Abstract
Prostate cancer is the second leading cause of cancer related death in American men. Androgen deprivation therapy (ADT) is used to treat patients with aggressive prostate cancers. After androgen deprivation therapy, prostate cancers slowly progress to an androgen-independent status. Taxanes (e.g., docetaxel) are used as standard treatments for androgen-independent prostate cancers. However, these chemotherapeutic agents will eventually become ineffective due to the development of drug resistance. A microRNA (miRNA) is a small noncoding RNA molecule, which can regulate gene expression at the post-transcription level. miRNAs elicit their effects by binding to the 3′-untranslated region (3′-UTR) of their target mRNAs, leading to the inhibition of translation or the degradation of the mRNAs. miRNAs have received increasing attention as targets for cancer therapy, as they can target multiple signaling pathways related to tumor progression, metastasis, invasion, and chemoresistance. Emerging evidence suggests that aberrant expression of miRNAs can lead to the development of resistant prostate cancers. Here, we discuss the roles of miRNAs in the development of resistant prostate cancers and their involvement in various drug resistant mechanisms including androgen signaling, apoptosis avoidance, multiple drug resistance (MDR) transporters, epithelialmesenchymal transition (EMT), and cancer stem cells (CSCs). In addition, we also discuss strategies for treating resistant prostate cancers by targeting specific miRNAs. Different delivery strategies are also discussed with focus on those that have been successfully used in human clinical trials.
Keywords: miRNA, prostate cancers, chemoresistance, cancer stem cells, epithelial-mesenchymal transition (EMT), androgen resistance, apoptosis, MDR transporters, delivery systems
Graphical Abstract
1. INTRODUCTION
Prostate cancer is the second leading cause of cancer related death in men in the United States. It is estimated that 238,590 new cases of prostate cancer were diagnosed in 2013 and that approximately 29,720 men died from this disease in 2013.1,2 Although a localized prostate cancer can be effectively treated with less difficulty, the treatment of aggressive and metastatic forms of prostate cancer is a significant challenge. Currently, androgen deprivation therapy (ADT) is used to treat patients with aggressive prostate cancers. Despite temporary response, most patients relapse and eventually progress to androgen-independent prostate cancers (AIPCs).3,4 AIPCs are developed because of the perturbation of androgen receptor (AR) signaling. Taxanes (e.g., docetaxel) are used as standard treatments for AIPCs.5 However, only half of the patients respond to docetaxel therapy. Even those who initially respond to docetaxel treatment will eventually become resistant.6–9
MicroRNAs (miRNAs) were first discovered in 1993 as endogenous small noncoding RNA molecules in Caenorhabditis elegans.10 miRNAs can regulate gene expression at the post-transcription level. Mature miRNAs of 18–22 nucleotides in length recognize and bind to the 3′-untranslated region (3′-UTR) of target mRNAs, leading to the degradation of target mRNAs or the inhibition of translation. miRNAs are being implicated in a range of physiological processes (e.g., development, cell differentiation, cell cycle control, and metabolism) as well as the pathogenesis of several human diseases, including prostate cancers.11,12 Deregulated miRNAs are involved in initiation, progression, and metastasis of prostate cancers. These miRNAs can be classified as oncogenic miRNAs, which can promote cancer progression, and tumor suppressor miRNAs, which can inhibit the progression of prostate cancers. Because of the significant role of miRNAs in prostate cancers, they have been extensively investigated as therapeutic targets for cancer treatment as well as biomarkers for diagnosis. The unique advantage of using miRNAs as targets for cancer therapy lies in the fact that a single miRNA can regulate multiple target genes in the same signaling pathway or even multiple signaling pathways and therefore can be more effective in treating cancers that are inherently heterogeneous and are resulted from the deregulation of multiple genes.
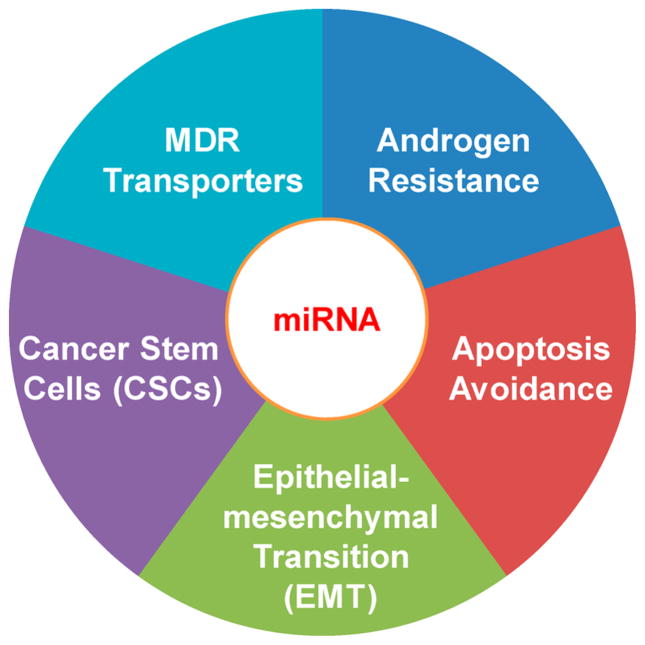
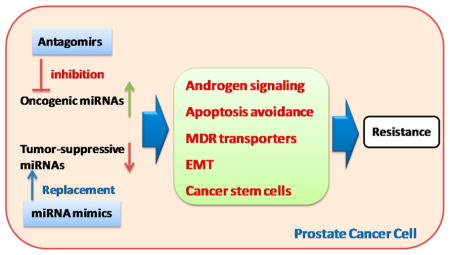
An increasing number of miRNAs has been discovered in prostate cancer cell lines as well as human tissue samples, indicating the significant functional roles of miRNAs in prostate cancers. Emerging evidence suggests that miRNAs are involved in the development of resistant prostate cancers through the regulation of androgen signaling pathway, apoptosis, drug transporters, epithelialmesenchymal transition (EMT), and cancer stem cells (CSCs) (Figure 1). Here, we discuss the regulatory roles of miRNAs in the above cellular processes and their involvement in the development of resistant prostate cancers. The use of these miRNAs as potential therapeutic targets for treating resistant prostate cancers and the design of appropriate therapeutic approaches are also discussed. Different delivery strategies are also discussed with focus on those that have been successfully used in human clinical trials.
Figure 1.
miRNAs regulate prostate cancer drug-resistance through different mechanisms.
2. ROLE OF MIRNAS IN DIFFERENT DRUG RESISTANCE MECHANISMS
2.1. Androgen Signaling
Early stage localized prostate cancers need androgen for proliferation. Therefore, androgen ablation is a standard treatment for recurred prostate cancers after radiation therapy. However, most prostate cancers eventually obtain the capacities to survive without androgens and develop resistance to hormone therapy. At this stage, prostate cancers are defined as AIPCs or hormone refractory prostate cancers (HRPCs). AR, which is a nuclear hormone receptor, plays a significant role in the development of AIPCs. The role of AR signaling in the progression of prostate cancers to androgen independence has been previously discussed.13 Many studies have investigated the interaction between AR signaling and miRNAs as well as the involvement of miRNAs in the progression of AIPCs. Evidence indicates that miRNAs are critical modulators of androgen signaling (Table 1).
Table 1.
Deregulated miRNAs in AIPCs
| miRNA | targets | refs |
|---|---|---|
| Up-Regulated | ||
| miR-125b | P53, Puma, BAK1 | 14–16 |
| miR-221/-222 | P27/Kip1, HECTD2, RAB1A | 17–19 |
| miR-32 | BTG2 | 22 |
| miR-21 | PDCD4, maspin | 20,22 |
| miR-148a | PIK3IP1 | 22 |
| miR-616 | TFPI-2 | 23 |
| miR-375 378 141 | 24 | |
| Down-Regulated | ||
| miR-146a | ROCK1, EGFR, MMP2 | 22,23 |
| let-7c | AR | 24,25 |
| miR-124 | AR, p53 | 29 |
| miR-34a,c | AR, PSA, Notch-1 | 27,28 |
| miR-148a | MSK1 | 32 |
| miR-31 | AR, E2F1, E2F2, EXO1, FOXM1, MCM2 | 33 |
| miR-200b-3p | 34 | |
| miR-185 | AR, CDC6 | 35 |
| miR-205 | AR, PSA | 36 |
2.1.1. Up-Regulation of miRNAs in AIPCs
miR-125b is overexpressed in AIPCs. The overexpression of miR-125b confers the resistance of LNCaP prostate cancer cells to androgen withdrawal. Prostate cancer cells can be sensitized to chemotherapy through the inhibition of miR-125b. The target genes regulated by miR-125b include p53, Puma, and Bak1.14–16 Genome-wide expression profile studies showed that miR-221/-222 were also up-regulated in AIPCs as well as bone metastatic tumor specimens.17 The analysis of human tumor specimens indicated that 90% of the AIPCs had up-regulated miR-221/-222 expression.18 The overexpression of miR-221/-222 in AIPCs (e.g., LNCaP and LAPC-4) could promote androgen-independent cell growth.17 miR-221 also promotes androgen resistance through the down-regulation of HECTD2 and Ras-related protein Rab-1A (RAB1A).19 miR-21 is also overexpressed in AIPCs.20–22 A microarray analysis showed that miR-21 was an AR responsive miRNA. AR regulates the transcription of miR-21 by binding to its promoter. Overexpression of miR-21 in AIPCs could make them resistant to androgen ablation. In addition, the reduction of miR-21 could inhibit androgen-induced prostate cancer growth.20,21 miR-616 induces androgen-independent growth of prostate cancers by suppressing the expression of tissue factor pathway inhibitor (TFPI-2).23 Circulating miRNAs are also associated with the progression of AIPCs. A recent study showed that serum samples from a patient with AIPCs had high levels of miR-375, miR-378*, and miR-141, indicating their roles in the development of AIPCs. These signature circulating miRNAs have the potential to be used as biomarkers for prostate cancer diagnosis.24
2.1.2. Down-Regulation of miRNAs in AIPCs
Several tumor suppressive miRNAs are down-regulated in AIPCs, including miR-146a,25,26 let-7C,27,28 miR-124,29 miR-34a, miR-34c,30,31 miR-148a,32 miR-31,33 miR-200b-3p,34 miR-185,35 and miR-205.36 Down-regulation of these tumor suppressive miRNAs in prostate cancer may lead to cancer progression, increased aggressiveness, and resistance to androgen deprivation therapy. For example, down-regulation of miR-205 is also correlated with the poor therapeutic outcome of prostate cancer patients.36 Restoration of these miRNAs in AIPCs can inhibit cell growth, reduce cell invasion, and prevent metastasis. AR is negatively regulated by several miRNAs including let-7C, miR-124, miR-34a, miR-34c, miR-31, miR-183, and miR-205. Down-regulation of these miRNAs results in the overexpression of AR, which is an important risk factor for androgen independence.13 These miRNAs also regulate other targets in AIPCs. The targets of miR-146a include Rho-associated, coiled-coil containing protein kinase 1 (ROCK1), epidermal growth factor receptor (EGFR), and matrix metalloproteinase-2 (MMP2). Down-regulation of miR-146a is critical for the overexpression of EGFR in AIPCs.25,26 miR-124 can increase p53 levels.29 miR-34a can inhibit prostate-specific antigen (PSA) and Notch-1, thus preventing cell proliferation and decreasing the self-renewal capacity of prostate cancers.30,31 miR-148a can inhibit mitogen and stress-activated protein kinase (MSK1).32 miR-31 can inhibit cell-cycle regulators such as E2F1, E2F2, EXO1, FOXM1, and MCM2.33 These miRNAs (e.g., miR-124 and miR-34a) are down-regulated due to the hypermethylation of their promoters.29,30 In addition, let-7C is negatively regulated by Lin28.27,28 miR-200b-3p expression level is correlated with that of p73 protein, which is related to p53 protein and is considered as a tumor suppressor. The interaction between miR-200b-3p and p73 can also promote the proliferation of AIPCs.34
2.2. Apoptosis Avoidance
Apoptosis is a process of programed cell death with defined unique morphology and biochemical changes of cells in response to apoptotic signals including death ligands, DNA damage, irradiation, and chemotherapy drugs. The ability to avoid apoptosis is developed during the tumorigenesis and is a hallmark of cancers.37 There are two major apoptotic pathways: extrinsic pathway and intrinsic pathways.38,39 These two pathways have considerable cross-talk and may proceed concurrently. The apoptosis defect and dysregulation of apoptotic mediators is an important mechanism leading to the chemoresistance of prostate cancers. Recent evidence has indicated the roles of miRNAs in the regulation of apoptosis and their association with the development of resistant prostate cancers (Table 2 and Figure 2).
Table 2.
miRNAs Involved in Apoptosis Avoidance
| miRNA | targets | refs |
|---|---|---|
| Up-Regulated | ||
| miR-21 | PDCD4, TPM1, MARCKS, PTEN | 53–57 |
| miR-32 | BCL2L11 (Bim) | 46 |
| miR-106b | E2F1, p21/WAF1 | 46 |
| miR-125b | P14(ARF), mdm2, p53, BAK1, Puma | 14–16,47 |
| miR-19b,23b,26a,92a | PTEN, PI3K/Akt, Cyclin D1 | 52 |
| miR-20a | E2F1 | 51 |
| Down-Regulated | ||
| miR-15a/16-1 | BCL2, CCND1, WNT3A | 43 |
| miR-31 | E2F6 | 44 |
| miR-205 | BCL2 | 42,44 |
| miR-34a | BCL2, SIRT1 | 40 |
| miR-34c | BCL2, E2F3 | 41 |
| miR-574-3p | BCl-XL | 45 |
Figure 2.
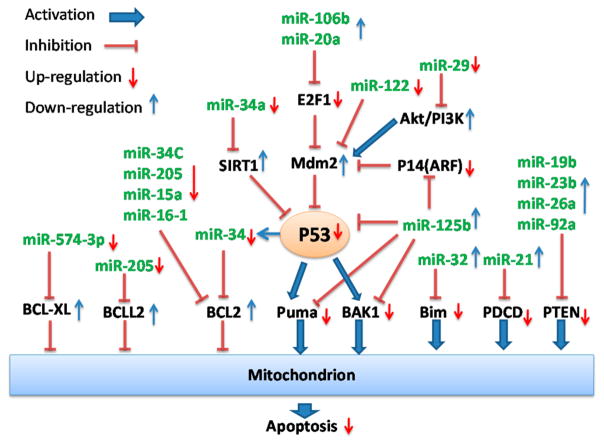
Aberrant expression of miRNAs targeting apoptotic pathways in prostate cancer leads to avoidance of apoptosis.
Bcl-2 family proteins are important regulators of apoptosis. Overexpression of antiapoptotic proteins (e.g., Bcl-2 and Bcl-XL) or underexpression of pro-apoptotic proteins [e.g., BAX, Puma (p53 up-regulated modulator of apoptosis), and Bim (Bcl-2-interacting mediator of cell death)] has contributed to the resistance of prostate cancers to chemotherapy. Recent studies have demonstrated the role of miRNAs in the regulation of Bcl-2 family proteins. These studies showed that Bcl-2 was regulated by miR-34a,40 miR-34c,41 miR-205,42 and miR-15a/miR-16-1.43 Bcl2L2 was regulated by miR-205;44 and Bcl-XL was regulated by miR-574-3p.45 Underexpression of these miRNAs results in the overexpression of pro-survival Bcl-2 family proteins. In contrast, miR-32 and 125b regulate pro-apoptotic Bcl-2 family proteins such as Bcl2L11/Bim, Puma/BBC3, and Bak1.15,16,46 Overexpression of miR-32 or 125b causes down-regulation of above pro-apoptotic proteins. Either overexpression of pro-apoptotic proteins or down-regulation of antiapoptotic proteins can promote cell apoptosis.
P53 is a tumor suppressor protein and a critical mediator of cell cycle and apoptosis in response to different stress signals, including hypoxia, DNA damage, and free radical formation. It initiates apoptosis by activating pro-apoptotic proteins and inhibiting anti-apoptotic proteins. Several miRNAs are involved in the regulation of p53. The up-regulation of miR-125b can inhibit p53 through the up-regulation of mouse double minute 2 homologue (MDM2), which is a negative regulator of p53.16,47 miR-29 can up-regulate p53 through the PI3K-AKT-MDM2 pathway. miR-122 activates p53 through the inhibition of cylclin G1/pp2A-MDM2 pathway.48 At the same time, p53 also regulates certain miRNAs (e.g., miR-145, miR-34, miR-192, and miR-215) to affect cell survival and proliferation.48 For example, the expression of miR-34a is transcriptionally regulated p53. miR-34a is regulating genes controlling cell cycle and apoptosis. The overexpression of miR-34a can inhibit cell growth, induce apoptosis, and sensitize resistant cells to camptothecin, indicating miR-34a as a potential target for p53-defective prostate cancer treatment.49 Similar studies were reported by Lodygin et al., who showed that miR-34a was a target of p53. In their studies, miR-34a overexpression in prostate cancers induced cell senescence and cell cycle arrest though targeting cyclin-dependent kinase 6 (CDK6).50
miRNAs also regulate apoptosis by targeting E2F transcription factors. E2F6 is a target of miR-31. The over-expression of miR-31 can increase the level of E2F6 and induce apoptosis.44 E2F1 are regulated by miR-106b46 and miR-20a.51 Up-regulation of miR-106b or miR-20a may lead to down-expression of E2F1 transcription factors and thus reduce apoptosis.
Several other pro-apoptotic proteins are also inhibited by miRNAs. Phosphatase and tensin homologue (PTEN) can be inhibited by miR-19b, miR-23b, miR-26a, miR-92a, or miR-21.52,53 Programmed cell death 4 (PDCD4) is a target of miR-21.53–57 miR-21 can inhibit PDCD4 and results in the resistance of PC3 prostate cancer cells to docetaxel.56 Antisense oligonucleotide mediated inhibition of miR-21 in DU145 and PC3 prostate cancer cells has increased their sensitivity to apoptosis.54
2.3. MDR Transporters
Overexpression of multiple drug resistance (MDR) transporters, such as P-glycoprotein (Pgp), breast cancer resistance protein (BCRP), and multiple drug resistance proteins (MRPs), is an important reason for chemoresistance.58–68 These transporters function as drug efflux pumps, which reduce intracellular accumulation of chemotherapeutic agents. The overexpression of MDR transporters correlates negatively with the sensitivity of prostate cancers to chemotherapy drugs.59 For example, Pgp and other MDR transporters were up-regulated in two paclitaxel-resistant cell lines (DU145-TXR and PC3-TXR), leading to the resistance of these cells to paclitaxel and other chemo-therapeutic agents.61 Many studies have demonstrated the regulatory roles of miRNAs on various MDR transporters.
Pgp/MDR1 is one of the most important MDR transporters. MDR1 is responsible for the resistance to various chemotherapy drugs including taxol, doxorubicin, etoposide, and vinblastine. The regulation of MDR1 by miRNAs has been demonstrated in multiple studies. miR-9 is involved in the Pgp expression and drug resistance. Inhibition of miR-9 with anti-miR-9 could sensitize brain cancer cells to Temozolomide.69 miR-381 and miR-495 are also down-regulated in resistant cancer cells with up-regulated MDR1.70 Down-regulation of let-7 family is associated with the resistance of cancer cells to taxol. Let-7 enhances cell resistance through the up-regulation of its target IGF2 mRNA-binding protein 1 (IMP-1), which, in turn, can stabilize MDR1.71 miR-122 can sensitize hepatocellular carcinoma to chemotherapy by the down-regulation of MDR1, MRP, and Bcl-w.72
The role of miR-27a and miR-451 on the modulation of Pgp has been reported in several papers with conflicting results.73–77 Some studies indicated that these two miRNAs were down-regulated in resistant cancer cells and were negative regulators of Pgp.73–75 Kocalchuk et al. reported that miR-451 was a negative regulator of Pgp. Doxorubicin resistant cells could be sensitized by the transfection of miR-451.73 Similarly, Feng et al. reported that miR-27a and miR-331-5p were down-regulated in leukemia cell lines. Their down-regulation was associated with Pgp overexpression in these cells.74 Chen et al. also reported that miR-27a was down-regulated in resistant hepatocellular carcinoma cells.75 In contrast, several other studies showed that both miR-27a and miR-451 were overexpressed in resistant cells and caused the overexpression of Pgp.76,77 Zhu et al. showed that both miR-451 and miR-27a were up-regulated in MDR cancer cell lines, such as A2780DX5 and KB-V1. The overexpression of these two miRNAs resulted in a high level of Pgp.76 Li et al. also showed that both miR-27a and Pgp were up-regulated in drug resistant ovarian cancer cells.77 These studies were carried out on different cell lines, indicating the importance of cellular context for miRNA functions. It is worthwhile to further investigate the role of miR-451 and miR-27a in regulating Pgp expression and their effects on drug resistance.
BCRP/ABCG2 is negatively regulated by miR-328 and miR-519.78,79 Overexpression of miR-328 could down-regulate BCRP in breast cancer cells and thus increase their sensitivity to mitoxantrone.78 However, the expression of BCRP is significantly up-regulated when the miRNA binding site at the 3′-UTR of BCRP mRNA is missing.80
MRP1/ABCC1 is up-regulated in VP-16-resistant breast cancer cells (MCF-7/VP).81 MRP-1 is negatively regulated by miR-326. The down-regulation of miR-326 has been observed in resistant cancer cells with MRP-1 overexpression. MRP4/ABCC4 is another drug efflux transporter that is negatively regulated by miR-124a and miR-506.82
2.4. Epithelial-Mesenchymal Transition
Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells assume a mesenchymal phenotype. During the EMT process, cells demonstrate elongated fibroblast morphology. Cell adhesions between epithelial cells are lost. Actin cytoskeleton is reorganized. Epithelial markers (e.g., E-cadherin) are down-regulated, while mesenchymal markers and matrix metalloproteinases (MMPs) are up-regulated. The EMT process is regulated by multiple transcription factors including N-cadherin, Snail, zinc-finger E-box binding homeo-box 1 (ZEB1), ZEB2, and Slug. EMT process is activated in the initiation of cancer metastasis and during the development of chemoresistance.83–88 EMT is associated with the resistance of several different cancers including gemcitabine-resistant pancreatic cancers,89 lapatinib-resistant gastric cancers,90 and taxol-resistant ovarian cancers.91,92 Emerging evidence suggests that miRNAs play critical roles in the regulation of EMT process (Table 3).93–96
Table 3.
miRNAs Regulate EMT in Prostate Cancers
| miRNAa | functions | refs |
|---|---|---|
| miR-143 | enhance E-cadherin; reduce fibronectin |
97 |
| miR-145 | enhance E-cadherin; reduce fibronectin; inhibit HEF1 |
97,98 |
| miR-29b | enhance E-cadherin; reduce N-cadherin, Twist, and Snail |
99 |
| miR-34b | enhance E-cadherin; reduce vimentin, ZO1, N-cadherin, and Snail |
100 |
| miR-200c, 205 | enhance E-cadherin; reduce ZEB1, ZEB2, and vimentin |
101 |
| miR-200 | enhance E-cadherin; reduce N-cadherin, Snail, ZEB1, ZEB2, Slug |
102,103 |
| miR-203 | reduce N-cadherin and vimentin; modulate Wnt signaling pathway |
104 |
| miR-23b/27b | reduce E-cadherin; inhibit Rac1 activity |
105 |
These miRNAs are down-regulated during EMT.
The following miRNAs are down-regulated in prostate cancers and are associated with the EMT process: miR-143,97 miR-145,97,98 miR-29b,99 miR-34b,100 miR-200 family,101–103 miR-205,101 miR-203,104 and miR-23b/27b.105 The down-regulation of these miRNAs results in high levels of mesenchymal markers and low levels of epithelial markers, which can promote EMT process and increase the aggressiveness of prostate cancers. For example, the down-regulation of miR-143 and miR-145 has contributed to the bone metastasis of prostate cancers.97 These miRNAs regulate EMT through the modulation of their targets. miR-145 suppresses EMT through the inhibition of its direct target HEF1.98 miR-29b increases E-cadherin expression, while reducing N-cadherin, Twist, and Snail expression.99 miR-34b inhibits EMT by reducing mesenchymal markers including vimentin, ZO1, N-cadherin, and Snail, while increasing epithelial markers such as E-cadherin.100 The levels of miR-200C and miR-205 are reduced in docetaxel-resistant prostate cancer cells. These cells are undergoing EMT with decreased E-cadherin and increased mesenchymal markers. Restoration of miR-200C and miR-205 through transfection can result in the re-expression of E-cadherin.101 miR-1, miR-200, and Slug form a regulatory loop. Slug is a direct repressor of these two miRNAs.106 The targets for miR-200 family include ZEB1 and ZEB2. Overexpression of miR-200 reverses EMT in PC3 PDGF-D cells through the down-regulation of ZEB1, ZEB2, and Snail.103 The treatment with erismodegib (a smoothened inhibitor) could regulate miR-200 and reverse EMT through the inhibition of transcription factors such as Snail, Slug, and ZEB1.102 miR-203 reduces cancer cell invasion and metastasis through the inhibition of Wnt signaling pathway.104 miR-23b/27b cluster is down-regulated in metastatic, castration-resistant cancer. They could inhibit Ras-related C3 botulinum toxin substrate 1 (Rac1) activity and increase the level of E-cadherin. The ectopic expression of miR-23B/27B could suppress metastasis of castration-resistant prostate cancers.105
2.5. Cancer Stem Cells
Cancer stem cells (CSCs), also known as tumor-initiating cells, are a subgroup of less differentiated cancer cells. Although only a small percentage of CSCs are present in tumors, CSCs are critical for cancer progression, metastasis, and chemoresistance. CSCs can self-renew, differentiate, and drive the expansion of cancers. They can be isolated from bulk tumor cells based on characteristic cell surface markers. CD44 has been used as a marker either alone or in combination with other CSCs markers.107–109 CD44+ prostate cancer cells demonstrated CSC-like properties such as increased clonogenic potential, tumorigenecity, and metastasis. Most current therapeutic agents are targeting at bulk tumor cells but not at CSCs. The use of these agents can effectively kill bulk tumor cells but are ineffective in the elimination of CSCs. The spared CSCs may cause cancer recurrence and drug resistance. The exact mechanism accounting for the development of drug resistant properties in CSCs is still unclear. The differentiation of CSCs under the selection pressure of chemotherapy drugs may generate drug resistant daughter cells and thus acquire drug resistance. Some CSCs have a high level of expression of MDR drug efflux transporters. For example, MRP1/ABCC1 overexpression in prostate cancers has resulted in the resistance of these cells to arsenic treatment.110 In another study, prostate CSCs showed resistance to several chemotherapeutic agents including cisplatin, doxorubicin, paclitaxel, and methotrexate.111
miRNAs have a pivotal role in the regulation of CSCs. For example, miR-34a is down-regulated in CD44+, CD133+, or α2β1+ prostate CSCs sorted from prostate cancer xenograft and prostate tumor patient tissues. Overexpression of miR-34a in prostate cancer cells or isolated CD44+ CSCs cells can significantly reduce tumor growth and metastasis. miR-34a inhibits prostate CSCs through the inhibition of CD44. Down-regulation of miR-34 in CD44− prostate cancer cells enhances the aggressiveness of tumors. miR-34a is a negative regulator of CD44+ prostate CSCs.112,113 Similar studies showed multiple miRNAs were down-regulated in prostate CSCs (e.g., miR-34a, let-7b, miR-106a, and miR-141), while other miRNAs were up-regulated (e.g., miR-301 and miR-452). The overexpression of let-7 could inhibit the prostate cancer growth through their effects on CSCs.114 miR-320 has been identified as a negative regulator of CD44+ prostate CSCs. miR-320 inhibits β-catenin expression and suppresses prostate CSCs through the inhibition of the Wnt/beta-catenin signaling pathway. Down-regulation of miR-320 enhances CSC properties such as tumor sphere formation, chemoresistance, and tumorigenicity. These CSC properties could be inhibited by the overexpression of miR-320.115 Enhancer of zeste homologue 2 (EZH2) is involved in CSC proliferation and differentiation. It is up-regulated in CD44+/CD133+ prostate CSCs and is negatively regulated by miR-101.116
2.6. Interrelationship between EMT, CSCs, Chemo-resistance, and miRNAs
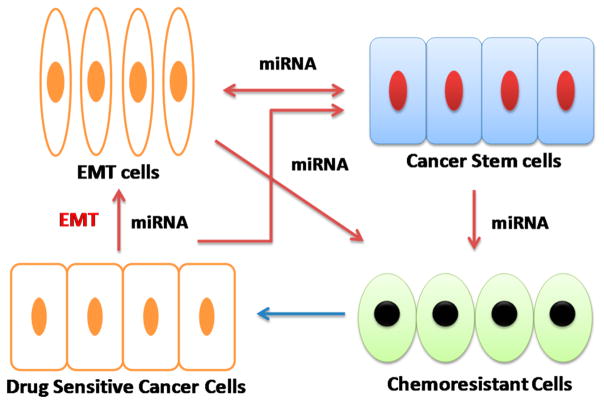
Growing evidence suggests the connections among EMT, CSCs, chemoresistance, and miRNAs.117–119 EMT cells have CSC-like properties such as self-renewal and mammosphere formation, while CSCs have mesenchymal-like features. EMT cells and CSCs have demonstrated chemo-resistance phenotypes, while a higher percentage of EMT cells and CSCs are identified in drug resistant prostate cancers. In addition, EMT and CSC development shares common signaling pathways, including Wnt, Notch, and hedgehog (Hh) pathways.120 EMT process is in parallel with the acquisition of “stemness”. Drug resistant cancers are usually developed through EMT. miRNAs play a pivotal role in the regulation of both EMT process and the CSC proliferation. miRNAs are the common thread connecting EMT, CSCs, and drug resistance (Figure 3). Therefore, targeting specific miRNAs involved in EMT or CSCs will be a promising cancer therapy approach, which could effectively eliminate CSCs and EMT cells and overcome drug resistance.
Figure 3.
Interrelationship between epithelial-mesenchymal transition (EMT), cancer stem cells (CSCs), chemoresistance, and miRNAs. EMT cells, CSCs, and chemoresistant prostate cancer cells are connected and regulated by miRNAs (ref 125).
In a recent study, erismodegib (a smoothened inhibitor) demonstrated the ability to inhibit EMT as well as CSC growth. The treatment with erismodegib may lead to the change of several miRNAs (miR-21, miR-128, and miR-200 family). Erismodegib can increase the levels of PDCD4 and promote apoptosis through the inhibition of miR-21. Erismodegib can also inhibit Bmi-1 through the up-regulation of miR-128. Bmi-1 plays an important role in CSCs. The inhibition of Bmi-1 as well as several other proteins is associated with the inhibition of prostate CSCs. In addition, erismodegib inhibits EMT through the miR-200 family, which can up-regulate E-cadherin and down-regulate N-cadherin, Snail, Slug, and ZEB1.102 In another study, miR-203 was suppressed during the EMT process through the methylation of the promoter. The deregulation of miR-203 not only promoted EMT but also enhanced stemness of the cancer cells. miR-203 inhibited the invasion and metastasis of cancers by inhibiting the Wnt signaling pathway.104 Docetaxel-resistant prostate cancer cells are undergoing EMT and have increased CSC populations. miR-200C and miR-205 are key regulators of EMT and CSCs. These two miRNAs are reduced in the above resistant cells. Reintroduction of these miRNAs can reverse EMT. This study has demonstrated the role of EMT and CSCs in docetaxel resistance and their regulation by miR-200C and miR-205.101
3. THERAPEUTIC STRATEGIES
Many miRNAs are aberrantly expressed in resistant prostate cancer cells and cause the resistance of cancer cells through the modulation of their targets. Because of their important roles in the development of chemoresistance, these miRNAs can be used as potential therapeutic targets for overcoming drug resistance. Here, we discuss different therapeutic agents that can be used to target miRNAs for treating resistant prostate cancers.
3.1. Macromolecule Therapeutics
Two categories of macromolecule therapeutics can be utilized to correct the miRNA expression levels in resistant cancers. Antagomirs (also known as anti-miRs)121 and miRNA sponges (mRNAs with multiple targeting sites for a specific miRNA)122,123 are usually used to reduce oncogenic miRNAs that are up-regulated in resistant cancers. An antagomir is a chemically modified synthetic RNA that is complementary to a specific miRNA target and used to silence endogenous miRNAs. The chemical modifications of antagomir are usually used to increase its resistance to nuclease degradation. Tumor suppressive miRNAs are down-regulated in resistant cancers. The levels of tumor suppressive miRNAs can be restored by using synthetic miRNAs (miRNA mimics) or genetic precursors (vector based miRNAs).124 The restoration of down-regulated tumor suppressive miRNAs is also called miRNA replacement therapy. Although a variety of therapeutic strategies have been developed and demonstrated therapeutic efficacies, anti-miRs and miRNA mimics are the two most advanced approaches to enter clinical trials.
3.2. Small Molecule Drugs
A growing body of studies have discovered the activities of small molecule drugs in the regulation of miRNAs and demonstrated their potential for overcoming drug resistance in prostate cancers. In our study, we have used the combination of paclitaxel and Hh pathway inhibitor cyclopamine to treat paclitaxel resistant prostate cancers. The combination therapy has successfully restored the expression of miR-220C and miR-34a in prostate cancer cells, thus effectively reversing chemoresistance and eliminating CSC side population. This study has demonstrated the feasibility of using small molecule agents to overcome drug resistance through the modulation of miRNA expression.125 Several chemicals derived from natural products have also been utilized to sensitize cancers by targeting miRNAs.126–128 For example, the treatment with 3,3′-diindolylmethane (DIM) and isoflavone increased the expression of miR-200 and let-7, which, in turn, reversed EMT process and increased the sensitivity of cancer cells to gemcitabine.126 A curcumin analogue, EF24, can effectively induce apoptosis in DU145 prostate cancer cells. It works through the inhibition of miR-21, thus enhancing the expression of miR-21 target genes such as PDCD4 and PTEN.129 Similarly, resveratrol can also reduce prostate cancer growth and metastasis by targeting miR-21.21 Genistein, a soy isoflavone, has shown anticancer effects on prostate cancer cells through the up-regulation of miR-34a.130 The advantages of small molecule drugs are their favorable in vivo stabilities and pharmacokinetics (PK) profiles, thus having less challenge in drug delivery.
3.3. Combination Therapy
The correction of aberrant miRNAs in resistant prostate cancers has been demonstrated to be an effective approach for sensitizing resistant prostate cancer to chemotherapy. For example, the transfection of miR-148 or miR-34a can sensitize paclitaxel resistant PC3 cells to paclitaxel treatment.32,40,49 The overexpression of miR-143 has also shown the ability to enhance the sensitivity of prostate cancer cells to docetaxel.131 Therefore, the combination of small molecule chemotherapeutic agents and miRNA therapeutics can be a promising strategy for treating resistant prostate cancers. The rationale of this combination therapy is that the correction of aberrant miRNA levels can sensitize resistant prostate cancer cells to small molecule drugs. A delivery system that could simultaneously deliver miRNA and small molecule drugs will be of great interest. In the past few years, many delivery systems were investigated for the codelivery of small molecule drugs and gene medicines (including miRNA, siRNA, and DNA) for cancer therapy.132–137
4. DELIVERY STRATEGIES FOR MIRNA THERAPEUTICS
Despite promising results in the development of miRNA therapeutics and successes on in vitro cell based studies, very limited progress has been made at in vivo studies or clinical trials. The major obstacle for miRNA-based therapy is the in vivo delivery of miRNA therapeutics.138 Significant efforts have been made for the delivery and targeting of miRNA therapeutics. Various delivery strategies have been successfully developed for miRNA therapeutics. There is still only a limited success with miRNA delivery and targeting and will continue being a great challenge. Although a variety of miRNA therapeutics have been studied, many of them are not suitable for human use. For example, vector-based systems rely on plasmids or viral vectors to deliver and express miRNA in cells. The safety concerns restrict their application in human as a drug candidate even though they can be very useful systems for in vitro cell-based studies. miRNA mimic (a double-strand RNA) is the most promising miRNA therapeutic, which has advanced to clinical trials. For example, MRX34 is the first miRNA mimic that has entered phase I clinical trial as a miRNA replacement therapy to treat liver cancers.139 miRNA inhibitory short single-strand oligonucleotide (e.g., antagomir) is another promising approach, which achieves therapeutic effects through the down-regulation of target miRNA. Therefore, in this section, we will discuss the most promising delivery strategies that have advanced into clinical trials. miRNA therapeutics have large molecular weight and are hydrophilic. They have very poor ability to cross cell membranes, which are composed of lipophilic phospholipids bilayers. In addition, they are unstable when exposed to nucleases in the blood. A successful in vivo delivery approach should be able to (1) protect miRNA therapeutics against serum nucleases, avoid renal clearance, prolong circulation time, and minimize the nonspecific interaction with nontarget cells or organs; (2) enhance the accumulation of miRNA therapeutics in target tissues and facilitate their uptake by target cells and their release inside the cells. Here, we discuss miRNA delivery strategies including (1) chemical modification, (2) lipid-based particles, (3) cyclo-dextrin polymer nanoparticle, and (4) bioconjugates. All of these approaches have been successfully used for in vivo delivery of siRNAs. Because of the similarity between siRNAs and miRNA mimics (both are double-strand RNA), these delivery strategies can be transferred for miRNA delivery.
4.1. Chemical Modification
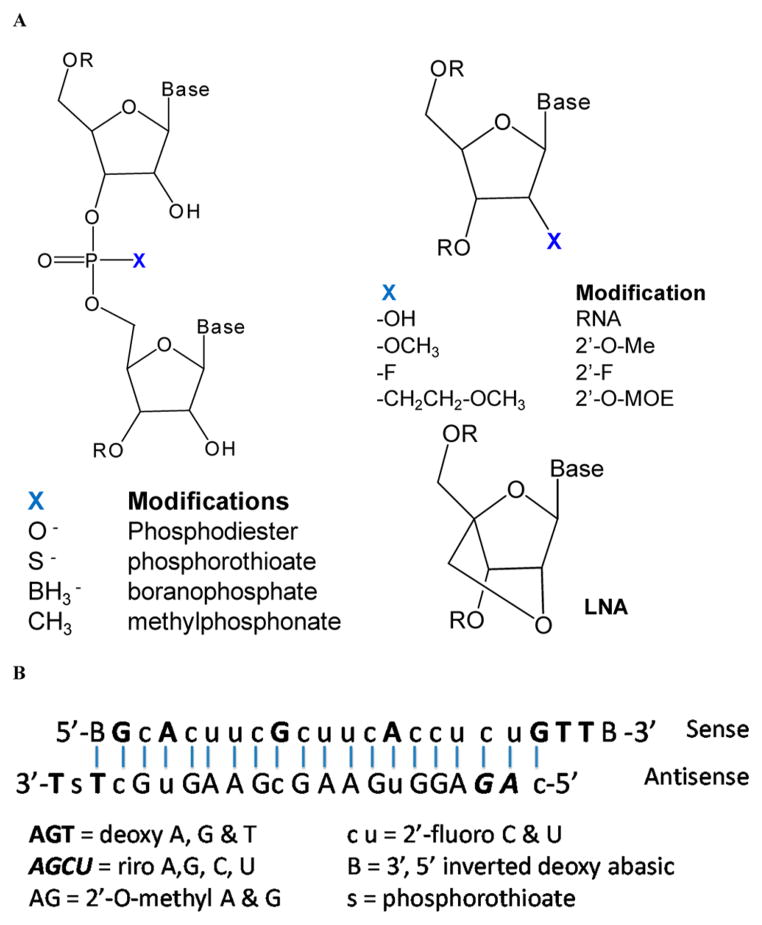
To increase the stability in serum, a variety of methods have been developed for the chemical modification of RNAs (Figure 4). Both phosphodiester and ribose can be potentially modified. The nonbridging oxygen in the phosphodiester can be replaced with sulfur (phosphorothioate), boron (boranophosphate), or methyl (methylphosphonate) groups. The 2′-position of the ribose can also be modified to 2′-fluoro (2′-F), 2′-O-methyl (2′-OMe), 2′-O-(2-methoxyethyl) (MOE), 2′-O-fluoro-β-D-arabi-nonucleotide (FANA), and locked nucleic acid (LNA). Optimized chemical modifications can increase the resistance to serum nuclease, avoid the activation of innate immune system, and reduce off-target effects.140 Miravirsen is a good example of a chemically modified miRNA inhibitor, which was developed by Santaris Pharma A/S. Miravirsen is composed of locked nucleic acid (LNAs) RNA interspaced throughout a phosphorothioate oligonucleotide. It is complementary to the 5′-end of miR-122 and can thus hybridize to miR-122. It inhibits Hepatitis C virus (HCV) by blocking the interaction between HCV RNA and miR-122. The chemical modifications in miravirsen can provide nuclease resistance and enhance its affinity to its target. Miravirsen is the first miRNA targeting drug that enters clinical trials and is currently in phase 2 trials for treating patients with HCV.141,142 The same platform has also been used by miRagen Therapeutic in the development of three drugs including MGN-9103 (targeting miR-208), MGN-1374 (targeting miR-15 and miR-195), and MGN-4893 (targeting miR-451).143 These drugs are being developed in collaboration with Santaris Pharma A/S and are still at the preclinical stage. Chemical modification has also been widely used for siRNAs. For example, siRNA targeting Hepatitis B virus (HBV) has been chemically modified to improve its stability.144,145 Although chemical modification can increase the stability of siRNAs or miRNA mimics, a synthetic carrier is usually needed for their in vivo delivery and targeting.144,145
Figure 4.
(A) RNA molecules can be modified by changing the nonbridging oxygen in the phosphodiester (left) or 2′-position of the ribose (right). (B) The use of a combination of different modifications to improve the in vivo stability of a siRNA (ref 150).
4.2. Lipid-Based Particles
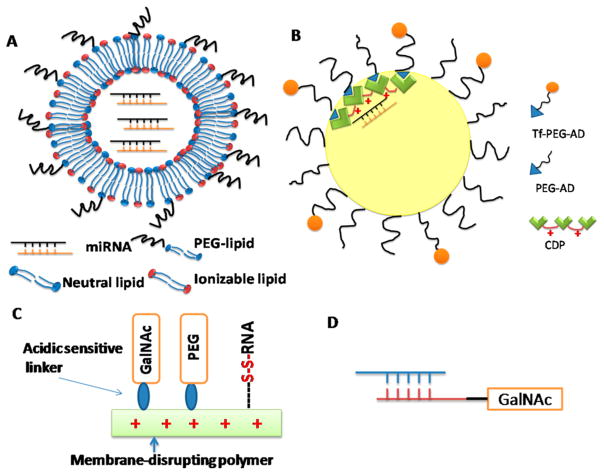
Lipid-based particles (or liposomes) have been used for decades for the delivery of gene medicines including plasmids, antisense oligonucleotides, and siRNAs. A number of lipid-based particle systems have been developed for delivery of siRNAs or miRNA mimics and used in clinical trials (Figure 5A).146,147
Figure 5.
Types of miRNA delivery systems: (A) lipid particles; (B) cyclodextrin polymer nanoparticles (CDP); (C) dynamic polyconju-gate (DPC); and (D) GalNAc-siRNA.
Stable nucleic acid-lipid particle (SNALP) is the most popular siRNA delivery system, which was originally developed by Tekira, and has been used as the delivery system for several products in human clinical trials (e.g., TKM-PLK1 and ALN-TTR02).148,149 A typical SNALP formulation includes ionizable lipids, shielding lipids, cholesterols, and targeting ligands. (1) Ionizable lipids in SNALP formulation are quite similar to those in Smarticles. The ionization status and thus the charge of the lipids are pH dependent. The positive charge at acidic pH can enhance encapsulation of RNA in the liposome, enhance cellular uptake, and facilitate endosomal escape. These lipids are unionized and neutral at physiological pH, thus avoiding nonspecific interaction and preventing rapid elimination of lipid particles. DLinDMA (1,2-dilinoleyloxy-N,N-dimethyl-3-amino-propane) is the first generation ionizable lipid used in SNALP.150 The second generation of ionizable lipids including Dlin-KC2-DMA and Dlin-MC3-DMA were discovered through extensive structure-activity relationship (SAR) studies.151 The pKa of the lipids is critical for siRNA delivery. Optimization of the pKa of the ionizable lipid head groups could enhance their membrane fusion and endosomal escape properties. In addition, the delivery efficiency is also affected by the structure of lipids including amine head groups, linkers, and hydrophobic tails.152 ReLNP (rapid eliminated LNP) developed by Alnylam is the third generation of SNALP, which uses degradable lipids derived from Dlin-MC3-DMA. The use of biodegradable lipids can reduce toxicity and improve biocompatibility.153 (2) Shielding lipids are PEG-lipid conjugates used to make stealth liposomes. Because of the steric effect of PEG coating, the PEGylation of lipid particles can reduce nonspecific interaction with serum components, avoid uptake by the cells of reticuloendothelial system (RES), minimize the kidney elimination, and increase the circulation time. However, shielding lipids can reduce the cellular uptake of particles in target cells and negatively affect the endosomal release of siRNAs, for PEG can sterically block the interaction of liposomes with cell membranes or endosome membranes. This issue can be addressed by using acid-sensitive shielding lipids, which can be detached from lipid particles inside the endosome, which has an acidic pH environment.154,155 (3) Both endogenous and exogenous targeting ligands can be utilized to enhance the delivery of liposomes into target cells or organs. Neutral liposomes often bind to serum proteins and thus are directed by the coated serum proteins to their target cells. For example, lipoprotein Apo-E has been used as a targeting ligand to enhance the uptake of liposomes in hepatocytes.156,157 Exogenous ligands can also be used to enhance the delivery of liposomes into target cells. These targeting ligands include antibodies, peptides, and small molecules.
Smarticles is a proprietary liposomal delivery system from Marina Biotech (Bothell, WA) and has been used for delivery of MRX34, which is the first miRNA mimic that entered clinical trials.146 MRX34 is a double-strand RNA to treat liver cancers. The ionizable lipid is a key component for enhancing the in vivo delivery of miRNA mimics or siRNAs. Because of the presence of ionizable lipid, smarticles is anionic at normal physiological pH but becomes cationic at lower pH. This pH-dependent change of surface charge is critical for minimizing nonspecific interaction during circulation in the blood but has efficient cellular uptake and endosomal escape ability in target cells. Smarticles is also used for delivery of PNR2258, which is an anti-Bcl-2 cancer drug developed by ProRNAi Therapeutics and is currently in phase II clinical trials.147
4.3. Cyclodextrin Polymer Nanoparticle (CDP)
RONDEL (RNAi/oligonucleotide nanoparticle delivery) is the delivery platform for Arrowhead Research’s CALAA-01. It is the first targeted delivery of siRNA entering the human trials. RONDEL delivery system has three major components: (1) a water-soluble positive charged cyclodextrin-containing polymer (CDP); (2) a PEG-adamantane conjugate (PEG-AD); and (3) a transferrin decorated PEG-AD (Tf-PEG-AD) (Figure 5B). When the siRNA solution is mixed with RONDEL before, targeted nanoparticles with a size of 50–70 nm are formed through self-assembly.158 The RONDEL delivery system has several advantages including the simple formulation preparation process, optimal particle size for enhanced permeability, and retention (EPR) effect, and inclusion of tumor targeting ligands (transferrin) for active tumor targeting. However, adverse events were observed among some patients during the phase I studies. Arrowhead Research has decided not to advance the CALAA-01 into phase II trials.159
4.4. Bioconjugates
Dynamic polyconjugate (DPCs) is a multiple component conjugate composed of an amphiphilic endosomolytic polymer, PEG, targeting ligand (GalNac), and siRNA (Figure 5C).160 In the first generation of DPC, polymer poly(butyl amino vinyl either) (PBAVE) is selected as the amphiphilic endosomolytic polymer. Both PEG and targeting ligand (GalNac) are conjugated to the polymer through an acid-sensitive linker. GalNac can enhance the targeting and cellular uptake of DPC by hepatocytes. Membrane distrusting PBAVE is masked by attached shielding PEGs during the circulation. After cellular uptake, PEGs are released and the exposed PBAVE can promote endosomal escape. Double-strand RNAs are attached to PBAVE polymer via a degradable disulfide linker, which can be cleaved in the reducing environment of cytoplasm to release RNAs. The small size of DPC (5–20 nm) is also helpful for tumor targeting. A new generation of DPC polymer has also been synthesized through an improved polymerization process to produce homogeneous polymers. In a recent study, siRNA-cholesterol conjugates were coinjected with DPC instead of using siRNA-DPC conjugates. The coinjection approach has also shown effective gene silencing in the liver.161 The coinjection approach is currently used by Arrowhead Research in their flagship pipeline, ARC-520, which is in phase I trials for the treatment of HBV. For ARC-520, a melittin-like peptide is used, which is similar to PBAVE and also has reversibly masked endosomolytic activities.162
GalNAc-siRNA is a liver-targeted conjugate delivery platform used by Alnylam in several drug candidates (ALN-TTRsc, ALN-AT3, and ALN-PCSsc).163 In this delivery system, three targeting ligands (GalNAc) are attached to the 3′-terminus of the passenger strand of siRNA via a triantennary spacer. GalNAc can bind to the asialoglycoprotein receptor (ASGPR) overexpressed in hepatocytes and facilitate the cellular uptake through endocytosis (Figure 5D).
In summary, the delivery systems discussed above have all demonstrated in vivo delivery efficacy and have been used in human clinical trials. They are diverse in the working mechanism and have different design features including size, chemical structure, and preparation method. “All roads lead to Rome” is a good summary of the current status of RNA therapeutics delivery approaches. At the same time, we also notice that a successful delivery system usually includes multiple functional elements to overcome various barriers to their in vivo delivery and has a good biocompatibility and safety profile for human use.
5. CONCLUSIONS AND PERSPECTIVES
miRNAs play significant roles in the development of resistant prostate cancers by targeting multiple signaling pathways or mechanisms. Deregulated miRNAs are associated with the occurrence of chemoresistant prostate cancers. Therefore, correction of aberrant miRNAs could be a strategy to overcome resistance in prostate cancer therapy. Many miRNAs related to drug resistance have been discovered, and additional ones are expected to be identified in the future. We are expecting to have a better understanding regarding the mechanisms of drug resistance in prostate cancers. The identification of the most critical miRNA(s) that are associated with drug resistance will be the cornerstone for the design of therapeutics. It is also noticed that, although many miRNAs have difierential expression between normal cells and cancer cells, not all these miRNAs are the cause of cancer development and thus cannot be used as therapeutic targets. In addition, most current miRNA targets are discovered in prostate cell lines because they are more accessible than human prostate cancer tissues. These miRNA targets need to be further validated with translational research or clinical studies before they can become clinically useful therapeutic targets. More miRNA targets will be discovered from human prostate cancer tissues, which will be clinically relevant and more valid.
Antagomirs and miRNA mimics are the two most promising miRNA-based therapeutics. miRNA mimics have demonstrated their great potential for treating cancers, especially drug-resistant cancers, by its ability to simultaneously inhibit multiple oncogenes or signaling pathways. Since the development of cancers and their resistance to treatments are usually caused by the aberrant expression of multiple genes and signaling pathways, the use of siRNAs or small molecule inhibitors targeting a single mRNA (or other intracellular target) can merely induce a modest therapeutic response. However, miRNA mimics can restore the level of an underexpressed miRNA, repress multiple oncogenes and pathways regulated by these miRNA, and thus effectively treat resistant cancers. At the same time, we also need to be cautious about the broad effects of miRNA mimics. Since multiple targets or complex signaling networks are regulated by a single miRNA mimic, a nontraditional approach must be used to evaluate their therapeutic effects by monitoring a broad gene expression profile rather than focusing on a single gene or protein. The prediction of side effects might also be a challenge due to the broad inhibition effects of miRNAs.
Off-target effects are side effects caused during the use of RNAi due to the unintended interactions between RNAi molecules and cellular components. Off-target effects can be divided into two categories based on their mechanisms: (1) specific off-target effects (caused by limited degree of complementarity between siRNA and nontargeting mRNAs); and (2) nonspecific off-target effects (toxicities caused by the activation of Toll-like receptor mediated innate immune response or due to the saturation of cellular RNAi machinery).164 As we discussed before, miRNA inhibition and miRNA replacement are two major approaches of miRNA-based therapeutics. Antagomirs can be designed to reduce the aberrantly up-regulated oncogenic miRNAs by being perfectly complementary to the specific miRNA target by mispairing at the cleavage site of Ago2. Antagomir recognizes and irreversibly binds to specific target miRNA. Antagomirs work similar to siRNAs in silencing target mRNAs. Therefore, off-target effects will be an issue for the use of antagomir or other similar miRNA inhibitors because they may bind to nontarget miRNAs or even mRNAs or induce innate immune responses. Approaches for minimizing off-target effects include the optimization of antagomir sequences and chemical modifications of its backbone and structure. MiRNA replacement therapy refers to the use of miRNA mimics to restore the down-regulated tumor suppressor miRNAs in cancer cells. Unlike siRNA, miRNA mimics are usually present in cells and are considered as “endogenous” molecules. A miRNA mimic has the same sequence as an endogenous miRNA and thus will not cause off-target effects. Since the same miRNAs are also present in normal cells, the introduction of miRNA mimics into normal cells will be well-tolerated. However, the use of miRNA mimics may still cause side effects. Since our knowledge about the expression levels of particular miRNAs is still very limited and their intracellular levels vary among different cells and same cell exposed to different environment, the delivery of too much miRNAs may cause side effects while too less will not be able to achieve sufficient therapeutic effects.
Significant progress has been made in the target identification and the design of miRNA based therapeutics because of the availability of bioinformatics tools and high throughput research methods. The identification of miRNA therapeutic targets and design of miRNA therapeutics is no longer a significant bottleneck. However, it is widely accepted that the delivery of miRNA therapeutics is still a challenge because of their poor in vivo stability, large molecular weight, hydrophilicity, and other in vivo delivery obstacles. Delivery systems discussed in this review have all demonstrated in vivo efficacy and have been pursued in human clinical trials. Although they are diverse in design features, all have demonstrated great potentials in addressing the delivery challenge. Liposomal systems are the most advanced formulation with several products in the development pipeline. Bioconjugate is also a promising system, which has precisely controlled structure and function. These delivery platforms will jump-start the clinical development of miRNA therapeutics, and some of them will finally become available as approved medications. Also, more delivery systems will be developed through the efforts from researchers from different scientific disciplines including chemistry, polymer sciences, engineering, pharmaceutical sciences, and biology. However, it is worth mentioning that most of the current miRNA delivery systems in clinical trials are to deliver therapeutic agents to the liver through either using liver targeting ligands or relying on the passive accumulation of delivery systems in the liver. The delivery into the liver is relatively less difficult because liver is a well-perfused tissue and has discontinuous endothelium. However, miRNA delivery to hepatocytes remains a challenge as liposomal and polymeric particualtes carrying miRNA are likely to be taken up by Kuffer cells before reaching the hepatocytes.165 The delivery to prostate cancer or other less accessible tissues will be more difficult. Additional efforts are needed to modify the current systems to make them more efficient in delivering miRNA therapeutics into prostate cancers. In addition to the general properties needed for siRNA delivery, additional features such as prolonged circulation for EPR effects and prostate cancer targeting ligands for active targeting are needed.
In conclusion, recent research and development in the understanding of miRNAs has demonstrated their great therapeutic potential for resistant prostate cancers. Further clinical or translational research will lead to the identification of miRNA targets and design of effective miRNA-based therapeutics. The recent progress achieved by using several different delivery systems is inspiring; however, delivery still remains a great challenge.
Acknowledgments
We thank the following grants for support: Hampton University Faculty Research Award and AACP New Investigator Award (to L.F.); and an Idea Award (W81XWH-10-1-0969) from the Department of Defense Prostate Cancer Research Program (to R.I.M.).
Footnotes
Notes: The authors declare no competing financial interest.
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. American Cancer Society; Atlanta, GA: 2013. [Google Scholar]
- 2.National Cancer Institute. Prostate Cancer. http://www.cancer.gov/cancertopics/types/prostate.
- 3.Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50(1):93–9. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 4.Sharifi N, Dahut WL, Steinberg SM, Figg WD, Tarassoff C, Arlen P, Gulley JL. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96(7):985–9. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 5.Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, McDermott R, Hervonen P, Ginman C, Luukkaa M, Nyandoto P, Hemminki A, Nilsson S, McCaffrey J, Asola R, Turpeenniemi-Hujanen T, Laestadius F, Tasmuth T, Sandberg K, Keane M, Lehtinen I, Luukkaala T, Joensuu H. 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14(2):117–124. doi: 10.1016/S1470-2045(12)70537-5. [DOI] [PubMed] [Google Scholar]
- 6.Theyer G, Schirmbock M, Thalhammer T, Sherwood ER, Baumgartner G, Hamilton G. Role of the MDR-1-encoded multiple drug resistance phenotype in prostate cancer cell lines. J Urol. 1993;150(5 Pt 1):1544–7. doi: 10.1016/s0022-5347(17)35838-x. [DOI] [PubMed] [Google Scholar]
- 7.van Brussel JP, Mickisch GH. Multidrug resistance in prostate cancer. Onkologie. 2003;26(2):175–81. doi: 10.1159/000071510. [DOI] [PubMed] [Google Scholar]
- 8.Hwang C. Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol. 2012;4(6):329–40. doi: 10.1177/1758834012449685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 11.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 12.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Saraon P, Jarvi K, Diamandis EP. Molecular alterations during progression of prostate cancer to androgen independence. Clin Chem. 2011;57(10):1366–75. doi: 10.1373/clinchem.2011.165977. [DOI] [PubMed] [Google Scholar]
- 14.DeVere White RW, Vinall RL, Tepper CG, Shi XB. MicroRNAs and their potential for translation in prostate cancer. Urol Oncol. 2009;27(3):307–11. doi: 10.1016/j.urolonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, de Vere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA. 2007;104(50):19983–8. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi XB, Xue L, Ma AH, Tepper CG, Kung HJ, White RW. miR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate. 2011;71(5):538–49. doi: 10.1002/pros.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun T, Wang Q, Balk S, Brown M, Lee GS, Kantoff P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69(8):3356–63. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun T, Yang M, Chen S, Balk S, Pomerantz M, Hsieh CL, Brown M, Lee GS, Kantoff PW. The altered expression of MiR-221/-222 and MiR-23b/-27b is associated with the development of human castration resistant prostate cancer. Prostate. 2012;72(10):1093–103. doi: 10.1002/pros.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun T, Wang X, He HH, Sweeney CJ, Liu SX, Brown M, Balk S, Lee GS, Kantoff PW. MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene. 2013 doi: 10.1038/onc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69(18):7165–9. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7(12):e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Janne OA, Seppala J, Lahdesmaki H, Tammela TL, Visakorpi T. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene. 2012;31(41):4460–71. doi: 10.1038/onc.2011.624. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Chan YP, Kwan PS, Lee TK, Yan M, Tang KH, Ling MT, Vielkind JR, Guan XY, Chan KW. MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res. 2011;71(2):583–92. doi: 10.1158/0008-5472.CAN-10-2587. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen HC, Xie W, Yang M, Hsieh CL, Drouin S, Lee GS, Kantoff PW. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate. 2013;73(4):346–54. doi: 10.1002/pros.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14(3):417–24. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu B, Wang N, Wang X, Tong N, Shao N, Tao J, Li P, Niu X, Feng N, Zhang L, Hua L, Wang Z, Chen M. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate. 2012;72(11):1171–8. doi: 10.1002/pros.22466. [DOI] [PubMed] [Google Scholar]
- 27.Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX, Chen H, Zhang J, Chen X, Luo J, de Vere White RW, Kung HJ, Evans CP, Gao AC. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS One. 2012;7(3):e32832. doi: 10.1371/journal.pone.0032832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadiminty N, Tummala R, Lou W, Zhu Y, Zhang J, Chen X, de Vere White RW, Kung HJ, Evans CP, Gao AC. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287(2):1527–37. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung HJ, de Vere White RW. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene. 2013;32(35):4130–8. doi: 10.1038/onc.2012.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res. 2012;4(4):432–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Ostling P, Leivonen SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71(5):1956–67. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 32.Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, Ito M. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem. 2010;285(25):19076–84. doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin PC, Chiu YL, Banerjee S, Park K, Mosquera JM, Giannopoulou E, Alves P, Tewari AK, Gerstein MB, Beltran H, Melnick AM, Elemento O, Demichelis F, Rubin MA. Epigenetic repression of miR-31 disrupts androgen receptor homeo-stasis and contributes to prostate cancer progression. Cancer Res. 2013;73(3):1232–44. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He M, Liu Y, Deng X, Qi S, Sun X, Liu G, Zhao M. Down-regulation of miR-200b-3p by low p73 contributes to the androgen-independence of prostate cancer cells. Prostate. 2013;73(10):1048–56. doi: 10.1002/pros.22652. [DOI] [PubMed] [Google Scholar]
- 35.Qu F, Cui X, Hong Y, Wang J, Li Y, Chen L, Liu Y, Gao Y, Xu D, Wang Q. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem. 2013;377(1–2):121–30. doi: 10.1007/s11010-013-1576-z. [DOI] [PubMed] [Google Scholar]
- 36.Hagman Z, Haflidadottir BS, Ceder JA, Larne O, Bjartell A, Lilja H, Edsjo A, Ceder Y. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer. 2013;108(8):1668–76. doi: 10.1038/bjc.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 40.Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70(14):1501–12. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- 41.Hagman Z, Larne O, Edsjo A, Bjartell A, Ehrnstrom RA, Ulmert D, Lilja H, Ceder Y. miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int J Cancer. 2010;127(12):2768–76. doi: 10.1002/ijc.25269. [DOI] [PubMed] [Google Scholar]
- 42.Verdoodt B, Neid M, Vogt M, Kuhn V, Liffers ST, Palisaar RJ, Noldus J, Tannapfel A, Mirmohammadsadegh A. MicroRNA-205, a novel regulator of the anti-apoptotic protein Bcl2, is downregulated in prostate cancer. Int J Oncol. 2013;43(1):307–14. doi: 10.3892/ijo.2013.1915. [DOI] [PubMed] [Google Scholar]
- 43.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, De Maria R. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14(11):1271–7. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 44.Bhatnagar N, Li X, Padi SK, Zhang Q, Tang MS, Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, Saini S, Arora S, Deng G, Shahryari V, Chang I, Tanaka Y, Tabatabai ZL, Enokida H, Seki N, Nakagawa M, Dahiya R. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS One. 2013;8(3):e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68(15):6162–70. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amir S, Ma AH, Shi XB, Xue L, Kung HJ, Devere White RW. Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer. PLoS One. 2013;8(4):e61064. doi: 10.1371/journal.pone.0061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3(1):44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377(1):114–9. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 50.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 51.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282(4):2135–43. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 52.Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS One. 2013;8(9):e75885. doi: 10.1371/journal.pone.0075885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Folini M, Gandellini P, Longoni N, Profumo V, Callari M, Pennati M, Colecchia M, Supino R, Veneroni S, Salvioni R, Valdagni R, Daidone MG, Zaffaroni N. miR-21: an oncomir on strike in prostate cancer. Mol Cancer. 2010;9:12. doi: 10.1186/1476-4598-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383(3):280–5. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 55.Yang CH, Yue J, Fan M, Pfeffer LM. IFN induces miR-21 through a signal transducer and activator of transcription 3-dependent pathway as a suppressive negative feedback on IFN-induced apoptosis. Cancer Res. 2010;70(20):8108–16. doi: 10.1158/0008-5472.CAN-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi GH, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin. 2010;31(7):867–73. doi: 10.1038/aps.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284(39):26533–46. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discovery. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez C, Mendoza P, Contreras HR, Vergara J, McCubrey JA, Huidobro C, Castellon EA. Expression of multidrug resistance proteins in prostate cancer is related with cell sensitivity to chemotherapeutic drugs. Prostate. 2009;69(13):1448–59. doi: 10.1002/pros.20991. [DOI] [PubMed] [Google Scholar]
- 60.Zalcberg J, Hu XF, Slater A, Parisot J, El-Osta S, Kantharidis P, Chou ST, Parkin JD. MRP1 not MDR1 gene expression is the predominant mechanism of acquired multidrug resistance in two prostate carcinoma cell lines. Prostate Cancer Prostatic Dis. 2000;3(2):66–75. doi: 10.1038/sj.pcan.4500394. [DOI] [PubMed] [Google Scholar]
- 61.Takeda M, Mizokami A, Mamiya K, Li YQ, Zhang J, Keller ET, Namiki M. The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate. 2007;67(9):955–67. doi: 10.1002/pros.20581. [DOI] [PubMed] [Google Scholar]
- 62.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 63.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22(47):7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 64.Gardner ER, Ahlers CM, Shukla S, Sissung TM, Ockers SB, Price DK, Hamada A, Robey RW, Steinberg SM, Ambudkar SV, Dahut WL, Figg WD. Association of the ABCG2 C421A polymorphism with prostate cancer risk and survival. BJU Int. 2008;102(11):1694–9. doi: 10.1111/j.1464-410X.2008.07913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding XW, Wu JH, Jiang CP. ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010;86(17–18):631–7. doi: 10.1016/j.lfs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Van Brussel JP, Jan Van Steenbrugge G, Van Krimpen C, Bogdanowicz JF, Van Der Kwast TH, Schroder FH, Mickisch GH. Expression of multidrug resistance related proteins and proliferative activity is increased in advanced clinical prostate cancer. J Urol. 2001;165(1):130–5. doi: 10.1097/00005392-200101000-00032. [DOI] [PubMed] [Google Scholar]
- 67.Munoz M, Henderson M, Haber M, Norris M. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life. 2007;59(12):752–7. doi: 10.1080/15216540701736285. [DOI] [PubMed] [Google Scholar]
- 68.Grzywacz MJ, Yang JM, Hait WN. Effect of the multidrug resistance protein on the transport of the antiandrogen flutamide. Cancer Res. 2003;63(10):2492–8. [PubMed] [Google Scholar]
- 69.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, Ohms SJ, Li Z, Wang Q, Gong G, Hu Y, Mao Z, Shannon MF, Fan JY. Changes in the expression of miR-381 and miR-495 are inversely associated with the expression of the MDR1 gene and development of multi-drug resistance. PLoS One. 2013;8(11):e82062. doi: 10.1371/journal.pone.0082062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyerinas B, Park SM, Murmann AE, Gwin K, Montag AG, Zillhardt M, Hua YJ, Lengyel E, Peter ME. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int J Cancer. 2012;130(8):1787–97. doi: 10.1002/ijc.26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z, Liu J, Cui Y, Bian X, Bie P, Qian C. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011;310(2):160–9. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 73.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152–9. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 74.Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, Chen YQ. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15(10):2164–75. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Z, Ma T, Huang C, Zhang L, Lv X, Xu T, Hu T, Li J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/beta-catenin pathway in hepatocellular carcinoma cells. Cell Signalling. 2013;25(12):2693–701. doi: 10.1016/j.cellsig.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76(5):582–8. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, Wang Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119(1):125–30. doi: 10.1016/j.ygyno.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75(6):1374–9. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory micro-RNAs (hsa-miR-328, -519c and -520(h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81(6):783–92. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28(17):5147–61. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79(6):817–24. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 82.Markova SM, Kroetz DL. ABCC4 is Regulated by microRNA-124a and microRNA-506. Biochem Pharmacol. 2013;87:515–22. doi: 10.1016/j.bcp.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 85.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–8. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, Tanabe KK. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68(7):2391–9. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 87.Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh IT, De K, Sun LZ. Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PLoS One. 2010;5(4):e10365. doi: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Update. 2008;11(4–5):123–51. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69(6):2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2013 doi: 10.1038/onc.2013.285. [DOI] [PubMed] [Google Scholar]
- 91.Du F, Wu X, Liu Y, Wang T, Qi X, Mao Y, Jiang L, Zhu Y, Chen Y, Zhu R, Han X, Jin J, Ma X, Hua D. Acquisition of paclitaxel resistance via PI3Kdependent epithelialmesenchymal transition in A2780 human ovarian cancer cells. Oncol Rep. 2013;30(3):1113–8. doi: 10.3892/or.2013.2567. [DOI] [PubMed] [Google Scholar]
- 92.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31(2):277–83. [PubMed] [Google Scholar]
- 93.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bracken CP, Gregory PA, Khew-Goodall Y, Goodall GJ. The role of microRNAs in metastasis and epithelial-mesenchymal transition. Cell Mol Life Sci. 2009;66(10):1682–99. doi: 10.1007/s00018-009-8750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31(3–4):653–62. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104(1):3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 97.Peng X, Guo W, Liu T, Wang X, Tu X, Xiong D, Chen S, Lai Y, Du H, Chen G, Liu G, Tang Y, Huang S, Zou X. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One. 2011;6(5):e20341. doi: 10.1371/journal.pone.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem. 2013;114(7):1606–15. doi: 10.1002/jcb.24502. [DOI] [PubMed] [Google Scholar]
- 99.Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012;11(5):1166–73. doi: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- 100.Majid S, Dar AA, Saini S, Shahryari V, Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, Deng G, Dahiya R. miRNA-34b inhibits prostate cancer through demethyla-tion, active chromatin modifications, and AKT pathways. Clin Cancer Res. 2013;19(1):73–84. doi: 10.1158/1078-0432.CCR-12-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol. 2012;181(6):2188–201. doi: 10.1016/j.ajpath.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Nanta R, Kumar D, Meeker D, Rodova M, Van Veldhuizen PJ, Shankar S, Srivastava RK. NVP-LDE-225 (Erismodegib) inhibits epithelial-mesenchymal transition and human prostate cancer stem cell growth in NOD/SCID IL2Rgamma null mice by regulating Bmi-1 and microRNA-128. Oncogenesis. 2013;2:e42. doi: 10.1038/oncsis.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27(8):1712–21. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taube JH, Malouf GG, Lu E, Sphyris N, Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, Herschkowitz JI, Rosen JM, Issa JP, Calin GA, Chang JT, Mani SA. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci Rep. 2013;3:2687. doi: 10.1038/srep02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishteiwy RA, Ward TM, Dykxhoorn DM, Burnstein KL. The microRNA -23b/-27b cluster suppresses the metastatic phenotype of castration-resistant prostate cancer cells. PLoS One. 2012;7(12):e52106. doi: 10.1371/journal.pone.0052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene. 2013;32(3):296–306. doi: 10.1038/onc.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 108.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 109.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67(14):6796–805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 110.Tokar EJ, Qu W, Liu J, Liu W, Webber MM, Phang JM, Waalkes MP. Arsenic-specific stem cell selection during malignant transformation. J Natl Cancer Inst. 2010;102(9):638–49. doi: 10.1093/jnci/djq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu T, Xu F, Du X, Lai D, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340(1–2):265–73. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 112.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71(18):5950–4. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res. 2012;72(13):3393–404. doi: 10.1158/0008-5472.CAN-11-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ, Lee KH, Yeh SD, Hong TM, Chen YL. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by down-regulating the Wnt/beta-catenin signaling pathway. Carcinogenesis. 2013;34(3):530–8. doi: 10.1093/carcin/bgs371. [DOI] [PubMed] [Google Scholar]
- 116.Li K, Liu C, Zhou B, Bi L, Huang H, Lin T, Xu K. Role of EZH2 in the growth of prostate cancer stem cells isolated from LNCaP cells. Int J Mol Sci. 2013;14(6):11981–93. doi: 10.3390/ijms140611981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S, Sarkar FH. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Update. 2010;13(4–5):109–18. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Danquah M, Singh S, Behrman SW, Mahato RI. Role of miRNA and cancer stem cells in chemoresistance and pancreatic cancer treatment. Expert Opin Drug Delivery. 2012;9(12):1443–7. doi: 10.1517/17425247.2012.722079. [DOI] [PubMed] [Google Scholar]
- 120.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):235–52. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 121.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 122.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 123.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16(11):2043–50. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Z. The guideline of the design and validation of MiRNA mimics. Methods Mol Biol. 2011;676:211–23. doi: 10.1007/978-1-60761-863-8_15. [DOI] [PubMed] [Google Scholar]
- 125.Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and hedgehog pathway. PLoS One. 2012;7(6):e40021. doi: 10.1371/journal.pone.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, VandenBoom TG, II, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69(16):6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7(3):464–73. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 128.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21(2):140–6. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Yang CH, Yue J, Sims M, Pfeffer LM. The curcumin analog EF24 targets NF-kappaB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One. 2013;8(8):e71130. doi: 10.1371/journal.pone.0071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y, Enokida H, Seki N, Nakagawa M, Dahiya R. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8(8):e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Hua L, Wang X. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350(1–2):207–13. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- 132.Kim C, Shah BP, Subramaniam P, Lee KB. Synergistic induction of apoptosis in brain cancer cells by targeted codelivery of siRNA and anticancer drugs. Mol Pharmaceutics. 2011;8(5):1955–61. doi: 10.1021/mp100460h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhi F, Dong H, Jia X, Guo W, Lu H, Yang Y, Ju H, Zhang X, Hu Y. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. PLoS One. 2013;8(3):e60034. doi: 10.1371/journal.pone.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ren Y, Kang CS, Yuan XB, Zhou X, Xu P, Han L, Wang GX, Jia Z, Zhong Y, Yu S, Sheng J, Pu PY. Co-delivery of as-miR-21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J Biomater Sci, Polym Ed. 2010;21(3):303–14. doi: 10.1163/156856209X415828. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–6. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 136.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 2008;3(6):761–76. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20(7):1298–304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31(7):577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- 140.Li F, Mahato RI. RNA interference for improving the outcome of islet transplantation. Adv Drug Delivery Rev. 2011;63(1–2):47–68. doi: 10.1016/j.addr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang S, Liu B, Gu C, Song Y, Qian C, Hu M, Chai L, Wang C. Self-similar evolution in a short fiber amplifier through nonlinear pulse preshaping. Opt Lett. 2013;38(3):296–8. doi: 10.1364/OL.38.000296. [DOI] [PubMed] [Google Scholar]
- 142.Yang TC, Chang CH, Liu YT, Chen YL, Tu PH, Chen HC. Predictors of shunt-dependent chronic hydrocephalus after aneurysmal subarachnoid haemorrhage. Eur Neurol. 2013;69(5):296–303. doi: 10.1159/000346119. [DOI] [PubMed] [Google Scholar]
- 143.He D, Lu W, Chang K, Liu Y, Zhang J, Zeng Z. Vascular endothelial growth factor polymorphisms and risk of Alzheimer’s disease: a meta-analysis. Gene. 2013;518(2):296–302. doi: 10.1016/j.gene.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 144.Liu F, Zhu ZJ, Li P, He YL. Creation of a female rabbit model for intrauterine adhesions using mechanical and infectious injury. J Surg Res. 2013;183(1):296–303. doi: 10.1016/j.jss.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 145.Niu H, Yuan R, Chai Y, Mao L, Liu H, Cao Y. Highly amplified electrochemiluminescence of peroxydisulfate using bienzyme functionalized palladium nanoparticles as labels for ultrasensitive immunoassay. Biosens Bioelectron. 2013;39(1):296–9. doi: 10.1016/j.bios.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 146.Shields N, Dodd KJ, Abblitt C. Do children with Down Syndrome perform sufficient physical activity to maintain good health? A pilot study. Adapt Phys Act Q. 2009;26(4):307–20. doi: 10.1123/apaq.26.4.307. [DOI] [PubMed] [Google Scholar]
- 147.Product Pipeline. http://www.pronai.com/pipeline/index.htm.
- 148.Pipeline Products. http://www.tekmira.com/pipeline/pipeline-products.php.
- 149.Alnylam 5 × 15. http://www.alnylam.com/product-pipeline/alnylam-5x15/
- 150.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–7. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 151.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–6. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 152.Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, Butler D, Eltepu L, Matsuda S, Narayanannair JK, Rajeev KG, Hafez IM, Akinc A, Maier MA, Tracy MA, Cullis PR, Madden TD, Manoharan M, Hope MJ. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem, Int Ed. 2012;51(34):8529–33. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W, Tam YK, Ansell SM, Kumar V, Qin J, Zhang X, Wang Q, Panesar S, Hutabarat R, Carioto M, Hettinger J, Kandasamy P, Butler D, Rajeev KG, Pang B, Charisse K, Fitzgerald K, Mui BL, Du X, Cullis P, Madden TD, Hope MJ, Manoharan M, Akinc A. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21(8):1570–8. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kolli S, Wong SP, Harbottle R, Johnston B, Thanou M, Miller AD. pH-triggered nanoparticle mediated delivery of siRNA to liver cells in vitro and in vivo. Bioconjugate Chem. 2013;24(3):314–32. doi: 10.1021/bc3004099. [DOI] [PubMed] [Google Scholar]
- 155.Lin SY, Zhao WY, Tsai HC, Hsu WH, Lo CL, Hsiue GH. Sterically polymer-based liposomal complexes with dual-shell structure for enhancing the siRNA delivery. Biomacromolecules. 2012;13(3):664–75. doi: 10.1021/bm201746t. [DOI] [PubMed] [Google Scholar]
- 156.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–64. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bisgaier CL, Siebenkas MV, Williams KJ. Effects of apolipoproteins A-IV and A-I on the uptake of phospholipid liposomes by hepatocytes. J Biol Chem. 1989;264(2):862–6. [PubMed] [Google Scholar]
- 158.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharmaceutics. 2009;6(3):659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 159.With Roche Delivery Tech Advancing, Arrowhead Drops First-Generation Cancer Drug. http://www.genomeweb.com/rnai/roche-delivery-tech-advancing-arrowhead-drops-first-generation-cancer-drug.
- 160.Lewis D. Dynamic Polyconjugates (DPC) Technology: An Elegant Solution to the siRNA Delivery Problem. http://www.arrowheadresearch.com/sites/default/files/udocs/Arrowhead_Research_Corporation-DPC_Technology_White_Paper.pdf.
- 161.Wong SC, Klein JJ, Hamilton HL, Chu Q, Frey CL, Trubetskoy VS, Hegge J, Wakefield D, Rozema DB, Lewis DL. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012;22(6):380–90. doi: 10.1089/nat.2012.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, Hegge JO, Klein JJ, Wakefield DH, Oropeza CE, Deckert J, Roehl I, Jahn-Hofmann K, Hadwiger P, Vornlocher HP, McLachlan A, Lewis DL. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21(5):973–85. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maier M. Advances in Systemic Delivery of RNAi Therapeutics. http://www.alnylam.com/Files/Presentations/ALNY-12thUS-Japan%20Symp-on%20DDS-131220.pdf.
- 164.Singh S, Narang AS, Mahato RI. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28(12):2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- 165.Mahato RI, Kawabata K, Nomura T, Takakura Y, Hashida M. Physicochemical and pharmacokinetic characteristics of plasmid DNA/cationic liposome complexes. J Pharm Sci. 1995;84(11):1267–71. doi: 10.1002/jps.2600841102. [DOI] [PubMed] [Google Scholar]