Abstract
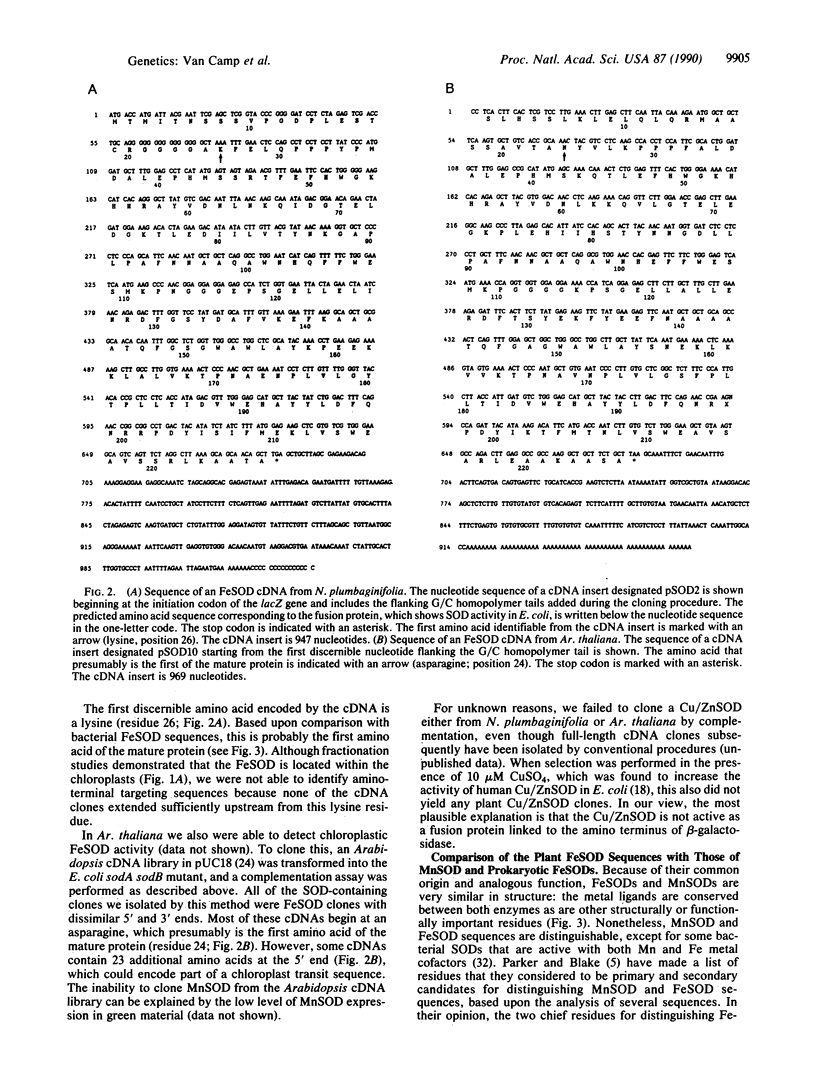
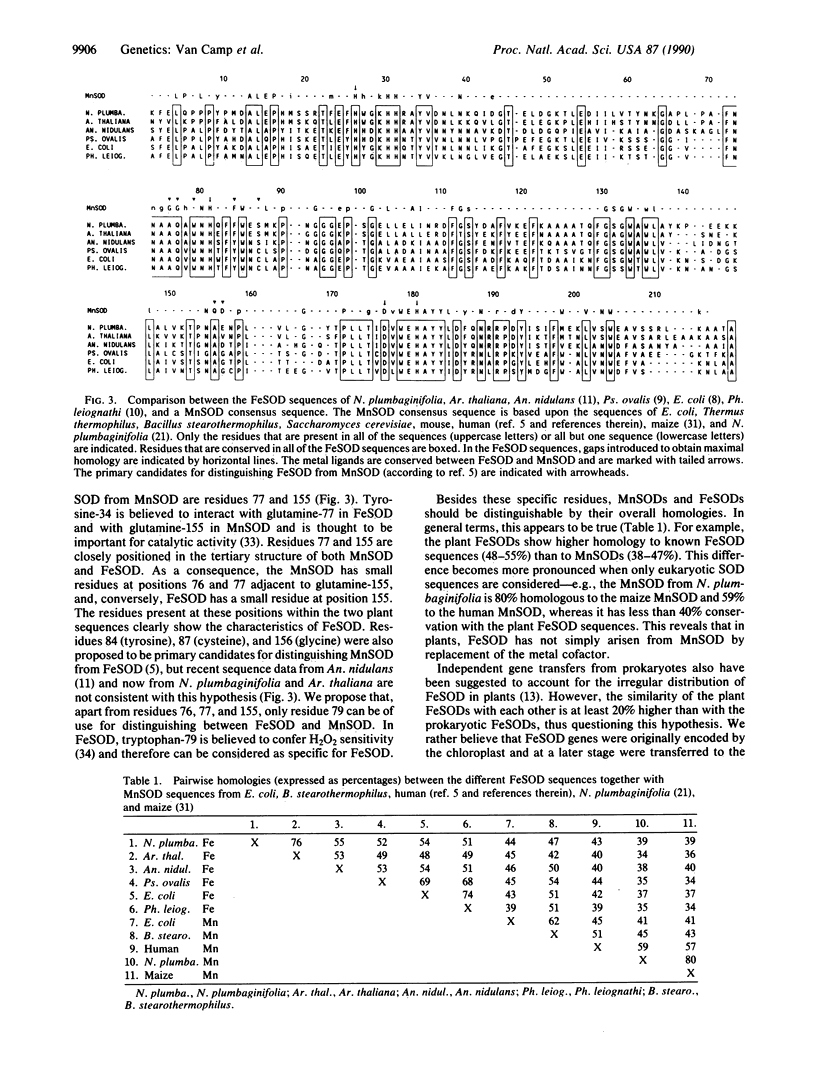
The inability of superoxide dismutase (SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1)-deficient mutants of Escherichia coli to grow aerobically on minimal medium can be restored by functional complementation with a heterologous SOD-encoding sequence. Based upon this property, a phenotypic selection system has been developed for the isolation of clones containing eukaryotic SOD cDNAs. cDNA expression libraries from both Nicotiana plumbaginifolia and Arabidopsis thaliana were transformed into a SOD-deficient E. coli strain by electroporation, and clones containing functional SODs were selected by growth on minimal medium. Analysis of these clones revealed the identity of cDNAs encoding the iron form of superoxide dismutase (FeSOD)--the first SODs of this type to be cloned from eukaryotes. The presence of this enzyme in these two divergent plant species challenges previous ideas that FeSOD is found in only a few plant families. In addition, these results show the potential for shotgun cloning of eukaryotic genes by complementation of bacterial mutants, particularly when it is combined with a highly efficient transformation method, such as electroporation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliotte T., Tiré C., Engler G., Peleman J., Caplan A., Van Montagu M., Inzé D. An Auxin-Regulated Gene of Arabidopsis thaliana Encodes a DNA-Binding Protein. Plant Physiol. 1989 Mar;89(3):743–752. doi: 10.1104/pp.89.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Barra D., Schininà M. E., Bannister W. H., Bannister J. V., Bossa F. The primary structure of iron-superoxide dismutase from Photobacterium leiognathi. J Biol Chem. 1987 Jan 25;262(3):1001–1009. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., De Loose M., Van Montagu M., Inzé D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1989 Jan;8(1):31–38. doi: 10.1002/j.1460-2075.1989.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., Van den Bulcke M., Bauw G., Vandekerckhove J., Van Montagu M., Inzé D. A plant manganese superoxide dismutase is efficiently imported and correctly processed by yeast mitochondria. Proc Natl Acad Sci U S A. 1989 May;86(9):3237–3241. doi: 10.1073/pnas.86.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Van Kaer L., Van Camp W., Van Montagu M., Inzé D., Dhaese P. Characterization of the Bacillus stearothermophilus manganese superoxide dismutase gene and its ability to complement copper/zinc superoxide dismutase deficiency in Saccharomyces cerevisiae. J Bacteriol. 1990 Mar;172(3):1539–1546. doi: 10.1128/jb.172.3.1539-1546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges S. M., Salin M. L. Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol. 1981 Aug;68(2):275–278. doi: 10.1104/pp.68.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loose M., Alliotte T., Gheysen G., Genetello C., Gielen J., Soetaert P., Van Montagu M., Inzé D. Primary structure of a hormonally regulated beta-glucanase of Nicotiana plumbaginifolia. Gene. 1988 Oct 15;70(1):13–23. doi: 10.1016/0378-1119(88)90100-x. [DOI] [PubMed] [Google Scholar]
- Droillard M. J., Bureau D., Paulin A., Daussant J. Identification of different classes of superoxide dismutase in carnation petals. Electrophoresis. 1989 Jan;10(1):46–48. doi: 10.1002/elps.1150100111. [DOI] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Fang Y. I., Muno D., Okuyama T., Ohmori D., Yamakura F. Amino acid sequence of iron-superoxide dismutase from Pseudomonas ovalis. FEBS Lett. 1987 Oct 19;223(1):92–96. doi: 10.1016/0014-5793(87)80516-1. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Kwiatowski J., Safianowska A., Kaniuga Z. Isolation and characterization of an iron-containing superoxide dismutase from tomato leaves, Lycopersicon esculentum. Eur J Biochem. 1985 Jan 15;146(2):459–466. doi: 10.1111/j.1432-1033.1985.tb08673.x. [DOI] [PubMed] [Google Scholar]
- Laudenbach D. E., Trick C. G., Straus N. A. Cloning and characterization of an Anacystis nidulans R2 superoxide dismutase gene. Mol Gen Genet. 1989 Apr;216(2-3):455–461. doi: 10.1007/BF00334390. [DOI] [PubMed] [Google Scholar]
- Le Trant N., Meshnick S. R., Kitchener K., Eaton J. W., Cerami A. Iron-containing superoxide dismutase from Crithidia fasciculata. Purification, characterization, and similarity to Leishmanial and trypanosomal enzymes. J Biol Chem. 1983 Jan 10;258(1):125–130. [PubMed] [Google Scholar]
- Natvig D. O., Imlay K., Touati D., Hallewell R. A. Human copper-zinc superoxide dismutase complements superoxide dismutase-deficient Escherichia coli mutants. J Biol Chem. 1987 Oct 25;262(30):14697–14701. [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 A resolution. J Mol Biol. 1988 Feb 20;199(4):649–661. doi: 10.1016/0022-2836(88)90308-7. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Isolation and Characterization of an Iron-Containing Superoxide Dismutase From Water Lily, Nuphar luteum. Plant Physiol. 1982 Jan;69(1):161–165. doi: 10.1104/pp.69.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schininà M. E., Maffey L., Barra D., Bossa F., Puget K., Michelson A. M. The primary structure of iron superoxide dismutase from Escherichia coli. FEBS Lett. 1987 Aug 31;221(1):87–90. doi: 10.1016/0014-5793(87)80357-5. [DOI] [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. Manganese and iron superoxide dismutases are structural homologs. J Biol Chem. 1984 Sep 10;259(17):10695–10699. [PubMed] [Google Scholar]
- Steinman H. M., Ely B. Copper-zinc superoxide dismutase of Caulobacter crescentus: cloning, sequencing, and mapping of the gene and periplasmic location of the enzyme. J Bacteriol. 1990 Jun;172(6):2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. Isolation and characterization of a cDNA for mitochondrial manganese superoxide dismutase (SOD-3) of maize and its relation to other manganese superoxide dismutases. Biochim Biophys Acta. 1988 Nov 10;951(1):61–70. doi: 10.1016/0167-4781(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Yamakura F. Destruction of tryptophan residues by hydrogen peroxide in iron-superoxide dismutase. Biochem Biophys Res Commun. 1984 Jul 31;122(2):635–641. doi: 10.1016/s0006-291x(84)80080-7. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]




