements using tissue-tracking CMR are summarized in Table 2. Patients with inappropriate shocks had lower LA total EF and active EF, lower Smax and SpreA, and lower absolute value of SRa, compared to those without inappropriate shocks.
TABLE 2.
Left Atrial Parameters by Inappropriate ICD Shocks
| No Inappropriate ICD Shock (N = 136) |
Inappropriate ICD Shocks (N = 26) |
P Value | |
|---|---|---|---|
| LAVImin, mL/m2 | 25.3 (17.9 – 38.9) | 35.8 (18.0 – 59.5) | 0.16 |
| LAVImax, mL/m2 | 42.5 (32.7 – 57.2) | 49.2 (30.2 – 68.7) | 0.39 |
| LAVIpreA, mL/m2 | 38.5 (28.3 – 53.0) | 42.5 (28.3 –67.4) | 0.31 |
| LASVI, mL/m2 | 16.0 ± 6.1 | 13.6 ± 5.8 | 0.09 |
| LA total EF, % | 38.0 (25.0 – 48.9) | 31.0 (16.7 – 43.5) | 0.049 |
| LA passive EF, % | 10.1 (4.9 – 16.2) | 9.6 (4.3 – 15.2) | 0.83 |
| LA active EF, % | 30.7 (16.6 – 39.8) | 25.7 (9.1 – 33.4) | 0.027 |
| LASmax, % | 20.4 (10.9 – 27.9) | 13.2 (6.5 – 21.0) | 0.014 |
| LASpreA, % | 12.9 (6.4 – 18.6) | 8.8 (2.8 – 14.0) | 0.006 |
| LASRs, 1/s | 0.78 (0.47 – 1.05) | 0.60 (0.29 – 0.94) | 0.051 |
| LASRe, 1/s | −0.46 (−0.75 – −0.29) | −0.45 (−0.64 – −0.21) | 0.44 |
| LASRa, 1/s | −1.1 (−1.64 – −0.64) | −0.79 (−1.1 – −0.25) | 0.004 |
Data are presented as median (interquartile range), or mean ± standard deviation.
EF = emptying fraction; LA = left atrium; Smax = maximum strain; SpreA = pre-atrial contraction strain; SRa = strain rate at atrial contraction; SRe = strain rate at left ventricular early diastole; SRs = maximum strain rate; SVI = stroke volume index; VImax = maximum indexed volume; VImin = minimum indexed volume; VIpreA = pre-atrial contraction indexed volume; Abbreviations as in Table 1.
Predictors of inappropriate ICD shocks
In univariable Cox models, significant predictors of inappropriate shocks included (Table 3): history of AF before ICD implantation, age <70 years, QRS duration <120ms, larger minimum LA volume, smaller LA stroke volume, lower LA EFs (total EF and active EF), lower LA strains (Smax and SpreA), and lower absolute values of LA strain rates (SRs and SRa). In multivariable models adjusting for history of AF, age <70 years, and QRS duration <120 msec, LA parameters which remained significant were lower Smax (HR: 0.96, p=0.044), lower SpreA (HR: 0.94, p=0.030), and lower absolute value of SRa (HR: 0.25, p<0.001). Lower Smax, SpreA, and absolute value of SRa were significantly associated with inappropriate shocks due to AF/AFL after multivariable-adjustment, but not due to SVT and abnormal lead sensing (online Data Supplement Table 1). The best cut-off values of LA parameters for predicting inappropriate shocks were 20.6% for Smax [area under the curve (AUC): 0.65, sensitivity: 77%, specificity: 48%], 14.6% for SpreA (AUC: 0.67, sensitivity: 89%, specificity: 41%), and 1.00 /s for absolute value of SRa (AUC: 0.73, sensitivity 77%, specificity: 62%). Sensitivity and specificity of history of AF prior to ICD implantation were 39% and 87%, respectively. Kaplan-Meyer curves for each parameter are shown in Figure 3.
TABLE 3.
Predictors of Inappropriate ICD Shocks
| Parameters | Univariable | Multivariable, Adjusted | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| History of AF | 3.80 | 1.66 – 8.31 | 0.002 | – | ||
| Age < 70 years | 3.82 | 1.13 – 23.8 | 0.028 | – | ||
| Sex (male) | 1.36 | 0.58 – 3.73 | 0.50 | – | ||
| QRS < 120ms | 2.53 | 1.02 – 7.63 | 0.046 | – | ||
| LAVImin, mL/m2 | 1.02 | 1.00 – 1.03 | 0.027 | 1.01 | 0.99 – 1.02 | 0.36 |
| LAVImax, mL/m2 | 1.01 | 0.99 – 1.03 | 0.097 | – | ||
| LAVIpreA, mL/m2 | 1.01 | 0.99 – 1.03 | 0.084 | – | ||
| LASVI, mL/m2 | 0.92 | 0.85 – 0.98 | 0.012 | 0.94 | 0.87 – 1.01 | 0.11 |
| LA total EF, % | 0.96 | 0.94 – 0.99 | 0.006 | 0.98 | 0.95 – 1.06 | 0.13 |
| LA passive EF, % | 0.98 | 0.93 – 1.02 | 0.36 | – | ||
| LA active EF, % | 0.96 | 0.93 – 0.99 | 0.004 | 0.98 | 0.95 – 1.00 | 0.11 |
| LASmax, % | 0.94 | 0.90 – 0.98 | 0.002 | 0.96 | 0.91 – 0.99 | 0.044 |
| LASpreA, % | 0.91 | 0.85 – 0.96 | 0.001 | 0.94 | 0.87 – 0.99 | 0.030 |
| LASRs, 1/s | 0.26 | 0.08 – 0.75 | 0.011 | 0.41 | 0.12 – 1.42 | 0.096 |
| LASRe, 1/s | 0.51 | 0.16 – 1.32 | 0.18 | – | ||
| LASRa, 1/s | 0.26 | 0.11 – 0.55 | 0.002 | 0.25 | 0.10 – 0.57 | <0.001 |
LASRe and LASRa are presented as absolute value. A Cox regression model was used. Multivariable modeling was performed by adjusting each variable for history of AF, age <70 years, and QRS <120ms which were significant in univariable analyses.
Figure 3.

Kaplan-Meier curves showing time to a first occurrence of inappropriate ICD shock according to left atrial (LA) parameters (A–C) and history of atrial fibrillation (AF) prior to ICD implantation (D). The cutoff of LA maximum strain (Smax), pre-atrial contraction strain (SpreA), and absolute value of strain rate in LA contraction (SRa) were ≦20.6% (A), ≦14.6% (B), and ≦1.0 /s (C) for the lower group, respectively.
Incremental value of LA function as a predictor of inappropriate ICD shocks
The AUC of LASRa added to the base model used for multivariable analyses was significantly higher than base model alone (0.79 vs. 0.69, p=0.033) (Figure 4). The AUC of Smax and SpreA added to the base model trended insignificantly higher than the base model alone. Kaplan-Meyer curves of combinations of AF history and LASRa, with a cut-off absolute value of 1.00 /s, are shown in Figure 5. The patients with higher absolute value of LASRa and no history of AF (Group 1 in Figure 5), representing 53% of the cohort, had the lowest incidence of inappropriate ICD shocks (1.0%/year) (p<0.05 by log-rank test). The patients with lower absolute value of LASRa and no history of AF (Group 2 in Figure 5), comprising 31% of the cohort, had as high an incidence of inappropriate shocks (8.3%/year) as those with AF history (9.7%/year; Group 3 and 4 in Figure 5; p>0.05 by log-rank test).
Figure 4.
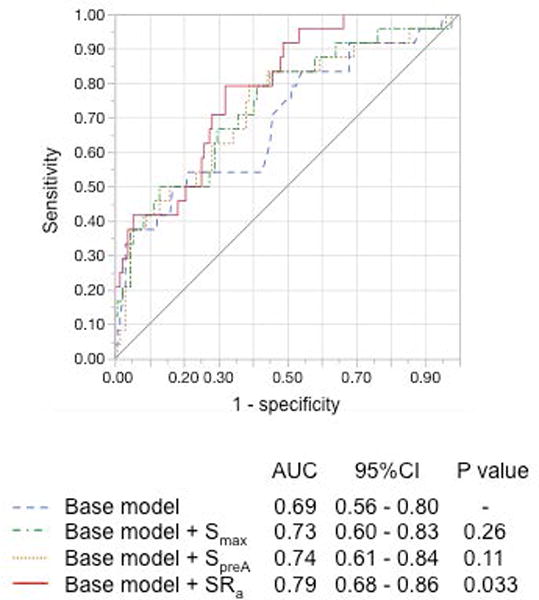
Receiver-operating characteristic curves of left atrial parameters to predict inappropriate ICD shocks over the base model including history of atrial fibrillation, age, and QRS duration. The area under the curve (AUC) of absolute value of strain rate in LA contraction (SRa) added to the base model was significantly higher than the base model alone.
Figure 5.
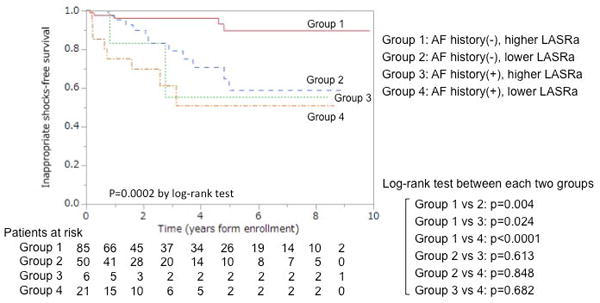
Kaplan-Meier curves showing time to a first occurrence of inappropriate ICD shock by combination of left atrial (LA) strain rate in LA contraction (SRa) and history of atrial fibrillation (AF) prior to ICD implantation. Group 1 included patients with higher absolute value of SRa and no history of AF, Group 2 with lower absolute value of SRa and no history of AF, Group 3 with higher absolute value of SRa and history of AF, and Group 4 with lower absolute value of SRa and history of AF. The SRa cutoff was ≦1.0 /s for the lower group.
Discussion
Summary of main findings
This is the first report to demonstrate an association between LA function and inappropriate shocks in primary prevention ICD recipients. The main findings are as follows: 1) the patient demographics that associated with inappropriate shocks included a history of AF prior to ICD implantation, age younger than 70 years, and QRS duration narrower than 120ms; 2) impaired LA function assessed by tissue-tracking CMR was an independent predictor of inappropriate shocks; and 3) strain rate in LA contraction (SRa) improved the predictive value of inappropriate shocks beyond the patient demographics.
Patient demographics
Our results are consistent with previous reports demonstrating AF as the most common risk factor for inappropriate shocks.3 In this study, the incidence of inappropriate shocks due to AF/AFL was 73%; however, the sensitivity of AF history prior to ICD implantation to predict inappropriate shocks was only 39%. In contrast, the specificity of AF history was relatively high at 87% but no prior history of AF would potentially miss the 61% incidence of inappropriate shocks. It is because AF history underdetects asymptomatic AF5 and new-onset AF. These results confirm the need for structural and functional precursors to AF that reflect the adverse atrial remodeling process.
Our results are also consistent with previous studies which identified a younger age as a independent predictor for inappropriate shocks.15 The potential mechanism is a higher ventricular rate due to faster atrioventricular conduction associated with younger age, despite a lower incidence of AF, compared with an older age.16 Our results also showed that the QRS duration narrower than 120 ms was associated with inappropriate shocks. This findings likely reflects that fact that the QRS duration wider than 120 ms indicates an underlying conduction system disease, which could be associated with reduction of the ventricular response rates to below the rate cutoffs.17 Thus, age less than 70 years and QRS duration less than 120ms could impact on the higher ventricular response during AF, which related to inappropriate shocks.
LA volume and function
Our results show that independent predictors of inappropriate shocks are not LA volumetric parameters, but LA functional parameters such as Smax, SpreA, and SRa, which reflect the reservoir (collection of pulmonary venous return during ventricular systole), the conduit (passage of blood to the LV during early diastole), and the booster pump function (augmentation of LV filling during late diastole), respectively. In particular, SRa improved the predict value of inappropriate ICD shocks, and the patients with lower absolute value of LA SRa (≦1.0 /s) had high incidence of inappropriate shocks with or without AF history. This finding highlights the critical importance of impaired LA function as a surrogate for LA fibrosis that harbors AF.18 This concept is supported by several lines of evidence. For example, Smax is an independent predictor of the maintenance of sinus rhythm after successful cardioversion in patients with recent-onset AF.19 In addition, LA booster pump function is an independent predictor of new-onset AF in a prospective cohort without a prior history of AF.7 SRa is also reduced in recent-onset AF despite normal LA size.20 Given the significant clinical impact of inappropriate shocks on medical and psychological prognosis of the patients, it is critically important to identify patients with a high risk of inappropriate shocks regardless of AF history.
Clinical implications
We found that LA functional parameters assessed by tissue-tracking CMR are independent predictors of inappropriate ICD shocks beyond demographical predictors. For candidates deemed at high risk for an inappropriate ICD shock, cardiac electrophysiologists can recommend a dual-chamber ICD21,22 with therapy reduction programing including combinations of higher detection rates and longer detection intervals, which have been shown to reduce inappropriate ICD shocks.23,24 Our findings expand upon the growing evidence supporting the utility of CMR as a valuable risk stratifier of outcomes in ICD recipients.11,25 Our results may support a routine use of speckle-tracking echocardiography to assess LA function prior to ICD implantation. The portability of echocardiography is an advantage over CMR that can be incorporated into a routine clinical workflow. However, a potential disadvantage of echocardiogram over CMR is longer time for off-line speckle-tracking analysis for strain and strain rate measurements due to a difference in baseline signal-to-noise ratio of the images and occasional inability to view the entire LA epicardium in both two-chamber and four-chamber views. To expand the routine clinical use of LA functional assessment prior to ICD implantation, further studies will be required demonstrating the improved clinical outcomes and cost-effectiveness.
Limitations
First, 162 patients were included in the study out of 1,189 patients initially enrolled, with a small number of inappropriate ICD shocks (n=26). Therefore, there is a non-negligible chance of selection bias which could have affected the results. Second, we used two different scanners, Siemens Avanto and GE Signa, for assessment of LA structure and function. However, MTT is cross-scanner, or scanner-independent, software, where strain and strain rate measurements are only influenced by temporal and spatial resolution of CMR images. Since we used the same pre-determined protocol in both the scanners, the difference in scanner should not have affected our strain and strain rate measurements. Third, the predictors of inappropriate ICD shocks are a function of the cohort characteristics. However, our cohort represents a clinically realistic sample of primary prevention ICD candidates, where AF was the most common cause of inappropriate shocks.3 Fourth, we do not have the detailed information of AF occurrence and the influence of additional therapies for inappropriate shocks for two reasons: 1) the endpoint of this study was the first occurrence of inappropriate shock; and 2) 57% of patients underwent single-chamber ICD implantation with which it was hard to detect AF during the follow-up. Although the sample size is relatively small, antiarrhythmic drugs may decrease inappropriate shocks in patients with a history of AF. Fifth, the patients without AF history prior to ICD implantation may have included those with clinically unrecognized AF. It is possible that more rigorous and continuous ECG monitoring may uncover clinically unrecognized AF and improve the predictive value of a history of AF. However, assessment of LA function is still advantageous in that it does not require lengthy monitoring periods and can provide clinically useful information about structural remodeling precursors to AF.
Conclusion
Impaired LA function assessed by tissue-tracking CMR is an independent predictor of inappropriate shocks in primary prevention ICD candidates. LA booster pump function assessed by SRa particularly improves the predictive value for inappropriate shocks beyond demographic predictors such as a history of AF and a younger age.
Supplementary Material
Acknowledgments
We thank the following for their invaluable contributions: Johns Hopkins research coordinators Jeannette Walker, BSN, Barbara Butcher, CCRN, and Sanaz Norgard, BA; and laboratory technologist Deborah DiSilvestre.
This work was supported by the National Institutes of Health [R01-HL103812 to K.C.W.]; the Japanese Heart Rhythm Society Medtronic fellowship 2015–2016 (to S.T.); the W.W. Smith Charitable Trust (to H.A.), the Magic That Matters Fund for Cardiovascular Research (to H.A.), the Zegar Family Foundation (to H.A.), and the Johns Hopkins University Institute of Clinical and Translational Research (to H.A.).
Footnotes
Disclosures: None
References
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 3.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turakhia MP, Zweibel S, Swain AL, Mollenkopf SA, Reynolds MR. Healthcare Utilization and Expenditures Associated With Appropriate and Inappropriate Implantable Defibrillator Shocks. Circ Cardiovasc Qual Outcomes. 2017;10:e002210. doi: 10.1161/CIRCOUTCOMES.115.002210. [DOI] [PubMed] [Google Scholar]
- 5.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, ASSERT Investigators Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 6.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: A prospective study. Eur J Echocardiogr. 2009;10:282–286. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 7.Hirose T, Kawasaki M, Tanaka R, Ono K, Watanabe T, Iwama M, Noda T, Watanabe S, Takemura G, Minatoguchi S. Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: Results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging. 2012;13:243–250. doi: 10.1093/ejechocard/jer251. [DOI] [PubMed] [Google Scholar]
- 8.Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, Almeida AL, Choi EY, Wu C, Alonso A, Heckbert SR, Bluemke DA, Lima JA. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis) Circ Cardiovasc Imaging. 2016;8 doi: 10.1161/CIRCIMAGING.115.004299. pii: e004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah ZA, Ellenbogen KA, Guallar E, Tomaselli GF. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:1–7. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Hear Rhythm. 2008;5:934–555. [Google Scholar]
- 11.Wu KC, Gerstenblith G, Guallar E, Marine JE, Dalal D, Cheng A, Marbán E, Lima JA, Tomaselli GF, Weiss RG. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5:178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai M, Ambale Venkatesh B, Samiei S, Donekal S, Habibi M, Armstrong AC, Heckbert SR, Wu CO, Bluemke DA, Lima JA. Multi-ethnic study of atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology. 2014;273:703–713. doi: 10.1148/radiol.14131971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JA, Venkatesh BA. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson. 2015;17:52. doi: 10.1186/s12968-015-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue YY, Alissa A, Khurram IM, Fukumoto K, Habibi M, Venkatesh BA, Zimmerman SL, Nazarian S, Berger RD, Calkins H, Lima JA, Ashikaga H. Quantitative Tissue-Tracking Cardiac Magnetic Resonance (CMR) of Left Atrial Deformation and the Risk of Stroke in Patients With Atrial Fibrillation. J Am Heart Assoc. 2015;4:e001844. doi: 10.1161/JAHA.115.001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biton Y, Huang DT, Goldenberg I, Rosero S, Moss AJ, Kutyifa V, McNitt S, Strasberg B, Zareba W, Barsheshet A. Relationship between Age and Inappropriate Implantable Cardioverter Defibrillator Therapy in MADIT-RIT (Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy) Heart Rhythm. 2016;13:888–893. doi: 10.1016/j.hrthm.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Taneja T, Mahnert BW, Passman R, Goldberger J, Kadish A. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol. 2001;24:16–21. doi: 10.1046/j.1460-9592.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Göteborg, Sweden. Eur Heart J. 2005;26:2300–2306. doi: 10.1093/eurheartj/ehi580. [DOI] [PubMed] [Google Scholar]
- 18.Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, Calkins H, Nazarian S. Association of Left Atrial Function and Left Atrial Enhancement in Patients With Atrial Fibrillation: Cardiac Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8:e002769. doi: 10.1161/CIRCIMAGING.114.002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, D’Onofrio A, Severino S, Calabró P, Pacileo G, Mininni N, Calabró R. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–395. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 20.Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M, Watanabe T, Noda T, Watanabe S, Takemura G, Minatoguchi S. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: Velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. 2012;13:227–234. doi: 10.1093/ejechocard/jer281. [DOI] [PubMed] [Google Scholar]
- 21.Friedman PA, McClelland RL, Bamlet WR, Acosta H, Kessler D, Munger TM, Kavesh NG, Wood M, Daoud E, Massumi A, Schuger C, Shorofsky S, Wilkoff B, Glikson M. Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113:2871–2879. doi: 10.1161/CIRCULATIONAHA.105.594531. [DOI] [PubMed] [Google Scholar]
- 22.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, 3rd, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W, MADIT-RIT Trial Investigators Reduction in Inappropriate Therapy and Mortality through ICD Programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 24.Saeed M, Hanna I, Robotis D, Styperek R, Polosajian L, Khan A, Alonso J, Nabutovsky Y, Neason C. Programming implantable cardioverter-defibrillators in patients with primary prevention indication to prolong time to first shock: Results from the PROVIDE study. J Cardiovasc Electrophysiol. 2014;25:52–59. doi: 10.1111/jce.12273. [DOI] [PubMed] [Google Scholar]
- 25.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019–1027. doi: 10.1093/eurjhf/hft053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


